Abstract
Background Primary sinonasal tract angiosarcoma are rare tumors that are frequently misclassified, resulting in inappropriate clinical management. There are only a few reported cases in the English literature. Materials and Methods Ten patients with sinonasal tract angiosarcoma were retrospectively retrieved from the Otorhinolaryngic Registry of the Armed Forces Institute of Pathology. Results Six males and four females, aged 13 to 81 years (mean, 46.7 years), presented with epistaxis and bloody discharge. Females were on average younger than their male counterparts (37.8 vs. 52.7 years, respectively). The tumors involved the nasal cavity alone (n = 8) or the maxillary sinus (n = 2), with a mean size of 4.3 cm; the average size was different between the genders: males: 2.8 cm; females: 6.4 cm. Histologically, all tumors had anastomosing vascular channels lined by remarkably atypical endothelial cells protruding into the lumen, neolumen formation, frequent atypical mitotic figures, necrosis, and hemorrhage. All cases tested (n = 6) demonstrated immunoreactivity with antibodies to Factor VIII-RA, CD34, CD31, and smooth muscle actin, while non-reactive with keratin and S-100 protein. The principle differential diagnosis includes granulation tissue, lobular capillary hemangioma (pyogenic granuloma), and Kaposi’s sarcoma. All patients had surgery followed by post-operative radiation (n = 4 patients). Follow-up was available in all patients: Six patients died with disease (mean, 28.8 months); two patients had died without evidence of disease (mean, 267 months); and two are alive with no evidence of disease at last follow-up (mean, 254 months). Conclusions Sinonasal tract angiosarcoma is a rare tumor, frequently presenting in middle-aged patients as a large mass usually involving the nasal cavity with characteristic histomorphologic and immunophenotypic features. Sinonasal tract angiosarcoma will often have a poor prognosis making appropriate separation from other conditions important.
Keywords: Angiosarcoma, Sinonasal tract, Nasal cavity, Vascular, Hemangioma, Sarcoma, Immunohistochemistry, Prognosis, Survival, Differential diagnosis
Introduction
Angiosarcomas are high-grade, malignant vascular tumors that make up only about 2% of all sarcomas [1, 2]. While angiosarcomas may occur in any region of the body, well over half occur in the head and neck, usually involving the skin and superficial soft tissues, particularly the scalp [1, 3–7]. Despite this fact, angiosarcoma accounts for less than 0.1% of all sinonasal tract malignancies [3, 8–13]. Primary sinonasal tract angiosarcomas are exceedingly uncommon and only a few cases have been reported in the English literature [9, 14–34]. The rarity of these tumors may result in the misclassification and subsequent inappropriate management. Further, many synonyms have been applied to angiosarcomas (epithelioid hemangioendothelioma; malignant hemangioendothelioma; malignant angioendothelioma; lymphangiosarcoma; hemangiosarcoma; hemangioblastoma), but the use of these terms in the sinonasal tract is discouraged, especially since hemangioendothelioma represents a unique entity. This report focuses on the clinical presentation, histologic features, immunohistochemical profiles, and therapeutic approaches of sinonasal angiosarcomas in relation to patient prognosis and outcome.
Material and Methods
Ten cases of angiosarcoma involving the involving the nasal cavity (n = 8) or paranasal sinuses (sphenoid, maxillary, ethmoid, and frontal sinuses; n = 2) were retrieved from the files of the Otorhinolaryngic-Head & Neck Tumor Registry of the Armed Forces Institute of Pathology (AFIP), Washington, DC, between 1970 and 1995. These tumors were chosen from a review of 20,156 (0.05%) benign or malignant primary sinonasal tract tumors seen in consultation during this time. All cases were obtained from civilian sources, including university medical centers.
Materials within the AFIP files were supplemented by a review of the patient demographics (gender, age, and race), symptoms at presentation (epistaxis, nasal obstruction, nasal discharge), including duration (Table 1). Follow-up information was obtained by direct written and oral communication with the referring pathologist, patient’s physicians, tumor registries, and patients or patient’s family members. Follow-up data was available for all ten patients and included information regarding exact tumor site, specific treatment modalities used, the presence or absence of recurrent or metastatic disease, and the current status of the disease and patient. It is important to add that we conducted this research from a tertiary pathology review center, conducting a retrospective review of these patients and we did not treat the patients. As we did not prosect the specimen, we had to rely on the contributing pathologist for an accurate assessment of the margins of resection. Submitted diagnoses included juvenile nasopharyngeal angiofibroma, hemangioma, hemangiosarcoma, malignant vascular tumor, malignant hemangiopericytoma, and hemangioendothelioma. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of the Code of Federal Regulations, Title 45, Part 46, and the Department of Defense Directive 3216.2 relating to human subjects in research.
Table 1.
Clinical characteristics
| Clinical characteristics | Number |
|---|---|
| Gender | |
| Females | 4 |
| Males | 6 |
| Age (in years) | |
| Range | 13–81 |
| Mean | 46.7 |
| Women (mean) | 37.8 |
| Men (mean) | 52.7 |
| Symptoms | |
| Duration (range, in months) | 2–24 |
| Duration (mean, in months) | 10.7 |
| Epistaxis | 6 |
| Obstructive symptoms | 3 |
| Nasal discharge | 1 |
| Anatomic site | |
| Nasal cavity alone | 8 |
| Maxillary sinus alone | 2 |
| Size (cm) | |
| Range | 1.8–8 |
| Mean | 4.3 |
| Female (mean) | 6.4 |
| Male (mean) | 2.8 |
| Maxillary sinus | 8.0 |
| Nasal cavity | 2.9 |
Hematoxylin and eosin-stained slides from all cases were reviewed to confirm that the established histopathologic criteria for the diagnosis of angiosarcoma were met. A number of macroscopic and histologic observations were recorded for each of the tumors as follows: tumor location (Fig. 1); tumor size (greatest dimension in centimeters); extravasated blood (absent or present [Fig. 2]); respiratory epithelium (present of absent); anastomosing vascular channels (Fig. 3); pleomorphism (moderate or severe [Fig. 3]); tumor cell spindling; neolumen formation (Figs. 2, 4, 5); mitotic figures (number of mitotic figures per 10 high power fields [magnification at 40× with a 10× objective lens using Olympus BX40 microscope]); atypical mitotic figures (present or absent, and defined by abnormal chromosome spread, tripolar or quadripolar forms, circular forms, or indescribably bizarre [Fig. 5]); necrosis (absent or present); and the presence of other microscopic pathologic findings.
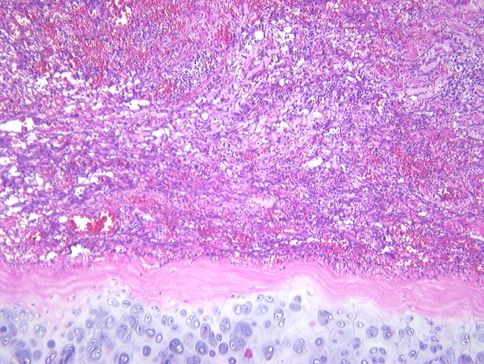
Fig. 1.
A vascular neoplasm abuts the nasal cartilage in this angiosarcoma of the nasal cavity
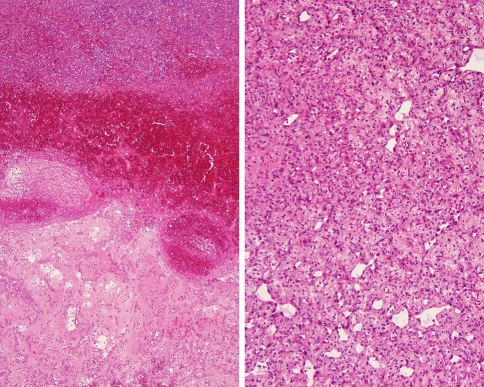
Fig. 2.
Blood with degeneration is the dominant finding (left), while a more bland cytologic appearance is seen in a different angiosarcoma (right)
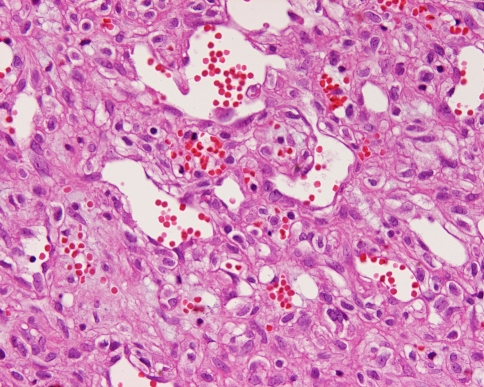
Fig. 3.
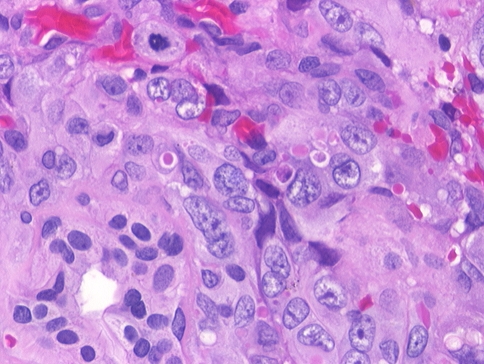
Dilated open vascular channels with moderately pleomorphic nuclei are noted. Extravasated erythrocytes and small areas of neolumen formation are seen
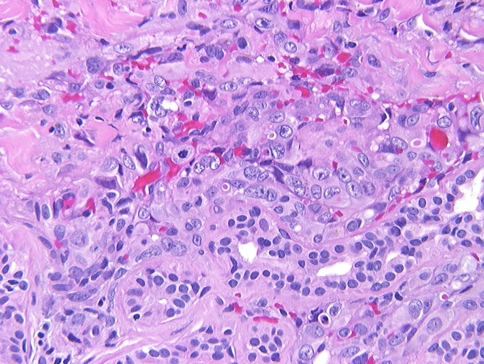
Fig. 4.
A more solid pattern of growth is appreciated as the malignant endothelial cells wrap around minor mucoserous glands. Mitotic figures and neolumen are seen
Fig. 5.
High power illustrating an atypical mitotic figure and neolumen formation within the cytoplasm of a malignant endothelial cells. Uninvolved minor mucoserous glands are seen
Immunophenotypic analysis was performed in all cases with suitable material by a standardized Envision™ method employing 4 μm-thick, formalin fixed, paraffin embedded sections. Table 2 documents the pertinent, commercially available immunohistochemical antibody panel used. The analysis was performed on a single representative block for each tumor. When required, proteolytic antigen retrieval was performed by predigestion for 3 min with 0.05% Protease VIII (Sigma Chemical Co., St. Louis, MO) in a 0.1 M phosphate buffer, pH of 7.8, at 37°C. Heat induced epitope retrieval was performed, as required, by using formalin-fixed, paraffin-embedded tissue treated with a buffered citric acid solution pH 6.0 (Citra, Dako Corporation, Carpinteria, CA) and heated for 20 min in a steamer. Standard positive controls were used throughout, with serum used as the negative control. The antibody reactions were described as either positive or negative. The Ki-67 antibody reaction was recorded as the fraction of positive cells, separating them into four groups: <10%, 11–50%, 51–90%, and >90%.
Table 2.
Immunohistochemical panel
| Antigen/antibody | Type | Company | Dilution | Antigen recovery |
|---|---|---|---|---|
| Factor VIII-RA | rp | Dako, Carpinteria, CA | 1:50 | n/a |
| CD34 | mm | BioGenex Labs, San Ramon CA | 1:40 | Steam |
| CD31 | mm | Dako | 1:100 | Steam |
| Cytokeratin (AE1/AE3 and LP34) |
mm | Boehringer Mannheim Biochemicals, Indianapolis, IN, and Dako | 1:50 1:200 |
Protease digestion |
| Epithelial membrane antigen | mm | Dako | 1:100 | Protease digestion |
| Muscle specific actin | mm | Ventana, Tucson, AZ | Neat | Protease digestion |
| Smooth muscle actin | mm | Sigma Chemical, St. Louis, MO | 1:400 | n/a |
| S-100 protein | rp | Dako | 1:800 | n/a |
| Ki-67 | mm | Immunotech, Westbrook, ME | 1:20 | Steam |
mm: mouse monoclonal; rp: rabbit polyclonal
A review of the English literature based on a MEDLINE search from 1966 to 2007 was performed and all cases involving the nasal cavity and/or paranasal sinuses were included in the review, the majority of which were single case reports. Clinical series of “head and neck angiosarcomas” were selected if critical information about sinonasal tract lesions was included. No foreign language articles were included.
Categorical variables were analyzed using chi-square tests to compare observed and expected frequency distributions. Comparison of means between groups were made with unpaired t-tests or one-way analysis of variance, depending on whether there were two groups or more than two groups, respectively. Multiple comparisons were analyzed using the Tukey method. Linear regression was used to investigate two measured variables, and Pearson correlation coefficients were generated to measure the strength of the association. Confidence intervals of 95% were generated for all positive findings. The alpha level was set at P < 0.05. All analyses were conducted using Statistical Package for the Social Sciences (SPSS) software (version 8.0 for PC; Chicago, IL).
Results
Clinical
The patients included 4 women and 6 men (Table 1) who ranged in age from 13 to 81 years (mean, 46.7 years); females were on average younger than their male counterparts (37.8 vs. 52.7 years, respectively), although there are insufficient cases to reach statistical significance. Patients presented with symptoms of epistaxis, nasal discharge and nasal obstruction with a duration that ranged from 2 months up to 2 years. The mean duration was 10.7 months. Two patients reported additional primary tumors, unrelated to the sinonasal tract lesion: one female with a breast carcinoma and one male with a lung carcinoma. None of the patients had any syndrome, specifically Kasabach–Merritt syndrome.
Pathologic Features
Macroscopic
The tumors involved the nasal cavity alone (n = 8) (Figure 1) or the maxillary sinus alone (n = 2), with the mean size of 4.3 cm (range, 1.8 to 8 cm). The average size varied with gender: 2.8 cm for males; 6.4 cm for females, although there were insufficient cases to reach statistical significance (Table 1). There was a statistical difference in the mean size of tumors which involved specific sites, nasal cavity alone (mean, 2.9 cm) and maxillary sinus (mean, 8.0 cm; P = 0.01). Further, maxillary sinus tumor patients were more likely to die from their disease in a shorter time than those with nasal cavity tumors (P = 0.039). Furthermore, the larger the overall size of the lesion (>4.0 cm) the more likely the patient was to have a poor clinical outcome (P = 0.02) The tumors were described as nodular and polypoid, soft and friable, purple to red, and often ulcerated with associated hemorrhage or clot and necrosis.
Microscopic
The majority of tumors demonstrated overlying respiratory surface epithelium (n = 9). Necrosis and hemorrhage (Fig. 2) were easily identified in most cases (necrosis, n = 7; hemorrhage, n = 10). Angiosarcoma has vasoformative neoplastic cells that infiltrate into adjacent soft and hard tissues. Most tumors had anastomosing vascular channels (n = 7), that appeared as tortuous, irregular vascular channels dissecting the stroma with cleft-like spaces, rudimentary vessels, and small to large cavernous spaces. These spaces are filled with erythrocytes and lined by plump, enlarged, atypical, spindled (n = 7) or epithelioid endothelial cells which protruded into the vascular spaces in multiple layers or papillae (Fig. 3). Intracytoplasmic vacuoles, or neolumen, were identified (n = 8) and contained erythrocytes (Figs. 3, 4, and 5). This feature was more characteristic of the epithelioid growth pattern. The endothelial cells demonstrated pleomorphic nuclei (severe, n = 3; moderate, n = 7) with coarse and heavy nuclear chromatin deposition, irregular nuclear contours, and prominent nucleoli (Fig. 3). Mitotic figures (Fig. 4) were seen in all of the cases and were easily identified. Additionally, atypical mitotic figures were present in most of the tumors (n = 8) (Fig. 5). No extracellular eosinophilic hyaline globules were identified. Inflammatory cells were present to a variably degree in all cases, although without a dominant cell type identified.
Immunohistochemical Results
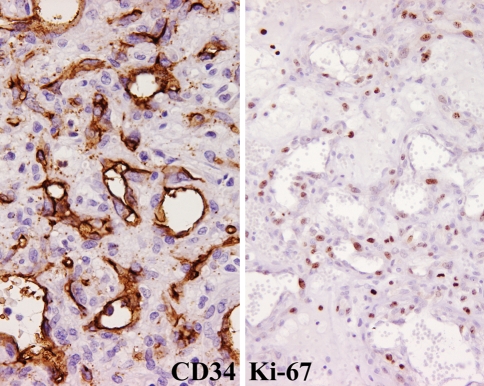
All tested cases (n = 6) demonstrated immunoreactivity with antibodies to Factor VIII-RA, CD34 (Fig. 6), CD31, and smooth muscle actin, while non-reactive with keratin and S-100 protein (Table 3). Epithelial membrane antigen (n = 1) and muscle specific actin (n = 2) showed varied immunoreactivity. Ki-67 was reactive, at >10% in all the tested cases (Fig. 6).
Fig. 6.
CD34 strongly stained the neoplastic cells’ cytoplasm and was distributed in a haphazard fashion (left). Ki-67 strongly and diffuse reacted with the nuclei in many cases (right)
Table 3.
Pathology findings
| Microscopic characteristic | Number of cases |
|---|---|
| Respiratory epithelium present | 9 |
| Anastomosing vascular channels | 7 |
| Tumor cell spindling | 7 |
| Mitotic activity | 1–18 |
| Atypical mitotic figures present | 8 |
| Pleomorphism | |
| Moderate | 7 |
| Severe | 3 |
| Neolumen formation | 8 |
| Necrosis present | 7 |
| Extravasated blood present | 10 |
| Immunohistochemical results | |
| Factor VIII-RA | 100% |
| CD34 | 100% |
| CD31 | 100% |
| Keratin | 0% |
| Epithelial membrane antigen | 20% |
| Muscle specific actin | 33% |
| Smooth muscle actin | 100% |
| S-100 protein | 0% |
| Ki-67 (>10%) | 100% |
Treatment and Follow-up
All patients were treated with surgery alone or with surgery followed by post-operative radiation (n = 4) and chemotherapy (n = 2). Follow-up was available in all ten patients (mean follow-up, 121 months; Table 4). Six patients died with disease (mean, 28.4 months), 2 were alive with no evidence of disease (mean, 254 months), and 2 were dead with no evidence of their disease (mean, 267 months) and died as the result of other primary malignancies.
Table 4.
Patient outcome
| All patients | A, NED | D, NED | D, D | |
|---|---|---|---|---|
| All patients with follow-up (years) | 10 (10.1) | 2 (21.2) | 2 (22.3) | 6 (2.4) |
| Follow-up range (years) | 0.1–32.7 | 9.6–32.8 | 18.7–25.8 | 0.1–6.3 |
| Gender | ||||
| Males (years) | 6 (12.6) | 2 (21.2) | 1 (25.8) | 3 (2.4) |
| Females (years) | 4 (6.5) | n/a | 1 (18.7) | 3 (2.4) |
| Age | ||||
| <40 years | 5 (12.1) | 1 (32.8) | 1 (18.7) | 3 (3.1) |
| ≥40 years | 5 (8.1) | 1 (9.6) | 1 (25.8) | 3 (1.7) |
| Size* | ||||
| <4.0 cm | 4 (13.3) | 2 (21.2) | n/a | 2 (5.4) |
| ≥4.0 cm | 3 (0.4) | n/a | n/a | 3 (0.4) |
| Anatomic site | ||||
| Nasal cavity alone | 8 (12.5) | 2 (21.2) | 2 (22.3) | 4 (3.4) |
| Maxillary sinus alone | 2 (0.4) | n/a | n/a | 2 (0.4) |
| Treatment received | ||||
| Surgery alone | 6 (11.9) | 1 (32.8) | 1 (25.8) | 4 (3.3) |
| Surgery with radiation | 2 (5.0) | 1 (9.6) | n/a | 1 (0.4) |
| Surgery with radiation/chemotherapy | 2 (9.7) | n/a | 1 (18.7) | 1 (0.7) |
A, NED: Alive, No Evidence of Disease; D, NED: Dead, No Evidence of Disease; D, D: Dead of Disease, * Size was not reported in all cases; n/a: not applicable
The four female patients (mean follow-up, 78 months; 3 dead with disease, mean 29 months) tended to have a poorer outcome than the six male patients (mean follow-up 151 months, 3 dead with disease, mean 29 months), but this may be related to the size of the tumors. A male and a female patient each died with no evidence of the primary angiosarcoma, but succumbed to other primary malignancies (mean follow-up, 267 months). Radiation and radiation with chemotherapy did not alter the patient outcome by a statistically identifiable amount. Age did not reach statistical significant as a predictor of patient outcome. Size of greater than or equal to 4 cm correlated with poor outcome (P = 0.02). Finally, tumors involving the maxillary sinuses tended to do worse than those which involved the nasal cavity alone (P = 0.039).
Discussion
Despite the fact that the majority of angiosarcomas affect the skin and soft tissues of the head and neck, angiosarcomas within the sinonasal tract account for <0.1% of all malignancies in this region [1, 3, 3–13]. This study reports the largest single series of sinonasal tract angiosarcomas reported in the English language to date, with the vast majority of studies reporting only a single case [9, 14–34]. Angiosarcoma has been reported to develop in nearly all anatomic sites, but when this high-grade vascular neoplasm occurs in the sinonasal tract, a number of differential diagnostic considerations are raised, along with a different outcome than primary angiosarcomas in other anatomic sites.
Retrospective analysis of any disease is a difficult undertaking in modern medicine, and even more so when the entity is rare. Terminology has evolved over the past few decades, rendering the many names used in the past for angiosarcoma surfeit. Clinical presentation, gender differences, anatomic site of distribution, size, histologic and immunohistochemical features, and patient outcome has not been well characterized by the many single case reports. The information in this study is combined with that gleaned from the literature (Table 5) in an attempt to more fully elucidate the nature of this uncommon tumor and perhaps contribute to more meaningful clinical management.
Table 5.
| Characteristics | Number (39) |
|---|---|
| Gender | |
| Females | 12 |
| Males | 26 |
| Age (in years) | |
| Range | 8–82 |
| Mean | 46.8 |
| Women (mean) | 42.6 |
| Men (mean) | 48.8 |
| Symptoms* | |
| Duration (range, in months) | 0.3–96 |
| Duration (mean, in months) | 9.8 |
| Epistaxis | 20 |
| Obstructive symptoms | 18 |
| Nasal discharge | 5 |
| Anatomic site | |
| Nasal cavity alone | 14 |
| Paranasal sinus alone | 10 |
| Combination of sinuses & nasal cavity | 14 |
| Size (cm)* | |
| Range | 0.7–8 |
| Mean | 3.9 |
| Female (mean) | 6.0 |
| Male (mean) | 2.9 |
| Paranasal sinus alone | 6.8 |
| Nasal cavity | 2.2 |
| Combination of sinuses & nasal cavity | 4.4 |
| Patient survival (mean, months) | |
| A, NED | 21 (47) |
| A, D | 1 (2.0) |
| D, NED | 3 (187) |
| D, D | 14 (18.2) |
* Not reported for all cases; A, NED: Alive, No Evidence of Disease; A, D: Alive, with disease; D, NED: Dead, No Evidence of Disease; D, D: Dead of Disease
Clinical Information
In our series, sinonasal angiosarcomas were more common in men than women (male: female, 3:2), a finding supported by the literature (male: female, 2.2) (Table 5). It is interesting that this is similar to angiosarcoma of the skin, in which there is also a distinct male predilection [6, 35]. There is a wide age range at presentation (8–82 years), with a mean of 46.8 years, substantially younger than the 8th decade mean age at presentation for soft tissue and skin angiosarcomas [6, 8, 11, 36–39]. While women tended to be younger than men at presentation (42.6 versus 48.8 years), this difference was not statistically significant. The patients tended to have symptoms for an average of 9.8 months, with epistaxis and obstruction identified most frequently (20 and 18, respectively). Other symptoms included nasal discharge, expanding or enlarging mass, sinusitis, epiphora, pain (headache, otalgia, tooth-ache), diplopia, ptosis and headaches. Needless to say, none of these symptoms is specific for this tumor, although the high rate of epistaxis is probably related to the vascular nature of the neoplasm. In fact, we posit that the overall better clinical prognosis for sinonasal tract angiosarcomas when compared to their skin, soft tissue or visceral counterparts, may be due to the earlier stage at diagnosis because of epistaxis as a presenting symptom. This results in an earlier detection of the tumor, and possibly a better outcome than angiosarcomas in other anatomic sites [1, 9, 10, 12, 40–44]. Within the sinonasal tract, a single anatomic site is affected more commonly than multiple sites (nasal cavity alone = 14; single sinus = 14; multiple areas = 10). Any of the paranasal sinuses can be involved (maxillary, ethmoid, sphenoid, cavernous sinus), but the maxillary sinus seems to be involved more frequently than the others. It is curious that when multiple sites are involved by tumor, the mean size of tumor (4.4 cm) is less than if a single paranasal sinus is involved (mean, 6.8 cm). This discrepancy is accounted for by the overall lack of size data from the single case reports. Overall, the tumor size within the nasal cavity alone is less than the paranasal sinuses alone or if there is a combination of nasal cavity and paranasal sinus (2.2 cm vs. 6.8 cm vs. 4.4 cm, respectively). It is our impression from this data, that perhaps a lesion within the confines of the nasal cavity is more likely to be evaluated earlier than a lesion which affects the sinuses [16, 17, 26, 45]. Tumors in female patients tend to be larger (mean, 6.0 cm) than tumors in male patients (mean, 2.9 cm), although there are insufficient cases to reach statistical significance.
While no patients in our clinical series had any documented environmental exposure as a possible etiologic factor, three patients reported in the literature had prior radiation exposure [25, 34, 46], one patient reported working in a coal mine for decades [9], and one patient reported exposure to vinyl chloride [32]. Therefore, it is possible that rare cases may have an environmental etiology.
Radiographic Studies
Angiosarcoma is an aggressive infiltrative tumor that will often invade adjacent soft tissues, cartilage and bone (Fig. 1). Sinonasal tract angiosarcoma may be radiolucent or radio-opaque. A soft tissue density, it may be associated with bone erosion or occasionally have well-delimited borders. Because of its ability to erode bone, computed tomography (CT) may allow for an accurate determination of the extent of the mass, showing enhancement with contrast. Magnetic resonance imaging (MRI) shows the tumor to be bright on T2-weighted images. Angiography is an excellent modality to identify the extent of the tumor and show the feeder vessel(s) if they are present, while also allowing for pre-surgical angiographic embolization, if desired [19, 22, 29, 30, 34].
Pathology
Macroscopically, the tumors are nodular and polypoid, although with increased size, they tend to infiltrate the surrounding tissues. The tumors are soft and friable, purple to red, and often associated with hemorrhage, clot and necrosis.
Respiratory epithelium was present and intact in 9 of 10 cases in this series, a histologic finding similar to descriptions of intact epidermis overlying skin primary angiosarcomas [47, 48]. Ulceration, therefore, is not a common finding, except in lesions that are large or have involved more than one anatomic site [15, 22–24, 30, 32, 35]. As may be expected, all our cases and the majority of those in the literature demonstrated histologic evidence of blood or extravasated erythrocytes. The vasoformative pattern seems to be quite universal, with tortuous, irregular, freely anastomosing vascular channels dissecting through stroma and creating cleft-like spaces, rudimentary vessels, capillary-sized vessels and large cavernous spaces. These vascular spaces and channels were filled with erythrocytes and lined by plump, enlarged, atypical endothelial cells which protruded into the vascular spaces in multiple layers or papillae. An epithelioid appearance can be seen focally in many cases, but it is usually not the dominant pattern. Tumor cell spindling is also present, and may sometimes expand the differential diagnosis. Intracytoplasmic neolumen were identified in the majority of cases (8 of 10 cases), but this feature is not always histologically demonstrated or described in the case reports. Neolumina seem to be more easily identified in areas that are epithelioid in appearance. The presence of erythrocytes within these spaces certainly confirms the vasoformative nature of this tumor (Figs. 3 and 4).
The neoplastic cells usually show profound nuclear pleomorphism, with the nuclei showing enlargement with coarse and heavy nuclear chromatin distribution. The nuclear contours are frequently irregular or “moth-eaten.” Prominent, irregular nucleoli are seen (Fig. 3). Mitotic figures were easily identified in all cases, ranging from 1 to 18 figures per 10 high power fields. Atypical forms, also readily identifiable in most cases, consisted of abnormal chromosome spread, tripolar or quadripolar forms, and circular or indescribably bizarre forms (Figs. 4 and 5). None of the cases in this series showed extracellular, eosinophilic, hyaline globules, a finding which is more frequent in Kaposi sarcoma [41, 49]. Overall, all tumors could be classified as high grade, without any low-grade lesions identified.
Immunohistochemical Studies
Angiosarcomas of the sinonasal tract are essentially the same as those of visceral sites. They are immunoreactive with vimentin, CD34, CD31, and Factor VIII-RA, while focally reactive with keratin or epithelial membrane antigen and actins (smooth muscle actin or muscle specific actin). While Factor VIII-RA is the most specific, it is also the least sensitive vascular marker. The neoplastic cells are non-reactive with chromogranin, desmin, S-100 protein, and HHV-8 [12, 22, 26, 33, 47, 50–53].
In this series, all tested cases demonstrated immunoreactivity with antibodies to CD34, similar to visceral angiosarcomas [54], but different from cutaneous angiosarcomas which are less likely to react with CD34. This suggests that sinonasal tract angiosarcomas are more closely associated with “angio-derived” endothelium, rather than “lymph-derived” endothelium of cutaneous primaries [51, 55, 56]. All cases in this series reacted with CD34, CD31 and Factor VIII-RA. Whereas as a single marker confirms the diagnosis, a panel approach does allow for a greater degree of certainty. Actins are a reflection of smooth muscle identified in the vascular wall of the tumor cells rather than within the endothelial cells. Their reactivity in this series is consistent with that reported in the literature [12, 22, 26, 43, 48, 52, 55, 57, 58].
While the cases in this series were non-reactive with the keratin cocktail (AE1/AE3), reactivity with epithelial membrane antigen was expressed in 20% of these cases. It is well known that epithelioid vascular tumors will be immunoreactive with keratins of varying molecular weights, and also with endothelial markers [12, 43, 51–53, 55, 59]. It is important, therefore, to interpret keratin or EMA immunoexpression in conjunction with the characteristic histologic features of the tumor and in the setting of positive immunoreactivity with endothelial/vascular markers. The Ki-67 reaction highlighting greater than 10% of the neoplastic cells suggests a high proliferation rate in these tumors, but by itself is not of diagnostic utility, as granulation-tissue and even lobular capillary hemangioma can have an increased mitotic index.
While we did not perform molecular or cytogenetic studies in this series, there are no specific findings in sinonasal angiosarcomas, but instead very complex structural chromosomal abnormalities with multiple deletions and additions [33, 60].
Differential Diagnosis
The differential diagnosis for angiosarcoma in the sinonasal tract includes granulation tissue, intravascular papillary endothelial hyperplasia (Masson’s disease), hemangioma (including variants, such as lobular capillary hemangioma), juvenile nasopharyngeal angiofibroma, epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia), glomangiopericytoma (sinonasal-type hemangiopericytoma), Kaposi sarcoma, and mucosal melanoma [12, 38, 39, 61–71].
Juvenile nasopharyngeal angiofibroma is a cellular and richly vascularized mesenchymal neoplasm that arises in a specific location in the nasopharynx (pterygoid region) in young male patients exclusively. Histologically, there is a background fibrous connective tissue stroma in which are found many, variably sized, disorganized vessels with patchy muscle content. The endothelial cells may be plump, but are not atypical. Elastic tissue is lacking in the vessel walls. Mast cells are common [66, 72–75].
Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia or histiocytoid hemangioma) is rare in the sinonasal tract, although it is common in the skin of the head and neck. This lesion is not the same as Kimura disease, which involves lymph nodes and shows fibrosis, prominent germinal centers with folliculolysis and eosinophil microabscesses [76]. Epithelioid hemangioma has a prominent, distinctive, vascular proliferation in association with a heavy nodular to diffuse lymphocytic infiltrate, usually associated with eosinophils. The endothelial cells are usually enlarged, protruding into the lumen in a cobblestone or hobnail type fashion, often occluding the vessel lumen. Vacuoles may be seen within the cytoplasm. However, cytologic atypia is not present. Angiosarcoma is not usually associated with a heavy lymphocytic infiltrate or with eosinophils [77–79].
Glomangiopericytoma (sinonasal-type hemangiopericytoma) shows a diffuse growth of closely packed cells that are arranged in short interlacing fascicles (storiform or whorled) that are richly vascularized. These vascular channels range from capillary-size to large patulous spaces that may have a ramifying “staghorn” configuration. There is a characteristic and prominent peritheliomatous hyalinization. The neoplastic cells form a closely packed syncytium of uniform, monotonous, oval to slightly spindle-shaped cells with indistinct cell borders. The nuclei are vesicular to hyperchromatic and oval to spindle-shaped. Extravasated erythrocytes, mast cells, and eosinophils are almost always present. The tumor cells are immunoreactive with actins but not with the vascular markers CD34, CD31, or Factor VIII-RA [61, 65].
Mucosal malignant melanoma, when it is non-pigmented, may present with a peritheliomatous palisade of neoplastic cells. However, a freely anastomosing vascular pattern is not identified in melanomas of the sinonasal tract [62, 80].
Kaposi sarcoma primary in the sinonasal tract is rare, with only isolated clinical reports [81–83]. The histology is identical to other anatomic sites, although the plaque-tumor stage with its sieve-like vasoformative pattern, slightly atypical spindled tumor cells and with eosinophilic, glassy-hyaline intra- and extra-cellular globules (PAS positive) is most common. HHV-8 is usually positive, helping to confirm the diagnosis.
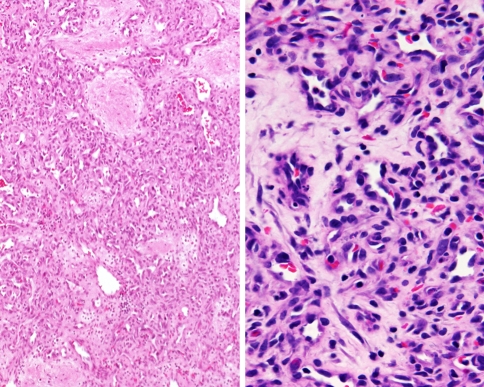
The term pyogenic granuloma is a misnomer, since it is not related to an infection and does not have granuloma formation histologically. The equivalent better term is lobular capillary hemangioma (LCH). The lesion is clinically polypoid. The low power view often shows surface epithelial ulceration and fibrinoid necrosis. LCH exhibits a distinct lobular architecture with a mixture of thin and thick blood vessels comprising the center the lesion (Fig. 7). The lobules are quite cellular and composed of small, closely packed capillaries with slit-like or indistinct lumina. The endothelial cells can be quite plump, but the nuclei are bland. Mitotic activity is usually brisk. The center and superficial portions of LCH show well-formed capillaries or large angulated vessels with branching lumina. These vessels may have thick walls resembling small arteries or venules. The stroma ranges from edematous to fibrotic. A variable inflammatory infiltrate composed of small lymphocytes, plasma cells, mast cells, and neutrophils is also present. The low power organization, lobular architecture, and lack of cytologic atypia helps to separate it from angiosarcoma [17, 63, 77, 84, 85]. The distinct separation of LCH from granulation tissue can at times be challenging. However, granulation tissue seems to have a perpendicular arrangement of the capillaries to the surface and is rich with inflammatory cells and histiocytes. Mitotic figures will be present, but are not atypical. There are no freely anastomosing vascular channels [67]. Recanalization of thrombosed vessels within the sinonasal tract may develop (Masson’s vegetant endothelial hyperplasia or intravascular papillary endothelial hyperplasia). The vessel wall is usually easy to identify, and there is no atypia of the endothelial cells as they line the papillary projections within the organizing spaces [4, 68, 86, 87].
Fig. 7.
The most difficult differential diagnosis is with lobular capillary hemangioma (pyogenic granuloma). The lobular configuration, granulation tissue and lack of atypical mitotic figures and no “neolumen” help to make the separation
Other entities may occasionally enter the differential diagnosis (angiomatous polyp [88–90], angiomyolipoma [91], arteriovenous malformation [92, 93], vascular leiomyoma [63, 94]), but these lesions do not have cytologic atypia or the complex arborizing architectural arrangement seen in angiosarcoma.
Treatment and Prognosis
Surgical resection with radiation and/or chemotherapy is the treatment of choice, although the majority of patients in this clinical series were managed by surgery alone. Recurrences developed in only two of our patients but are more common in the reported cases (38.4% in total). Radiation alone was used in approximately 41%, with radiation and chemotherapy in an additional 18%. In spite of the recurrence rate, there is an approximately 61% survival overall survival (Table 5), distinctly unique from the uniformly fatal prognosis within two years for skin and soft tissue angiosarcomas [3, 9, 14, 17, 19–21, 24, 25, 27–34, 95–97]. While many patients survive, the reported cases in the literature only follow the patient for the first 6–12 months, stating they are disease free at that time. In this clinical series, 60% of patient died with disease, an average of 2.4 years after initial surgery. Therefore, with longer follow-up, the death rate from disease may in fact be higher than presently reported in the literature.
While 69% of male patients are either alive or have died but without evidence of disease, only 42% of female patients fit in the same category. While this difference is noticeable, the actual overall follow-up time is similar: 64.8 and 67 months, respectively. Similarly, 58% of female patients died with evidence of disease, while only 31% of male patients did, but they both died within a similar time frame: 16.3 and 17.8 months, respectively, a finding that is not statistically significant. This suggests there is no alteration in prognosis based on gender.
When sinonasal angiosarcomas develop metastatic disease, it is usually to the lung, liver, spleen, and bone (marrow) [38]. Patients with a specific etiologic factor seem to have a shorter outcome [32, 34].
There is a trend for patients of greater than 50 years of age to have a worse clinical outcome than patients who are younger: 29.0 months versus 61.8 months, respectively. However, this data is not statistically meaningful since the mean follow-up for the patients in the literature is 19.5 months, while for this clinical series it is 121.3 months. There is a trend towards tumors of >4 cm tending to behave more aggressively than patients with tumors <4 cm: 13.9 months versus 81.9 months, respectively. However, the size information was not given in all reports and the short follow-up period may confound the data. However, size seems to play a role in long term patient outcome. Finally, there is a significantly greater survival for patients with tumors confined to the nasal cavity (n = 14; mean = 96.8 months) versus those with maxillary sinus only (n = 10; mean = 18.2 months) or with mixed sinus and nasal cavity (n = 14; mean = 15.8 months). Due to the limited number of patients, a multivariate analysis was not possible and so specific prognostic factors cannot be stated with certainty.
There is no accepted staging for sarcomas in the sinonasal tract, although lymph node and distant metastasis is not common at initial presentation. Likewise, while a grading system of grades 1 through 3 is used in soft tissue angiosarcomas, this system has not been applied to angiosarcomas of the sinonasal tract. Grading may not have clinical prognostic significance in sinonasal tract angiosarcomas, although further evaluation with a larger number of cases would be necessary [39].
Conclusion
Primary sinonasal angiosarcoma is a rare neoplasm-affecting males more frequently than females, although females tend to present at a slightly younger age than male patients. Clinical presentation overlaps with many other sinonasal tract lesions, and, therefore, angiosarcoma should be considered in the differential diagnosis of a sinonasal tract mass. Females tend to have larger tumors than male patients. Female patients tend to die more frequently of their tumors, but the overall survival rate is not altered. There is a wide age range at presentation, with a peak in the fifth decade, significantly younger than cutaneous angiosarcomas (7th and 8th decades). Patients who are older than 50 years at the time of diagnosis tend to survive for a shorter time than those who are younger than 50 years at the time of discovery. The tumors are vasoformative, with freely anastomosing vascular channels and local invasion. They are immunoreactive with CD31, CD34, and Factor VIII-RA. Sinonasal angiosarcoma appears to show a less aggressive clinical course in comparison to other types of angiosarcoma. Although recurrences develop in about 38% of patients, a combination of surgery with radiation therapy seems to yield an overall 61.5% survival. Prognosis seems to be better when tumors are confined to the nasal cavity and measure less than 4 cm, without any prognostic difference based on tumor morphology (necrosis or epithelioid appearance).
Acknowledgement
Presented at the 93rd Annual Meeting of the United States and Canadian Academy of Pathology, Vancouver, British Columbia, Canada, March 6–12, 2004.
References
- 1.Ordonez-Escalante KG, Mantilla-Morales A, Gallegos F. [Nasal cavity angiosarcoma: a case report and literature review] Gac Med Mex. 2006;142:155–8. [PubMed] [Google Scholar]
- 2.Fletcher CD. Distinctive soft tissue tumors of the head and neck. Mod Pathol. 2002;15:324–30. doi: 10.1038/modpathol.3880526. [DOI] [PubMed] [Google Scholar]
- 3.Bardwil JM, Mocega EE, Butler JJ, et al. Angiosarcomas of the head and neck region. Am J Surg. 1968;116:548–53. doi: 10.1016/0002-9610(68)90391-7. [DOI] [PubMed] [Google Scholar]
- 4.Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106–13. doi: 10.1002/1097-0142(197909)44:3<1106::AID-CNCR2820440345>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Jones EW. Dowling oration 1976. Malignant vascular tumours. Clin Exp Dermatol. 1976;1:287–312. doi: 10.1111/j.1365-2230.1976.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 6.Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907–21. doi: 10.1002/1097-0142(19811015)48:8<1907::AID-CNCR2820480832>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Matejka M, Konrad K. Cutaneous angiosarcoma of the face and scalp. Clin Exp Dermatol. 1984;9:232–42. doi: 10.1111/j.1365-2230.1984.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 8.Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943–51. doi: 10.1177/000348949710601110. [DOI] [PubMed] [Google Scholar]
- 9.Bankaci M, Myers EN, Barnes L, et al. Angiosarcoma of the maxillary sinus: literature review and case report. Head Neck Surg. 1979;1:274–80. doi: 10.1002/hed.2890010311. [DOI] [PubMed] [Google Scholar]
- 10.Loos BM, Wieneke JA, Thompson LD. Laryngeal angiosarcoma: a clinicopathologic study of five cases with a review of the literature. Laryngoscope. 2001;111:1197–202. doi: 10.1097/00005537-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973–8. doi: 10.1001/archotol.1993.01880210061009. [DOI] [PubMed] [Google Scholar]
- 12.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683–97. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Wanebo HJ, Koness RJ, MacFarlane JK, et al. Head and neck sarcoma: report of the Head and Neck Sarcoma Registry. Society of Head and Neck Surgeons Committee on Research. Head Neck. 1992;14:1–7. doi: 10.1002/hed.2880140102. [DOI] [PubMed] [Google Scholar]
- 14.Bomer DL, Arnold GE. Rare tumors of the ear, nose, and throat. Third series: uncommon malignant tumors of the head and neck. Acta Otolaryngol Suppl. 1971;289:1–25. [PubMed] [Google Scholar]
- 15.Dass AA, Saleem Y. Hemangioendothelioma of the maxillary sinus. Otolaryngol Head Neck Surg. 1995;112:735–7. doi: 10.1016/S0194-5998(95)70184-2. [DOI] [PubMed] [Google Scholar]
- 16.Di Girolamo A, Giacomini PG, Coli A, et al. Epithelioid haemangioendothelioma arising in the nasal cavity. J Laryngol Otol. 2003;117:75–7. doi: 10.1258/002221503321046711. [DOI] [PubMed] [Google Scholar]
- 17.Fu YS, Perzin KH. Non-epithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx: a clinicopathologic study. I. General features and vascular tumors. Cancer. 1974;33:1275–88. doi: 10.1002/1097-0142(197405)33:5<1275::AID-CNCR2820330513>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima K, DeJima K, Koike S, et al. A case of angiosarcoma of the nasal cavity successfully treated with recombinant interleukin-2. Otolaryngol Head Neck Surg. 2006;134:886–7. doi: 10.1016/j.otohns.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Tanaka S, Furukawa M. Angiosarcoma of the nasal cavity. J Laryngol Otol. 1992;106:368–9. doi: 10.1017/s0022215100119528. [DOI] [PubMed] [Google Scholar]
- 20.Kurien M, Nair S, Thomas S. Angiosarcoma of the nasal cavity and maxillary antrum. J Laryngol Otol. 1989;103:874–6. doi: 10.1017/s0022215100110369. [DOI] [PubMed] [Google Scholar]
- 21.Lanigan DT, Hey JH, Lee L. Angiosarcoma of the maxilla and maxillary sinus: report of a case and review of the literature. J Oral Maxillofac Surg. 1989;47:747–53. doi: 10.1016/s0278-2391(89)80020-5. [DOI] [PubMed] [Google Scholar]
- 22.Lopes M, Duffau H, Fleuridas G. Primary spheno-orbital angiosarcoma: case report and review of the literature. Neurosurgery. 1999;44:405–7. doi: 10.1097/00006123-199902000-00102. [DOI] [PubMed] [Google Scholar]
- 23.Maezaka A, Konishi T. Case of angiosarcoma of the maxillary sinus. Otorhinolaryngol Tokyo. 1963;35:943–5. [PubMed] [Google Scholar]
- 24.McClatchey KD, Batsakis JG, Rice DH, et al. Angiosarcoma of the maxillary sinus: report of case. J Oral Surg. 1976;34:1019–21. [PubMed] [Google Scholar]
- 25.Narula AA, Vallis MP, el-Silimy OE, et al. Radiation induced angiosarcomas of the nasopharynx. Eur J Surg Oncol. 1986;12:147–52. [PubMed] [Google Scholar]
- 26.Oliveira P, Correia R, Castro E, et al. Primary columellar angiosarcoma: a case report. Ear Nose Throat J. 2005;84:45–6, 51. [PubMed] [Google Scholar]
- 27.Sharma BG, Nawalkha PL. Angiosarcoma of the maxillary antrum: report of a case with brief review of literature. J Laryngol Otol. 1979;93:181–6. doi: 10.1017/S0022215100086916. [DOI] [PubMed] [Google Scholar]
- 28.Sobol SM, Matthieu DE, Agee JH. Angiosarcoma of the maxillary sinus. Ear Nose Throat J. 1990;69:813–8. [PubMed] [Google Scholar]
- 29.Solomons NB, Stearns MP. Haemangiosarcoma of the maxillary antrum. J Laryngol Otol. 1990;104:831–4. doi: 10.1017/s0022215100114045. [DOI] [PubMed] [Google Scholar]
- 30.Triantafillidou K, Lazaridis N, Zaramboukas T. Epithelioid angiosarcoma of the maxillary sinus and the maxilla: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:333–7. doi: 10.1067/moe.2002.126022. [DOI] [PubMed] [Google Scholar]
- 31.Velegrakis GA, Panayiotides JG, Skoulakis CE, et al. Angiosarcoma of the maxillary sinus. J Laryngol Otol. 2000;114:381–4. doi: 10.1258/0022215001905634. [DOI] [PubMed] [Google Scholar]
- 32.Williamson IG, Ramsden RT. Angiosarcoma of maxillary antrum–association with vinyl chloride exposure. J Laryngol Otol. 1988;102:464–7. doi: 10.1017/s0022215100105365. [DOI] [PubMed] [Google Scholar]
- 33.Wong KF, So CC, Wong N, et al. Sinonasal angiosarcoma with marrow involvement at presentation mimicking malignant lymphoma: cytogenetic analysis using multiple techniques. Cancer Genet Cytogenet. 2001;129:64–8. doi: 10.1016/S0165-4608(01)00431-9. [DOI] [PubMed] [Google Scholar]
- 34.Zakrzewska JM. Angiosarcoma of the maxilla–a case report and review of the literature including angiosarcoma of maxillary sinus. Br J Oral Maxillofac Surg. 1986;24:286–92. doi: 10.1016/0266-4356(86)90095-1. [DOI] [PubMed] [Google Scholar]
- 35.Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046–57. doi: 10.1002/1097-0142(19870301)59:5<1046::AID-CNCR2820590533>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851–6. doi: 10.1111/j.1365-4362.1991.tb04350.x. [DOI] [PubMed] [Google Scholar]
- 37.Girard C, Johnson WC, Graham JH. Cutaneous angiosarcoma. Cancer. 1970;26:868–83. doi: 10.1002/1097-0142(197010)26:4<868::AID-CNCR2820260421>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 38.Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381–4. doi: 10.1288/00005537-198612000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Weiss SW, Lasota J, Miettinen MM. Angiosarcoma of soft tissue. In: Fletcher CDM, Unni K, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone, World Health Organization Classification of Tumours, Kleihues P, Sobin LH, series editors. Lyon, France: IARC Press, 2002. p. 175–7.
- 40.Fanburg-Smith JC, Furlong MA, Childers EL. Oral and salivary gland angiosarcoma: a clinicopathologic study of 29 cases. Mod Pathol. 2003;16:263–71. doi: 10.1097/01.MP.0000056986.08999.FD. [DOI] [PubMed] [Google Scholar]
- 41.Kempson RL, Fletcher CDM, Evans HL, Hendrickson MR, Sibley RK. Tumors of soft tissue. 3. Washington, DC: American Registry of Pathology; 1998. [Google Scholar]
- 42.Mark RJ, Poen JC, Tran LM, et al. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer. 1996;77:2400–6. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2400::AID-CNCR32>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 43.Schammel DP, Tavassoli FA. Uterine angiosarcomas: a morphologic and immunohistochemical study of four cases. Am J Surg Pathol. 1998;22:246–50. doi: 10.1097/00000478-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Fedok FG, Levin RJ, Maloney ME, et al. Angiosarcoma: current review. Am J Otolaryngol. 1999;20:223–31. doi: 10.1016/S0196-0709(99)90004-2. [DOI] [PubMed] [Google Scholar]
- 45.Nishiwaki Y, Tada Y, Nakatani C, et al. A case of angiosarcoma of the nose. J Dermatol. 2002;29:593–8. doi: 10.1111/j.1346-8138.2002.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 46.Namyslowski G, Scierski W, Turecka L, et al. A very rare case of low-grade angiosarcoma of the nose and paranasal sinuses. Otolaryngol Pol. 2005;59:105–8. [PubMed] [Google Scholar]
- 47.Mentzel T, Kutzner H, Wollina U. Cutaneous angiosarcoma of the face: clinicopathologic and immunohistochemical study of a case resembling rosacea clinically. J Am Acad Dermatol. 1998;38:837–40. doi: 10.1016/S0190-9622(98)70470-0. [DOI] [PubMed] [Google Scholar]
- 48.Gallardo MA, Bosch RJ, Vidal L, et al. Angiosarcoma arising on rhinophyma. Eur J Dermatol. 2000;10:555–8. [PubMed] [Google Scholar]
- 49.McCarthy WD, Pack GT. Malignant blood vessel tumors. A report of 56 cases of angiosarcoma and Kaposi’s sarcoma. Surg Gynecol Obstet. 1950;91:465–82. [PubMed] [Google Scholar]
- 50.Lasota J, Miettinen M. Absence of Kaposi’s sarcoma-associated virus (human herpesvirus-8) sequences in angiosarcoma. Virchows Arch. 1999;434:51–6. doi: 10.1007/s004280050304. [DOI] [PubMed] [Google Scholar]
- 51.Mentzel T, Beham A, Calonje E, et al. Epithelioid hemangioendothelioma of skin and soft tissues: clinicopathologic and immunohistochemical study of 30 cases. Am J Surg Pathol. 1997;21:363–74. doi: 10.1097/00000478-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Al-Abbadi MA, Almasri NM, Al-Quran S, et al. Cytokeratin and epithelial membrane antigen expression in angiosarcomas: an immunohistochemical study of 33 cases. Arch Pathol Lab Med. 2007;131:288–292. doi: 10.5858/2007-131-288-CAEMAE. [DOI] [PubMed] [Google Scholar]
- 53.Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Hum Pathol. 2000;31:1062–7. doi: 10.1053/hupa.2000.9843. [DOI] [PubMed] [Google Scholar]
- 54.Ramani P, Bradley NJ, Fletcher CD. QBEND/10, a new monoclonal antibody to endothelium: assessment of its diagnostic utility in paraffin sections. Histopathology. 1990;17:237–42. doi: 10.1111/j.1365-2559.1990.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 55.Orchard GE, Zelger B, Jones EW, et al. An immunocytochemical assessment of 19 cases of cutaneous angiosarcoma. Histopathology. 1996;28:235–40. doi: 10.1046/j.1365-2559.1996.d01-411.x. [DOI] [PubMed] [Google Scholar]
- 56.Sauter B, Foedinger D, Sterniczky B, et al. Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells. Differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J Histochem Cytochem. 1998;46:165–76. doi: 10.1177/002215549804600205. [DOI] [PubMed] [Google Scholar]
- 57.Tabata M, Sugihara K, Matsui R, et al. Angiosarcoma of the tongue: report of a case with immunohistochemical findings. J Oral Pathol Med. 1999;28:92–5. doi: 10.1111/j.1600-0714.1999.tb02003.x. [DOI] [PubMed] [Google Scholar]
- 58.Traweek ST, Kandalaft PL, Mehta P, et al. The human hematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol. 1991;96:25–31. doi: 10.1093/ajcp/96.1.25. [DOI] [PubMed] [Google Scholar]
- 59.Wenig BM, Abbondanzo SL, Heffess CS. Epithelioid angiosarcoma of the adrenal glands. A clinicopathologic study of nine cases with a discussion of the implications of finding “epithelial-specific” markers. Am J Surg Pathol. 1994;18:62–73. [PubMed] [Google Scholar]
- 60.Mandahl N, Jin YS, Heim S, et al. Trisomy 5 and loss of the Y chromosome as the sole cytogenetic anomalies in a cavernous hemangioma/angiosarcoma. Genes Chromosom Cancer. 1990;1:315–6. doi: 10.1002/gcc.2870010410. [DOI] [PubMed] [Google Scholar]
- 61.Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003;27:737–49. doi: 10.1097/00000478-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Thompson LDR, Wieneke JA, Miettinen M. Sinonasal tract melanomas: A clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27:594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Thompson LDR, Fanburg-Smith JC. Tumors of the Nasal Cavity and Paranasal sinuses: Benign soft tissue tumours. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of tumours of the head and neck. World Health Organization Classification of Tumours., Kleihues P, Sobin LH, series editors. Lyon, France: IARC Press, 2005. p. 47–51.
- 64.Thompson LDR, Fanburg-Smith JC. Tumors of the Nasal Cavity and Paranasal sinuses: Malignant soft tissue tumours. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. World Health Organization Classification of Tumours. Kleihues P, Sobin LH, series editors. Lyon, France: IARC Press, 2005. p. 35–42.
- 65.Thompson LDR, Fanburg-Smith JC, Wenig BM. Tumors of the Nasal Cavity and Paranasal sinuses: borderline and low malignant potential tumours of soft tissues. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. World Health Organization Classification of Tumours. Kleihues P, Sobin LH, series editors. Lyon, France: IARC Press, 2005. p. 43–5.
- 66.Thompson LDR, Fanburg-Smith JC. Tumours of the Nasopharynx: Nasopharyngeal angiofibroma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. World Health Organization Classification of Tumours. Kleihues P, Sobin LH, series editors. Lyon, France: IARC Press, 2005. p. 102–3.
- 67.Alameda F, Fontane J, Corominas JM, et al. Reactive vascular lesion of nasal septum simulating angiosarcoma in a cocaine abuser. Hum Pathol. 2000;31:239–41. doi: 10.1016/S0046-8177(00)80226-9. [DOI] [PubMed] [Google Scholar]
- 68.Kuo T, Sayers CP, Rosai J. Masson’s “vegetant intravascular hemangioendothelioma:” a lesion often mistaken for angiosarcoma: study of seventeen cases located in the skin and soft tissues. Cancer. 1976;38:1227–36. doi: 10.1002/1097-0142(197609)38:3<1227::AID-CNCR2820380324>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 69.Wenig BL, Sciubba JJ, Cohen A, et al. Nasal septal hemangioma. Otolaryngol Head Neck Surg. 1985;93:436–41. doi: 10.1177/019459988509300329. [DOI] [PubMed] [Google Scholar]
- 70.Wenig BM, Heffner DK. Contact ulcers of the larynx. A reacquaintance with the pathology of an often underdiagnosed entity. Arch Pathol Lab Med. 1990;114:825–8. [PubMed] [Google Scholar]
- 71.Stern Y, Braslavsky D, Segal K, et al. Intravascular papillary endothelial hyperplasia in the maxillary sinus. A benign lesion that may be mistaken for angiosarcoma. Arch Otolaryngol Head Neck Surg. 1991;117:1182–4. doi: 10.1001/archotol.1991.01870220130024. [DOI] [PubMed] [Google Scholar]
- 72.Bremer JW, Bryan Neal H, Sando LW, et al. Angiofibroma: treatment trends in 150 patients during 40 years. Laryngoscope. 1986;96:222–31. doi: 10.1288/00005537-198612000-00001. [DOI] [PubMed] [Google Scholar]
- 73.Harrison DF. The natural history, pathogenesis, and treatment of juvenile angiofibroma. Personal experience with 44 patients. Arch Otolaryngol Head Neck Surg. 1987;113:936–42. doi: 10.1001/archotol.1987.01860090034015. [DOI] [PubMed] [Google Scholar]
- 74.Jacobsson M, Petruson B, Svendsen P, et al. Juvenile nasopharyngeal angiofibroma. A report of eighteen cases. Acta Otolaryngol. 1988;105:132–9. doi: 10.3109/00016488809119456. [DOI] [PubMed] [Google Scholar]
- 75.Neel HB, Whicker JH, Devine KD, et al. Juvenile angiofibroma. Review of 120 cases. Am J Surg. 1973;126:547–56. doi: 10.1016/S0002-9610(73)80048-0. [DOI] [PubMed] [Google Scholar]
- 76.Chen H, Thompson LD, Aguilera NS, et al. Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2004;28:505–13. doi: 10.1097/00000478-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Kapadia SB, Heffner DK. Pitfalls in the histopathologic diagnosis of pyogenic granuloma. Eur Arch Otorhinolaryngol. 1992;249:195–200. doi: 10.1007/BF00178468. [DOI] [PubMed] [Google Scholar]
- 78.Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. A clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781–96. doi: 10.1016/S0190-9622(85)70098-9. [DOI] [PubMed] [Google Scholar]
- 79.Fetsch JF, Weiss SW. Observations concerning the pathogenesis of epithelioid hemangioma (angiolymphoid hyperplasia) Mod Pathol. 1991;4:449–55. [PubMed] [Google Scholar]
- 80.Wenig BM, Dulguerow P, Kapadia SB, Prasad ML, Fanburg-Smith JC, Thompson LDR. Tumors of the nasal cavity and paranasal sinuses: neuroectodermal tumours. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of tumours of the head and neck, World Health Organization Classification of Tumours, Kleihues P, Sobin LH, series editors. Lyon, France: IARC Press, 2005. p. 65–75.
- 81.Wyatt ME, Finlayson CJ, Moore-Gillon V. Kaposi’s sarcoma masquerading as pyogenic granuloma of the nasal mucosa. J Laryngol Otol. 1998;112:280–2. doi: 10.1017/S0022215100158359. [DOI] [PubMed] [Google Scholar]
- 82.Zhang R, Huang X, Tan C. Kaposi’s sarcoma associated with HIV in otorhinolaryngology: report of 21 cases. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 1997;11:64–5. [PubMed] [Google Scholar]
- 83.Fliss DM, Parikh J, Freeman JL. AIDS-related Kaposi’s sarcoma of the sphenoid sinus. J Otolaryngol. 1992;21:235–7. [PubMed] [Google Scholar]
- 84.Kapella M, Panosetti E, Rombaux P, et al. Lobular capillary haemangioma of the nasal cavity: observation of three specific cases. Acta Otorhinolaryngol Belg. 2001;55:241–6. [PubMed] [Google Scholar]
- 85.Mills SE, Cooper PH, Fechner RE. Lobular capillary hemangioma: the underlying lesion of pyogenic granuloma. A study of 73 cases from the oral and nasal mucous membranes. Am J Surg Pathol. 1980;4:470–9. [PubMed] [Google Scholar]
- 86.Lancaster JL, Alderson DJ, Sherman IW, et al. Papillary endothelial hyperplasia (Masson’s tumour) of the maxillary sinus. J Laryngol Otol. 1998;112:500–2. doi: 10.1017/S0022215100140903. [DOI] [PubMed] [Google Scholar]
- 87.Torne ER, Umbert MP. Masson’s pseudoangiosarcoma of the tongue: report of two cases. J Cutan Pathol. 1985;12:66–71. doi: 10.1111/j.1600-0560.1985.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 88.Batsakis JG, Sneige N. Choanal and angiomatous polyps of the sinonasal tract. Ann Otol Rhinol Laryngol. 1992;101:623–5. doi: 10.1177/000348949210100716. [DOI] [PubMed] [Google Scholar]
- 89.Batsakis JG, Rice DH. The pathology of head and neck tumors: vasoformative tumours. 9A. Head Neck Surg. 1981;3:231–9. doi: 10.1002/hed.2890030311. [DOI] [PubMed] [Google Scholar]
- 90.Batsakis JG, Rice DH. The pathology of head and neck tumors: vasoformative tumours. 9B. Head Neck Surg. 1981;3:326–39. doi: 10.1002/hed.2890030408. [DOI] [PubMed] [Google Scholar]
- 91.Banerjee SS, Eyden B, Trenholm PW, et al. Monotypic angiomyolipoma of the nasal cavity: a heretofore undescribed occurrence [In Process Citation] Int J Surg Pathol. 2001;9:309–15. doi: 10.1177/106689690100900410. [DOI] [PubMed] [Google Scholar]
- 92.Cansiz H, Yener M, Kalekoglu N, et al. Arteriovenous malformation of the maxillary sinus and mandible: a case report. Ear Nose Throat J. 2003;82:608–10, 612, 614. [PubMed] [Google Scholar]
- 93.Rossi S, Fletcher CD. Angiosarcoma arising in hemangioma/vascular malformation: report of four cases and review of the literature. Am J Surg Pathol. 2002;26:1319–1329. doi: 10.1097/00000478-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 94.Hanna GS, Akosa AB, Ali MH. Vascular leiomyoma of the inferior turbinate-report of a case and review of the literature. J Laryngol Otol. 1988;102:1159–60. doi: 10.1017/s0022215100107595. [DOI] [PubMed] [Google Scholar]
- 95.Goldenberg AS. The symptoms of an angiosarcoma mimicking pulpal pain. J Endodontics. 1983;9:65–70. doi: 10.1016/S0099-2399(83)80078-8. [DOI] [PubMed] [Google Scholar]
- 96.Goudaert M, Leduc M, Vandenbussche F, et al. Angiosarcome du maxillaire superier. Rev Stomato-Odont. 1968;23:159–72. [PubMed] [Google Scholar]
- 97.Nishizawa S, Matsumoto K, Funasaka S, et al. Malignant fibrous histiocytoma of the nasal septum. A report of an unusual lesion. ORL J Otorhinolaryngol Relat Spec. 1982;44:335–9. doi: 10.1159/000275613. [DOI] [PubMed] [Google Scholar]