Abstract
The introduction of bisphosphonates has increased in the last decade following their indication for metastatic bone diseases, osteoporosis, hypercalcaemia of malignancy and Paget’s disease. Although bisphosphonates have been used clinically for more than three decades there have been no documented long-term complications of their effects on the jaws until recently, where there is now growing evidence of the influence of bisphosphonates on osteonecrosis of the jaws. The aim of this paper is to report a case of this newly described complication, to review this phenomenon, including the clinical implications and to reiterate current clinical guidelines for management of patients in which bisphosphonate therapy is indicated. To the best of our knowledge this is the first reported case of bisphosphonate-induced necrosis of the jaw in South Africa.
Keywords: Bisphosphonates, Osteonecrosis, Jaws
Introduction
The first published report on the effects of a bisphosphonate (BP) was in 1965 by Fleish et al. on the influence of pyrophosphates on calcification [1]. They found that pyrophosphate has a high affinity for calcium crystals and impairs its formation as well as its dissolution in vitro and inhibits its calcification in vivo. It was this action that lead to it being envisaged for treatment of ectopic calcification. However when taken orally, the pyrophosphates were rapidly metabolised and rendered inactive.
By substituting the oxygen molecule with a carbon in pyrophosphate, a class of bisphosphonates (BPs) was developed that was resistant to biological degradation. BPs also have two additional groups in their molecule that are attached to the central carbon, named R1 and R2 respectively. By varying the substitutions in the R2, a number of BPs can be created that vary in clinical and biological potency. These BPs are potent inhibitors of bone resorption. After being taken up they are embedded in bone exerting their action with long lasting effect. The skeleton has a large capacity to retain BPs [2, 3]. Generally, BPs are classified into two main classes dependent on the presence or absence of nitrogen in the R2. The nitrogen BPs (N-BPs) are the most potent and are represented by alendronate (Fosamax®, PO) [Merck, Whitehouse Station, NJ, USA], ibandronate (Bondronat®, PO, Boniva®, PO) [Roche, Nutley, NJ, USA], pamidronate (Aredia®, IV), zoledronic acid (Zometa®, IV) [Novartis, East Hanover, NJ, USA, and risedronate (Actonel®, PO) [Proctor & Gamble, Cincinnati, Ohio, USA], whilst the non N-BPs are mainly etidronate (Didronel®, PO) [Proctor & Gamble, Cincinnati, Ohio, USA], clodronate (Bonefos®, PO) [Aventis Pharma, Bridgewater, NJ, USA] and tiludronate (Skelid®, PO) [Sanofi, Bridgewater, NJ, USA].
Bone is homeostatically controlled by mineralisation and resorption by osteoblasts and osteoclasts respectively. Non N-BPs inhibit resorption by forming a toxic ATP analogue whilst N-BPs inhibit farsenylpyrophosphate synthase, both resulting in inactivation of osteoclasts as well as induction of their apoptosis [2–4]. These mechanisms result in decreased bone resorption and bone turnover. Recently it has also been suggested that BPs have antiangiogenic and antitumour properties [5, 6]. It is these precise mechanisms that have resulted in their indication for metastatic disease, multiple myeloma, and management of hypercalcaemia due to malignancy, Paget’s disease and osteoporosis. Their use has resulted in reduced bone pain and improved quality of life and skeletal related events for cancer patients.
Although BPs have been used clinically for more than three decades there have been no documented long-term complications until recently with the publication of a few reported cases of gastrointestinal and renal complications [7] and now growing evidence of the influence of BPs on osteonecrosis of the jaws [8–12]. Recently there have been reported cases of avascular necrosis of the hip in patients receiving BP treatment for multiple myeloma, suggestive of BP-induced osteonecrosis manifesting first in the jaws but being a truly systemic complication in patients who survive longer [13]. Concerns of oversuppression of bone turnover and accumulation of micro-damage with long term use of BPs have also been highlighted recently and raises concern of impaired bone strength following long term use [14–16]. There are however others who believe that this is merely theoretical with no clinical basis [17–19].
Classically, in BP-induced osteonecrosis, patients present with severe pain usually associated with a non-healing extraction socket with exposed jaw bone with or without sequestra, localised swelling and loosening of teeth [5, 10, 20, 21]. Approximately 75% of these signs and symptoms usually follow dental treatment, but they can appear spontaneously [22]. Treatment with intravenous BPs seem to have a higher risk of developing this disorder, with an incidence estimated to affect 1 per 10,000 patients [23, 24], although cases with oral administration have been reported [5]. The incidence for patients taking alendronate (Fosamax®), the most commonly prescribed oral BP, has been estimated to occur in approximately 0.7 per 100,000 person/years exposure [23] whilst, the incidence for other oral BPs, risedronate (Didronel®), and ibandronate (Bondronat®, Boniva®), cannot yet be quantified because too few cases have been reported [24].
Case report
The patient, a 67-year old female presented in November 2005 with a long time concern of worsening pain in her lower jaw. Eight months prior to her first attendance, the first lower right premolar tooth was extracted. The pain did not abate, even after two separate courses of antibiotics over a period of time. She gave a history of being a controlled diabetic; on Warfarin for previous deep vein thrombosis and medication for the management of breast carcinoma with secondaries to the bone and lung, which was diagnosed some 4-years previously. Following the diagnosis of breast carcinoma she was on monthly intravenous zoledronic acid (Zometa®) and oral Letrozole (Femara®, PO) [Novartis, East Hanover, NJ, USA]. There was no history of radiation therapy, especially none to the jaws.
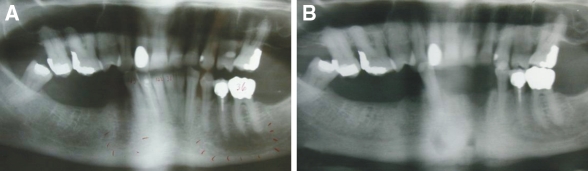
Signs and symptoms of myofacial pain were present bilaterally. There was no regional lymphadenopathy. Intraorally, the teeth appeared non-carious, exhibited incisal wear patterns and there was neither tooth mobility nor signs of gross periodontal disease. Two months after the initial consultation, the anterior mandibular first and second incisors and the left mandibular canine were extracted because of severe pain. The panoramic radiographs before and after the extractions of the teeth show no evidence of rarefraction or any increase or decrease in jaw bone density (Fig. 1A, B). The patient showed no gross exposure of the alveolar bone, however there was marked thinning and erythema of the alveolar mucosa with definitive alveolar bone necrosis, which had persisted for over 11 weeks. Bone exposure appeared imminent, with a focal area of necrotic bone exuding from an extraction site. Based on the guidelines outlined by the American Association of Oral and Maxillofacial Surgeons (AAOMS) in 2007 [25], a diagnosis of an early stage 2 BP-induced osteonecrosis of the jaw was favoured over a ‘conventional’ osteomyelitis.
Fig. 1.
Panoramic radiographs prior (A) and post extraction (B) show no rarefraction or any increase or decrease in jaw bone density
The patient was definitely within a risk category, being treated with IV BPs for breast carcinoma, without any radiation therapy. The dentoalveolar surgery appeared to have increased the risk of development of the BP-induced osteonecrosis rather than being the primary cause of the osteonecrosis. In view of the persistent presence of necrotic bone with the associated infection as evidenced by pain and erythema in the region of the imminent bone exposure, a BP-induced osteonecrosis of the jaw was favoured, regardless of the use of the oral BPs.
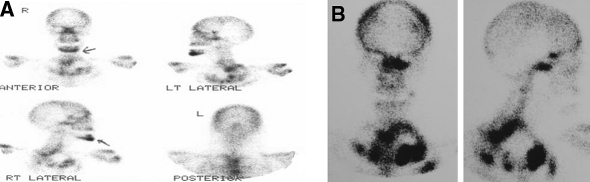
Even though it is clinically well recognised that inflammation and bone regeneration results in increased technetium uptake, a technetium bone scan was performed six months following the second set of extractions, in view of the severe anterior mandibular bone pain. This revealed marked activity, not only at the extraction site, which one would expect to be “hot”, but also in the symphyseal region of the mandible, with prominence in other bones of the skeleton. A previous technetium scan done in 2001 showed no activity within the anterior lower facial bones (Fig. 2 A, B).
Fig. 2.
Technetium scans taken in 2005 (A) and 2001 (B) respectively, indicating pronounced activity in the symphyseal region of the mandible in 2005
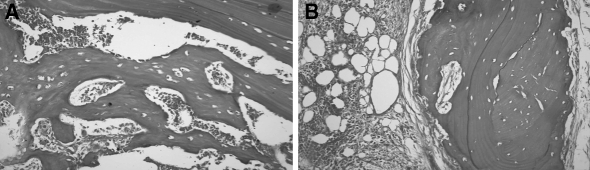
Bilateral symphyseal core biopsies were done through a vestibular incision to exclude the possibility of secondary metastatic tumour deposits. Microscopic examination of both biopsies revealed irregular trabeculae and sheets of non-vital bone, which were devoid of osteocytes. The bony margins were scalloped, however there was no osteoclastic activity observed. There were associated necrotic debris, haemorrhage, and foci of suppuration (Fig. 3A, B). Whilst very occasional filamentous bacterial elements were identified, microbiological culture was not performed to determine the exact nature of these organisms. This was due to the fact that at this stage metastatic breast carcinoma within the mandible, and not BP-induced osteonecrosis, was the primary diagnostic concern. In addition, the larger portion of bone showed cellular intervening fibrofatty marrow tissue that showed evidence of fibrosis. There was no representation of a draining sinus. Features of metastatic breast carcinoma were not observed in either of the biopsy specimens. This was confirmed with the negative immunostaining in the presence of adequate preparations and controls with a pan cytokeratin marker, MNF116 [Dako; Glostrup, Denmark] and a more specific breast marker, gross cystic disease fluid protein-15 (GCDFP-15) [Signet Laboratories, Inc.; Dedham, Massachusetts, USA]. An initial diagnosis of chronic suppurative osteomyelitis was made, which was later confirmed as being consistent with the use of BPs on combined review with the scans and history.
Fig. 3.
(A, B) Chronic suppurative osteomyelitis as evidenced by the sheets and irregular trabeculae of non-vital bone, with no evidence of metastatic carcinoma (Haematoxylin and eosin stain; ×64)
Following the diagnosis of BP-induced osteonecrosis, the zoledronic acid (Zometa®) was stopped in consultation with the oncologist and an international expert in this field, Prof. Robert Marx, chief surgeon from the Department of Surgery at the University of Miami. Penicillin (Len V.K.®, PO) [Aspen, Port Elizabeth, South Africa] four times a day was empirically prescribed for a period of 2 months with significant improvement in symptoms. The Femara® was continued. Eight months after the initial consultation a further technetium scan was obtained which showed distinct reduced activity in the symphysis (Fig. 2 A), keeping in mind that the use of the scan is non-specific and not pathognomonic for BP-induced osteonecrosis.
Discussion
To the best of our knowledge this is the first reported case of BP-induced osteonecrosis in South Africa. There have been many reported cases in the United States [8, 10, 12, 22], in Greece [4] and in Australia [11, 26, 27]. All cases presented with similar clinical presentations following BP therapy. The salient clinicopathologic features of these cases are summarised in Table 1.
Table 1.
Reported cases of bisphosphonate-induced osteonecrosis of the jaws
| Reference | Patients, n | Gender, n | Age | Site, n | BP medication (n) | Primary diagnosis (n) | Treatment (n) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | Mandible | Maxilla | Both | ||||||
| Diego [28] | 10 | 6 | 4 | 58–71 | 8 | 12 | ZA (10) | Multiple myeloma (3) | ABs; debridement (7) | |
| Breast carcinoma (1) | ABs; maxillectomy (2) | |||||||||
| Lung carcinoma (4) | Mandibular resection (1) | |||||||||
| Prostate carcinoma (2) | ||||||||||
| Magopoulos [29] | 60 | 28 | 32 | 47–76 | 30 | 22 | 8 | P; ZA (24) | Multiple myeloma (32) | Cessation of BP; ABs; surgery (9) |
| P; ZA; I (3) | Breast carcinoma (18) | HBO; ABs; surgery (6) | ||||||||
| ZA (33) | Lung carcinoma (2) | Cessation of BP; ABs (24) | ||||||||
| Prostate carcinoma (5) | ABs (11) | |||||||||
| Neuroendocrine carcinoma (1) | ABs; surgery (10) | |||||||||
| Fibrous dysplasia (1) | ||||||||||
| Amyloidosis (1) | ||||||||||
| Clarke [30] | 25 | 15 | 10 | 41–80 | 19 | 6 | P (10) | Multiple Myeloma (20) | Medical (12); +HBO (3) | |
| P; ZA (8) | Breast carcinoma (1) | Medical; surgical, not specified (10) | ||||||||
| ZA (5) | Prostate carcinoma (1) | Not specified (2) | ||||||||
| Metastatic lymphoma (1) | No treatment (1) | |||||||||
| Osteoporosis (1) | ||||||||||
| Mortensen [31] | 7 | 3 | 5 | 61–76 | 3 | 4 | P (3) | Multiple myeloma (4) | Maxillectomy; zygoma resection (1) | |
| ZA (4) | Breast carcinoma (3) | Partial maxillectomy (2) | ||||||||
| ABs; debridement; sequestrectomy (3) | ||||||||||
| Not specified (1) | ||||||||||
| Brooks [23] | 2 | 2 | 62; 70 | 1 | 1 | Risedronate (2) | Osteoporosis (2) | ABs; sequestrectomy (1) | ||
| ABs (1) | ||||||||||
| Rossi [32] | 10 | 6 | 4 | 64, mean | 7 | 2 | 1 | ZA (10) | Not specified | Debridement (7) |
| Partial maxillectomy (2) | ||||||||||
| Mandibular resection (1) | ||||||||||
| Dimitrakopoulos [4] | 11 | 5 | 6 | 47–76 | 7 | 3 | 1 | P; ZA (4) | Multiple myeloma (5) | Debridement (1) |
| ZA (6) | Breast carcinoma; lung carcinoma (1) | Sequestrectomy (3) | ||||||||
| P; ZA; I (1) | Prostate carcinoma (2) | Medical (2) | ||||||||
| Osteoporosis (1) | ||||||||||
| Neuroendocrine carcinoma (1) | ||||||||||
| Fibrous dysplasia (1) | ||||||||||
| Merigo [33] | 29 | 4 | 21 | 45–83 | 20 | 7 | 2 | P (11) | Multiple myeloma (12) | Medical; surgical; Nd:YAG biostimulation |
| ZA (15) | Breast carcinoma (12) | |||||||||
| A (3) | Prostate carcinoma (1) | |||||||||
| Osteoporosis (3) | ||||||||||
| Pleural mesothelioma (1) | ||||||||||
| Zarychanski [12] | 12 | 7 | 5 | 10 | 1 | 1 | P (12) | Multiple myeloma (10) | ||
| Breast carcinoma (1) | ||||||||||
| Renal carcinoma (1) | ||||||||||
| Bamias [34] | 17 | 10 | 7 | 43–72 | 14 | 3 | P; ZA (9) | Multiple myeloma (11) | Cessation of BP; ABs; oral rinses; minor debridement; HBO | |
| ZA (7) | Breast carcinoma (2) | |||||||||
| ZA; I (1) | Prostate carcinoma (3) | |||||||||
| Other neoplasm (1) | ||||||||||
| Bagan [35] | 20 | 5 | 15 | 36–80 | 11 | 1 | 8 | P (5) | Multiple myeloma (9) | ABs; minor surgery; bone resection; free grafts; microvascular surgery |
| P; ZA (6) | Breast carcinoma (10) | |||||||||
| ZA (9) | Prostate carcinoma (1) | |||||||||
| Bagan [36] | 10 | 2 | 8 | 36–80 | 5 | 5 | P (4) | Multiple myeloma (4) | Not specified | |
| P; ZA (4) | Breast carcinoma (6) | |||||||||
| ZA (2) | ||||||||||
| Cheng [11] | 5 | 3 | 2 | 57–84 | 4 | 1 | P (4) | Not specified | HBO; maxillectomy (1) | |
| A (1) | Local debridement; sequestrectomy (1) | |||||||||
| Local debridement ; HBO (1) | ||||||||||
| Partial maxillectomy (1) | ||||||||||
| Miglioratti [37] | 18 | 4 | 14 | 37–74 | P; ZA | Multiple myeloma (3) | Debridement (1) | |||
| Not specified | Not specified | Breast carcinoma (10) | ABs; bone smoothening (1) | |||||||
| Prostate carcinoma (2) | ABs; rinses (1) | |||||||||
| Ovarian carcinoma (1) | ABs; sequestrectomy (14) | |||||||||
| Ovarian; breast carcinoma (1) | ABs; sequestrectomy; HBO (1) | |||||||||
| Osteopenia (1) | ||||||||||
| Melo [38] | 11 | 7 | 4 | 59–83 | 8 | 2 | 1 | P (4) | Multiple myeloma (7) | Sequestrectomy (6) |
| P; ZA (3) | Breast carcinoma (3) | Partial mandibulectomy (1) | ||||||||
| ZA (4) | Lung carcinoma (1) | Partial maxillectomy (1) | ||||||||
| Conservative treatment (3) | ||||||||||
| Pires [39] | 14 | 4 | 10 | 43–84 | 9 | 4 | 1 | P (4) | Multiple myeloma (6) | ABs; debridement (11) |
| P; ZA (5) | Breast carcinoma (6) | ABs (3) | ||||||||
| ZA (3) | Lung carcinoma (1) | |||||||||
| Not specified | Prostate carcinoma (1) | |||||||||
| Carter [26] | 5 | 3 | 2 | 57–84 | 4 | 1 | P (4) | Multiple myeloma (2) | Cessation of BP (5) | |
| A (1) | Paget’s disease (3) | Local debridement (3) | ||||||||
| HBO (2) | ||||||||||
| Sequestrectomy (1) | ||||||||||
| Wide excision (1) | ||||||||||
| Le Fort 1 maxillectomy (1) | ||||||||||
| Purcell [27] | 13 | 7 | 6 | 42–83 | 4 | 2 | P (2) | Multiple myeloma (3) | ||
| Not specified | P; ZA (1) | Breast carcinoma (5) | ||||||||
| ZA (9) | Prostate carcinoma (4) | |||||||||
| A (1) | Osteoporosis (1) | |||||||||
| Marx [22] | 119 | 81 | 33 | 5 | P (32) | Multiple myeloma (62) | Cessation of BP; debridement; HBO; ABs; surgery | |||
| P; ZA (36) | Breast carcinoma (50) | |||||||||
| ZA (48) | Prostate carcinoma (4) | |||||||||
| A (3) | Osteoporosis (3) | |||||||||
| Ruggiero [10] | 63 | 24 | 39 | 43–89 | 39 | 23 | 1 | P (34) | Multiple myeloma (29) | Sequestrectomy (45) |
| P; ZA (13) | Breast carcinoma (21) | Marginal mandibulectomy (4); +HBO (2) | ||||||||
| ZA (9) | Lung carcinoma (1) | Segmental mandibular resection (6) | ||||||||
| A (5) | Prostate carcinoma (3) | Partial maxillectomy (5) | ||||||||
| A; ZA (1) | Osteoporosis (7) | Complete maxillectomy (1) | ||||||||
| Risedronate (1) | Leiomyosarcoma (1) | |||||||||
| Leukemia (1) | ||||||||||
| Estilo [40] | 13 | 4 | 9 | 6 | 5 | 2 | Intravenous forms | Multiple myeloma (4) | ||
| Not specified | Breast carcinoma (9) | |||||||||
| Marx [8] | 36 | 29 | 5 | 2 | P (24) | Multiple myeloma (18) | ABs; rinses; minor debridement | |||
| P; ZA (6) | Breast carcinoma (17) | |||||||||
| ZA (6) | Osteoporosis (1) | |||||||||
Bisphosphonates—BPs; Antibiotics—ABs; HBO—hyperbaric oxygen therapy; Zoledronic acid—ZA; Pamidronate—P; Ibanronate—I; A—Alendronate
An analysis of these cases [28–41], including the current case, reveals a female predominance (206 women, 147 men), with a male-to-female ratio of 0.7:1. The osteonecrosis involved patients over a wide age range, with patients ranging between the ages of 36 and 89 years. Most patients were within the seventh decade of life. The mandible (320 cases; 63.1%) was more commonly involved than the maxilla (146 cases; 28.8%), whilst some patients showed osteonecrosis of both jaws (41 cases; 8.1%). The primary pathology for which oral BPs were initially prescribed included multiple myeloma (244 cases; 49.1%), followed by breast carcinoma (176 cases; 35.4%), prostate carcinoma (29 cases; 5.8%), osteoporosis (20 cases; 4.0%), lung cancer (10 cases; 2.0%) and other specified conditions (16 cases; 3.2%). We have reviewed a total of 511 cases including the current case, and the clinico-pathologic features and treatment outcomes are in concordance with the findings of Woo et al. [41] who reviewed 368 published cases of BP associated osteonecrosis of the jaws. The average time between the use of the BP and the first evidence of bone exposure/necrosis ranged from about 9 to 14 months [22]. Regardless of the wide range of available treatment options, there appears to be stabilisation of necrosis in most patients following conservative rather than surgical management [29].
This disorder is refractory to the conventional treatment of debridement and bone contouring procedures. Attempts at debridement only increase the defect size and expose the patient to a greater risk of pathological fracture, despite the presence of vascularised bone at the resected margins. Surgery is thus generally counterproductive and hyperbaric oxygen therapy has yielded little success [10, 21, 22].
The actual mechanism of development of the BP-induced osteonecrosis has yet to be elucidated. Two theories have been put forward. Firstly, that BPs induce compromised vascularity of the jaws as they are known to be antiangiogenic, by decreasing vascular endothelial growth factor (VEGF), inducing apoptosis of the endothelial cells and inhibiting capillary neoangiogenesis [4, 9, 10, 22]. These events lead to avascular necrosis of the jaws. The apoptotic rate of both osteoclasts and keratinocytes have also been increased, which in turn may decrease the oral mucosa keratinocyte barrier and predispose the osteon to oral flora [4].
The second hypothesis is that the ischaemia is thought to be induced through dense, poorly formed bone via the anticlastic mechanism of BPs as they alter the cytoskeletal morphology of osteoclasts including integrin signalling, trafficking of endosomes and disruption of the ruffled border [30]. Here, the everyday extreme cycling of the jaws in masticatory function results in microstress and microfractures of the jaw bones. These in turn signal the osteoclastic and osteoblastic resorption and remineralisation of the damaged bone respectively. Due to the osteoclastic inhibition, there is no release of bone morphogenic protein, with no induction of osteoblastic differentiation resulting in an acellular and avascular osteon [5, 22].
Histologically the affected area presents as osseous necrosis with progression onto sequestration as noted in our patient. Interestingly it has been reported that bacterial cultures often reveal Actinomyces as the organism most cultured [13, 14, 22, 42, 43]. Other micro-organisms commonly implicated include Eikenella corrodens as well as other commensal oral flora [4, 22].
Clinical recommendations for the use of BPs in cancer patients have been suggested [44–46]. However, definitive recommendations for the prevention and detection of BP-induced osteonecrosis are not possible at present due to the lack of sufficient evidence based data. Although the incidence of BP-induced osteonecrosis is low, practitioners treating patients with bone disease need to be aware of the refractory nature of this condition. We recommend caution in management of patients utilising BP therapy.
A comprehensive dental examination and management should be undertaken prior to BP therapy [47]. Any tooth of questionable prognosis should be extracted. Oral hygiene and any restorative dental work should be optimised. Ill-fitting prostheses should be managed.
Once therapy has been initiated regular oral and dental follow up is essential. Intrabony biopsies should be avoided unless required to rule out metastatic spread. In such instances, bacterial cultures should be undertaken if possible. Patients presenting with osteonecrosis and currently on BP therapy should be largely managed with oral rinses, antibiotics and if necessary local debridement; palliation and control of infection being of primary concern [11, 21, 22]. Initially it was believed that cessation of BP administration was essential in the management of BP-induced osteonecrosis [4, 48]. However, current guidelines indicate that cessation of BPs is not recommended given their clinical benefit as well as their prolonged effect on the bone [5, 21, 22]. According to the AAOMS on BP-related osteonecrosis of the jaws [25] discontinuation of intravenous (IV) BPs offers no short-term benefit, whilst discontinuation of oral BP therapy in patients with BP-induced osteonecrosis has been associated with gradual improvement in clinical disease. Thus if systemic conditions permit, modification or cessation of both IV and oral BP therapy should be done as long-term discontinuation of BPs has been shown to be beneficial. A distinction between cancer and osteoporosis should be highlighted, whereas unlike the patient with malignancy, it may not be in the best interest of the patient with osteoporosis to continue with BP therapy, as alternative therapies are available and should be considered [24].
Conclusion
Awareness of this newly described complication of BP therapy is important to promote detection and prevention of the resulting devastating osteonecrosis. The significant benefits conferred to patients on BP therapy should however not be discounted, and its use should be reliably controlled by the attending oncologist. Further research on the pharmacokinetics and pharmacodynamics of BPs as well as on the epidemiologic factors, pathophysiology and microbiology of this particular type of osteonecrosis is necessary to opine true evidence based guidelines. Until then we recommend that the care be taken in the diagnosis and treatment of these patients to decrease the risk of this debilitating complication.
References
- 1.Fleish H, Schibler D, Maerki J, et al. Inhibition of aortic calcification by means of pyrophosphate and polyphosphates. Nature. 1965;207:1300–1. doi: 10.1038/2071300b0. [DOI] [PubMed] [Google Scholar]
- 2.Papapoulos SE. Bisphosphonate actions: physical chemistry revisited. Bone. 2006;38:613–6. doi: 10.1016/j.bone.2006.01.141. [DOI] [PubMed] [Google Scholar]
- 3.Zavras AI, Zhu S. Bisphosphonates are associated with increased risk for jaw surgery in medical claims data: is it osteonecrosis? J Oral Maxillofac Surg. 2006;64:917–23. doi: 10.1016/j.joms.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrakopoulos I, Magopoulos C, Karakasis D. Bisphosphonate-induced avascular osteonecrosis of the jaws: a clinical report of 11 cases. Int J Oral Maxillofac Surg. 2006;35:588–93. doi: 10.1016/j.ijom.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. 2006;7:508–14. doi: 10.1016/S1470-2045(06)70726-4. [DOI] [PubMed] [Google Scholar]
- 6.Lenz JH, Steiner-Krammer B, Schmidt W, et al. Does avascular necrosis of the jaws in cancer patients only occur following treatment with bisphosphonates? J Craniomaxillofac Surg. 2005;33:395–403. doi: 10.1016/j.jcms.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Groen PC, Lubbe DF, Hirsch LJ, et al. Esophagitis associated with the use of aledronate. N Engl J Med. 1996;335:1016–21. doi: 10.1056/NEJM199610033351403. [DOI] [PubMed] [Google Scholar]
- 8.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–7. doi: 10.1016/S0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 9.Migliorati CA. Bisphophonates and oral cavity avascular bone necrosis. J Clin Oncol. 2003;21:4253–4. doi: 10.1200/JCO.2003.99.132. [DOI] [PubMed] [Google Scholar]
- 10.Ruggiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Cheng A, Mavrokokki A, Carter G, et al. The dental implications of bisphosphonates and bone disease. Aust Dent J. 2005;50:S4–13. doi: 10.1111/j.1834-7819.2005.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 12.Zarychanski R, Elphee E, Walton P, et al. Osteonecrosis of the jaw associated with pamidronate therapy. Am J Hematol. 2006;81:73–5. doi: 10.1002/ajh.20481. [DOI] [PubMed] [Google Scholar]
- 13.Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol. 2006;24:945–52. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 14.Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 15.Ott SM. Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005;90:1897–9. doi: 10.1210/jc.2005-0057. [DOI] [PubMed] [Google Scholar]
- 16.Ott SM. Fractures after long-term alendronate therapy. J Clin Endocrinol Metab. 2001;86:1835–6. doi: 10.1210/jc.86.4.1835. [DOI] [PubMed] [Google Scholar]
- 17.Miller PD. Efficacy and safety of long-term bisphosphonates in postmenopausal osteoporosis. Expert Opin Pharmacother. 2003;4:2253–8. doi: 10.1517/14656566.4.12.2253. [DOI] [PubMed] [Google Scholar]
- 18.Management of Osteoporosis in Postmenopausal Women: 2006 Position Statement of The North American Menopause Society. Menopause 2006;13(3):340–67. [DOI] [PubMed]
- 19.Tonino RP, Santora A, Ross PD. Safety of long-term alendronate. J Clin Endocrinol Metab. 2001;86:1835–6. doi: 10.1210/jc.86.4.1835-a. [DOI] [PubMed] [Google Scholar]
- 20.Assael LA. A time for perspective on bisphosphonates. J Oral Maxillofac Surg. 2006;64:877–9. doi: 10.1016/j.joms.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Novartis Pharmaceuticals Corporation. Expert panel recommendation for the prevention, diagnosis and treatment of osteonecrosis of the jaw. 2005. Appendix 11. Available from: www.fda.gov/orhms/dockets/ac/05/briefing/2005-4095B2_02_12-Novartis-Zometa-App-11.htm. Accessed 2 February 2007.
- 22.Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention and treatment. J Oral Maxillofac Surg. 2005;63:1567–75. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Brooks JK, Gilson AJ, Sindler AJ, et al. Osteonecrosis of the jaws associated with use of risedronate: Report of 2 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:780–6. doi: 10.1016/j.tripleo.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Capsoni F, Longhi M, Weinstein R. Bisphosphonate-associated osteonecrosis of the jaw: the rheumatologist’s role. Arthritis Res Ther. 2006;8:219. doi: 10.1186/ar2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Association of Oral and Maxillofacial Surgeons Position Paper on Bisphosphonate-related Osteonecrosis of the Jaws. J Oral Maxillofac Surg. 2007;65:369–76. [DOI] [PubMed]
- 26.Carter G, Goss AN, Doecke C. Bisphosphonates and avascular necrosis of the jaw: a possible association. Med J Aust. 2005;182:413–5. doi: 10.5694/j.1326-5377.2005.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 27.Purcell PM, Boyd IW. Bisphosphonates and osteonecrosis of the jaw. Med J Aust. 2005;182:417–8. doi: 10.5694/j.1326-5377.2005.tb06762.x. [DOI] [PubMed] [Google Scholar]
- 28.Diego R, D’Orto O, Pagani D, et al. Bisphosphonate-associated osteonecrosis of the jaws: a therapeutic dilemma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e1–5. doi: 10.1016/j.tripleo.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Magopoulos C, Karakinaris G, Telioudis Z, et al. Am J Otolaryngol 2007;28:158–63. [DOI] [PubMed]
- 30.Clarke B, Boyette J, Vural E, et al. Bisphosphonate and jaw necrosis: the UMAS experience. Otolaryngol Head Neck Surg. 2007;136:396–400. doi: 10.1016/j.otohns.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Mortensen M, Lawson W, Montazem A. Osteonecrosis of the jaw associated with bisphosphonate use: presentation of seven cases and literature review. Laryngoscope. 2007;117:30–4. doi: 10.1097/01.mlg.0000240885.64568.9e. [DOI] [PubMed] [Google Scholar]
- 32.Rossi R, Dorto O, Agazzi A, et al. Bisphosphonate-vascular osteonecrosis of the jaws: medical or surgical approach? [Abstract] J Craniomaxillofac Surg. 2006;34:34. [Google Scholar]
- 33.Merigo E, Manfredi M, Meleti M, et al. Bone necrosis of the jaws associated with bisphosphonate treatment: a report of twenty-nine cases. Acta Biomed. 2006;77:109–117. [PubMed] [Google Scholar]
- 34.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580–7. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 35.Bagan JV, Jimenez Y, Murillo J, et al. Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions. Study of 20 cases [Letter] Oral Oncol. 2006;42:327–9. doi: 10.1016/j.oraloncology.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Bagan JV, Murillo J, Jimenez Y, et al. Avascular jaw osteonecrosis in association with cancer chemotherapy: series of 10 cases. J Oral Pathol. 2005;34:120–3. doi: 10.1111/j.1600-0714.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 37.Miglioratti CA, Shubert MM, Peterson DE, et al. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer. 2005;104:83–93. doi: 10.1002/cncr.21130. [DOI] [PubMed] [Google Scholar]
- 38.Melo MD, Obeid G. Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. J Am Dent Assoc. 2005;136:1675–81. doi: 10.14219/jada.archive.2005.0110. [DOI] [PubMed] [Google Scholar]
- 39.Pires FR, Miranda A, Cardoso ES, et al. Oral avascular bone necrosis associated with chemotherapy and bisphosphonate therapy. Oral Dis. 2005;11:365–9. doi: 10.1111/j.1601-0825.2005.01130.x. [DOI] [PubMed] [Google Scholar]
- 40.Estilo CS, Van Poznak CH, Williams T, et al. Osteonecrosis of the maxilla and mandible in patients treated with bisphosphonates: a retrospective study [Abstract]. Proc Am Soc Clin Oncol. 2004;22:750.
- 41.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 42.Hansen T, Kunkel M, Weber A, et al. Osteonecrosis of the jaws in patients treated with bisphosphonates—histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155–60. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 43.Thakkar SG, Isada C, Smith J, et al. Jaw complications associated with bisphosphonate use in patients with plasma cell dyscrasias. Med Oncol. 2006;23:51–6. doi: 10.1385/MO:23:1:51. [DOI] [PubMed] [Google Scholar]
- 44.Michaelson MD, Smith MR. Bisphosphonates for treatment and prevention of bone metastases. J Clin Oncol. 2005;23:8219–24. doi: 10.1200/JCO.2005.02.9579. [DOI] [PubMed] [Google Scholar]
- 45.Djulbegovic B, Wheatley K, Ross J, et al. Bisphosphonates in multiple myeloma. Cochrane Database Syst Rev 2002;(3):CD003188. [DOI] [PubMed]
- 46.Body JJ, Diel IJ, Lichinitser MR, et al. Intravenous ibandronate reduces incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol. 2003;14:1399–405. doi: 10.1093/annonc/mdg367. [DOI] [PubMed] [Google Scholar]
- 47.American dental association council on scientific affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006;137:1144–50. [DOI] [PubMed]
- 48.Farrugia MC, Summerlin DJ, Krowiak E, et al. Osteonecrosis of the mandible or maxilla associated with the new generation bisphosphonates. Laryngoscope. 2006;116:115–20. doi: 10.1097/01.mlg.0000187398.51857.3c. [DOI] [PubMed] [Google Scholar]