Abstract
Stroma desmoplasia was studied by immunohistochemistry for α-smooth muscle actin (α-SMA) in 17 instances of carcinoma ex-pleomorphic adenoma (CXPA) classified according to the presence of epithelial and myoepithelial cells and the degree of invasion: intracapsular, minimally and frankly invasive carcinoma. In “resident” pleomorphic adenoma, no desmoplasia was detected. In invasive areas of the intracapsular type of CXPA with only an epithelial component, desmoplasia started to be revealed by the presence of myofibroblasts close to the capsule. In the minimally invasive type, myofibroblasts were seen in the septum between islands of malignant cells and in focal peripheral areas of the tumor interpreted as the actual front of invasion. In the frankly invasive type of CXPA showing large blocks of cells, intense desmoplasia was seen, also separating the tumor cells from the neighboring normal tissue. In tumors with cords and/or small nests of cells, desmoplasia was very slight. In the invasive type of CXPA with a myoepithelial component, α-SMA expression was seen in the septum between the islands of cells. The expression was less intense and not present in all areas of the stroma. In CXPA with epithelial and myoepithelial cells, myofibroblasts were rarely seen in the septum separating sheets of cells. Thus, we may deduce that the presence of desmoplasia parallels the capacity of invasion of CXPA by epithelial cells, being minimum in the intracapsular and minimally invasive type of CXPA and increasing as the tumor becomes frankly invasive. Furthermore, we may also conclude that in CXPA with a myoepithelial component, desmoplasia is very rare.
Keywords: Carcinoma ex-pleomorphic adenoma, Malignant transformation of pleomorphic adenoma, Intracapsular carcinoma ex-pleomorphic adenoma, Invasive carcinoma ex-pleomorphic adenoma, Front of invasion, Desmoplasia, α-Smooth muscle actin, Myofibroblast, Immunohistochemistry
Introduction
Carcinoma ex-pleomorphic adenoma (CXPA) is a salivary gland tumor derived from the malignant transformation of a benign tumor, the pleomorphic adenoma, usually from a long-standing or a recurrent tumor. It comprises 3% of all salivary tumors, 12% of all salivary malignance, and 6.2% of all pleomorphic adenomas [1].
Our group has studied various aspects of this lesion, such as cytoskeleton and proteins involved in cell cycling. Recently, we demonstrated by immunohistochemistry that CXPA is composed of tumors with either epithelium alone or with epithelium and myoepithelium. These tumors were classified as intracapsular, minimally invasive carcinoma, and frankly invasive carcinoma. Furthermore, some of these neoplasms may present intra-ductal areas also called in situ carcinoma [2]. We studied the immunoprofile of reactive myoepithelial cells, which surrounded the malignant luminal epithelial cells, showing that these cells become more differentiated and produce proteins related to tumor suppressor function [3].
However, tumor growth is not only determined by the tumor cells themselves, but it is also the result of a complex and poorly understood interplay between tumor cells and surrounding stromal tissue [4].
Studies have focused on stromal features of various tumors, aiming to add more information to the diagnostic and prognostic prediction of neoplastic lesions. In particular desmoplasia, a process characterized by deposition of collagen by myofibroblasts, has been pointed out as a valuable prognostic factor [5]. Myofibroblasts are regarded as reparative cells, and the fibroblasts are thought to be their precursor. Differentiation of fibroblasts into myofibroblasts is due to growth factor and cytokine activities, amongst which, transforming growth factor-β1 (TGF-β1) is the main agent [6].
The present study investigated the presence of stromal desmoplasia by identification of myofibroblasts in CXPA of salivary glands, aiming to correlate the later with the subtype components and the invasion grade of CXPA.
Materials and Methods
The present study protocol was approved by the Ethics Committee of School of Medicine of the State University of Campinas, Brazil.
About 17 surgical samples from cases diagnosed as CXPA were retrieved from the files of the Department of Clinical Pathology at the State University of Campinas Medical School. The tumors were classified according to Brandwein et al. [7] taking into account the extension of invasion beyond the capsule of the previous pleomorphic adenoma as intracapsular (without invasion), minimally invasive (≤1.5 mm of invasion), and frankly invasive. The tumors were also classified according to the presence of epithelial and/or myoepithelial cells using immunohistochemistry for cytokeratins (epithelial cells), vimentin, and α-smooth muscle actin (α-SMA) (myoepithelial cells) as previously described [2] (Table 1).
Table 1.
Sex, age, localization, component, and degree of invasion of the carcinoma ex-pleomorphic adenoma
| Case | Sex | Age (years) | Salivary gland | Component | Degree of invasion |
|---|---|---|---|---|---|
| 1 | Female | 44 | Submandibular | Epithelial | Intracapsular |
| 2 | Male | 58 | Parotid | Epithelial | Intracapsular |
| 3 | Female | 50 | Parotid | Epithelial | Intracapsular |
| 4 | Female | 37 | Submandibular | Epithelial | Intracapsular |
| 5 | Female | 51 | Parotid | Epithelial | Intracapsular |
| 6 | Female | 65 | Parotid | Epithelial | Minimally invasive |
| 7 | Female | 43 | Parotid | Epithelial | Minimally invasive |
| 8 | Female | 89 | Submandibular | Epithelial | Minimally invasive |
| 9 | Male | 74 | Parotid | Epithelial | Minimally invasive |
| 10 | Female | 62 | Submandibular | Epithelial | Frankly invasive |
| 11 | Male | 66 | Parotid | Epithelial | Frankly invasive |
| 12 | Male | * | Parotid | Epithelial | Frankly invasive |
| 13 | Male | 44 | Parotid | Epithelial | Frankly invasive |
| 14 | Female | 55 | Palate | Myoepithelial | Frankly invasive |
| 15 | Male | 65 | Parotid | Myoepithelial | Frankly invasive |
| 16 | Female | 50 | Parotid | Myoepithelial | Frankly invasive |
| 17 | Female | 74 | Palate | Myoepithelial | Frankly invasive |
* Data not available
Serial sections, 3 μm in thickness, were obtained from paraffin-embedded samples and the dewaxed sections were processed for epitope desmasking (Table 2). Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide. After washing, the sections were incubated with primary anti-vimentin, anti-α-SMA, and anti-desmin (Table 2).
Table 2.
Clone, pretreatment for epitope desmasking, dilution and incubation times for primary antibodies
| Antibody | Clone | Pretreatment | Dilution | Incubation time (min) |
|---|---|---|---|---|
| Vimentina | V9 | Citrate 0.01 M; pH 6.0; 95°C; 30 min | 1:800 | 40 |
| α-Smooth muscle actina | 1A4 | No pretreatment | 1:300 | 40 |
| Desmina | DE-R-11 | Citrate 0.01 M; pH 6.0; 95°C; 30 min | 1:100 | 40 |
aDAKO, Carpinteria, CA, USA
Signal detection was performed using the DAKO EnVision Peroxidase procedure (DAKO, Carpinteria, CA, USA), followed by a diaminobenzidine chromogen solution and counterstaining with Mayer’s hematoxylin.
Wall vessel and myoepithelial cells of the normal salivary glands included in the tumoral specimens were used as positive internal control.
The labeled sections were qualitatively evaluated by two independent pathologists.
Results
In this investigation, the immunohistochemical expression of α-SMA was evaluated to detect myofibroblasts responsible for stromal desmoplasia. Our results disclosed that vimentin was positive for both fibroblasts and myofibroblasts; desmin was not detected in myofibroblasts. This expression was studied in CXPA tumors with an epithelial component—5 intracapsular, 4 minimally invasive, and 4 invasive—and 4 invasive tumors with both epithelial and myoepithelial cells or with only a myoepithelial cell component.
“Residual” Pleomorphic Adenoma
α-SMA expression was observed only in vessel walls and, in focal areas, in rare plasmacytoid and fusiform myoepithelial cells. Few myoepithelial cells surrounding ductal structures were also positive. No desmoplasia was detected in the stroma.
Carcinoma Ex-Pleomorphic Adenoma Without a Myoepithelial Component
Intracapsular
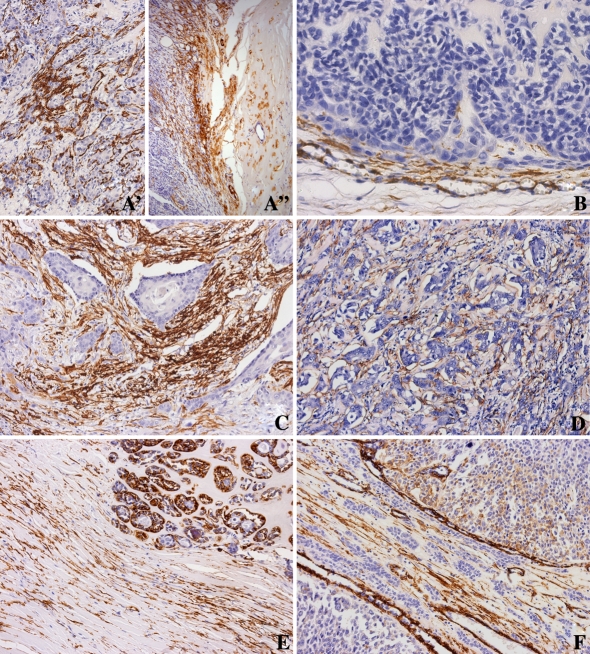
In areas of in situ carcinoma of the intracapsular type, there is a strong expression of α-SMA in benign myoepithelial cells around ductal structures (Fig. 1A′). In invasive areas of CXPA which are still inside the pleomorphic adenoma capsule, initiation of desmoplasia starts with the presence of myofibroblasts expressing α-SMA close to the capsule (Fig. 1A″).
Fig. 1.
Immunohistochemistry of α-SMA in CXPA. (A′) and (A″) Intracapsular type, with epithelial component. (A′) Strong expression of α-SMA in the benign myoepithelial cells surrounding the ductal structures of in situ areas. (A″) Presence of myofibroblasts positive for α-SMA close to the capsule. (B) Minimally invasive type, with epithelial component. Desmoplasia is observed in invasion front. (C) and (D) Frankly invasive type, with epithelial component. Strong expression of α-SMA in myofibroblasts revealing intense desmoplasia in septum among blocks of malignant epithelial cells (C) and discrete desmoplasia among small nests of cell (D). (E) Frankly invasive type, with epithelial and myoepithelial components. Expression of α-SMA is seen in a few myofibroblasts in the stroma. Observe the strong expression of α-SMA in malignant myoepithelial cells of ductal structures. (F) Frankly invasive type, with myoepithelial component. Presence of myofibroblasts is very rare in the tumoral stroma. α-SMA is detected in myoepithelial cells and wall vessel
Minimally Invasive
Myofibroblasts were seen in septae between blocks of malignant cells and in focal peripheral areas of the tumor interpreted as the invasion front (Fig. 1B).
Frankly Invasive
In invasive CXPA showing large islands of cells, intense desmoplasia was seen, separating the tumor cells from the neighboring normal tissue (Fig. 1C). However, when the tumor was represented by cords and/or small nests of cells, desmoplasia was very slight (Fig. 1D).
Carcinoma Ex-Pleomorphic Adenoma with Myoepithelial Component
Frankly Invasive
In CXPA with only myoepithelium, α-SMA expression was seen in some malignant myoepithelial cells and in the septum among the blocks of cells. The expression was less intense and not present in the entire stroma (Fig. 1E).
In CXPA composed of epithelial and myoepithelial cells, the expression of α-SMA was present only in myoepithelial malignant cells. No stromal myofibroblasts were seen in the majority of the specimens. Myofibroblasts expressing α-SMA were rarely seen in the septum separating sheets of cells (Fig. 1F).
Discussion
In the present study, we have analyzed the presence of desmoplasia along different stages of CXPA development. Our results showed that desmoplasia parallels the change in behavior of CXPA: at first there is no desmoplasia in pleomorphic adenoma; it starts appearing in invasive areas of intracapsular type, although still inside the PA capsule; and finally, the desmoplasia becomes intense in invasive type of CXPA with only epithelial cells but scarce in CXPA with myoepithelial cells. In tumors with a minimal degree of invasion, myofibroblasts were observed in focal peripheral areas interpreted as the real invasion front.
Tumor development and progression has been considered to be a consequence of an imbalance between apoptosis and proliferation of transformed cells. Proliferation is the result of genetic aberrations which trigger the activation of oncogenes and/or loss of tumor suppressor genes. However, progression towards a malignancy requires a dynamic interaction between tumor cells and the environment in which they thrive. This interaction promotes changes in the tumoral stroma, including the appearance of desmoplasia. An excellent review of the role of tissue stroma in cancer invasion has been presented by de Wever and Mareel [8].
Desmoplasia is characterized by collagen fibers plus myofibroblasts, mesenchimal cells sharing characteristics with smooth muscle cells and fibroblasts. Morphologically, this cell is defined by stress fibers and well-developed cell-matrix interactions (fibronexus). Myofibroblasts are large spindle-shaped cells with indented nuclei, reflecting their myogenic nature. Immunocytochemical characterization of the myofibroblast is based on a combination of different markers such as positivity for α-SMA, vimentin, prolyl 4-hydroxylase, and absence of cytokeratin [9–11]. Growth factors, such as transforming growth factor-β (TGF-β), produced by tumoral cells have been suggested to be responsible for the transdifferentiation of fibroblasts to myofibroblasts, first described in wound healing.
Myofibroblasts have been observed in tumors of various origins—colon [12], breast [13, 14], liver [15], lung [16], prostate [17], and pancreas [18]—mainly at the invasion front. Desmoplastic response in the invasion front was observed in more than 90% of pancreatic carcinoma [19]. The causal role of the myofibroblast in the transition from the non-invasive towards the invasive phenotype is suggested by the finding that the appearance of myofibroblasts precedes the invasive stage of the cancer. Thus, myofibroblasts are a common stromal element in the colon from patients that have developed familial adenomatous polyposis and larger villous adenomas which both frequently transit toward invasive carcinomas [20].
In this study, looking at the epithelial tumors with a minimally invasive degree of invasion, myofibroblasts started being observed in focal peripheral areas. The same pattern of expression by tenascin was demonstrated in a recent study suggesting that the alteration of stroma may play a role in invasion properties of this tumor [21]. We recall that both tenascin and transdifferentiation of fibroblasts to myofibroblasts are signalized by TGF-β.
Growth factors such as TGF-β, which is produced by tumoral cells, transdifferentiate fibroblasts to myofibroblasts, changing the cancer stroma which seems to be the first signal of tumor invasion [8]. Besides the transdifferentiation of fibroblasts into myofibroblasts, TGF-β in tumors has multiple activities: it leads to critical changes in cytokine balance, extracellular matrix proteins, proteinases, and their inhibitors and provides combinatorial information for invasion and survival, angiogenesis, and escape from immune surveillance [22–24].
Controversial findings are described in the literature about the role of desmoplasia in human tumors. It is not known whether it gives to the tumoral cells a growth advantage or it is a reaction of the host to inhibit cancer cell progression. Caporale et al. [25], studying desmoplasia in colorectal carcinoma, concluded that it may prevent cancer invasiveness by building a barrier against tumor diffusion. In salivary gland tumors, Sobral et al. [26] showed greater desmoplasia in low-grade mucoepidermoid carcinoma when compared with high-grade mucoepidermoid carcinoma. Vasudev and Harris [27] and Schurch et al. [28, 29] also related desmoplasia to low-grade tumors in breast adenocarcinomas.
Alternatively, myofibroblasts not only stimulate cancer cell invasion but also angiogenesis and shield the cancer cell from the immune response preventing physical contact between cancer cells and immune cells, an essential phenomenon for cancer cell destruction. The presence of myofibroblasts around progressive tumors is associated with the absence of immune and inflammatory cells within tumors [30].
It is also interesting to remember that TGF-β, which is responsible for the desmoplasia, is related to cancer invasion. Various studies have given support to this hypothesis: high levels of TGF-β associated with colon cancer progression in men [31]; over expression of TGF-β in prostatic cancer growth and invasion in vivo [32]; inhibition by anti-TGF-β monoclonal antibodies of lung metastasis of mammary cancer cells inoculated intraperitoneally into athymic mice [33]; and, in transgenic mice, keratinocyte targeted expression of TGF-β promotes cancer progression as evidenced by the conversion rates of non-invasive towards invasive lesions [34, 35].
The three cases of CXPA composed of epithelial and myoepithelial cells, as well as the case composed only of myoepithelial cells were all in the invasive stage, whereas these tumors are usually identified just when they have invaded outside the original or residual pleomorphic adenoma [2]. In these tumors, the desmoplasia characterized by myofibroblasts was rarely observed in the septum separating sheets of cells. This fact probably is related to the presence of myoepithelial cells, that also exhibit α-SMA filament. It is important to emphasize the role of myoepithelial cell as a tumor suppressor providing an important defense against cancer invasion [3]. This function was based on the capacity of myoepithelial cells to accumulate abundant extracellular matrix and secrete relatively low levels of matrix degradation proteinases but relatively high levels of maspin and various other anti-invasion proteinase inhibitors [36–38].
Based on our results, we may deduce that the presence of desmoplasia parallels the capacity of the invasion of CXPA composed by epithelial cells, starting in the invasion front of the minimally invasive type of CXPA and increasing as the tumor becomes frankly invasive. Furthermore, we conclude that in CXPA with a myoepithelial component, desmoplasia is very rare.
Acknowledgment
This work was supported by FAPESP—grant no. 04/07960-0.
References
- 1.Gnepp DR, Brandwein-Gensler MS, El-Naggar AK, et al. Carcinoma ex pleomorphic adenoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, et al., editors. World Health Organization classification of tumours. Pathology & genetics. Head and neck tumours. 1. Lyon: IARCPress; 2005. pp. 242–243. [Google Scholar]
- 2.Altemani A, Martins MT, Freitas L, et al. Carcinoma ex pleomorphic adenoma (CXPA): immunoprofile of the cells involved in carcinomatous progression. Histopathology. 2005;46:635–41. doi: 10.1111/j.1365-2559.2005.02157.x. [DOI] [PubMed] [Google Scholar]
- 3.Araujo VC, Altemani A, Furuse C, et al. Immunoprofile of reactive salivary myoepithelial cells in intraductal areas of carcinoma ex-pleomorphic adenoma. Oral Oncol. 2006;42:1011–6. doi: 10.1016/j.oraloncology.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 5.Rønnov-Jessen L, Petersen OW. Induction of α-smooth muscle actin by transforming growth factor-β1 in quiescent human breast gland fibroblasts: implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- 6.Desmoulière A, Geinoz A, Gabiani F, et al. Tranforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandwein M, Huvos AG, Dardick I, Thomas MJ, Theise ND. Noninvasive and minimally invasive carcinoma ex mixed tumor: a clinicopathologic and ploidy study of 12 patients with major salivary tumors of low (or no?) malignant potential. Oral Surg Oral Med Oral Pathol Oral Radiol. 1996;81(6):655–64. doi: 10.1016/S1079-2104(96)80071-0. [DOI] [PubMed] [Google Scholar]
- 8.Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–47. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 9.Lazard D, Sastre X, Frid MG. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA. 1993;90:999–1003. doi: 10.1073/pnas.90.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loop FT, Schaart G, Timmer ED, et al. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol. 1996;134:401–11. doi: 10.1083/jcb.134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama H, Enzan H, Miyazaki E, et al. The role of myofibroblasts at the tumor border of invasive colorectal adenocarcinomas. Jpn J Clin Oncol. 1998;28:615–20. doi: 10.1093/jjco/28.10.615. [DOI] [PubMed] [Google Scholar]
- 12.Miura S, Kodaira S, Hosoda Y. Immunohistologic analysis of the extracellular matrix components of the fibrous stroma of human colon cancer. J Surg Oncol. 1993;53:36–42. doi: 10.1002/jso.2930530111. [DOI] [PubMed] [Google Scholar]
- 13.Rønnov-Jessen L, Petersen OW, Koteliansky VE, et al. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–73. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobuzio-Donahue CA, Argani P, Hempen PM, et al. The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res. 2002;62:5351–7. [PubMed] [Google Scholar]
- 15.Neaud V, Faouzi S, Guirouilh J, et al. Human hepatic myofibroblasts increase invasiveness of hepatocellular carcinoma cells: evidence for a role of hepatocyte growth factor. Hepatology. 1997;26:1458–66. doi: 10.1002/hep.510260612. [DOI] [PubMed] [Google Scholar]
- 16.Doucet C, Jasmin C, Azzarone B. Unusual interleukin-4 and -13 signaling in human normal and tumor lung fibroblasts. Oncogene. 2000;19:5898–905. doi: 10.1038/sj.onc.1203933. [DOI] [PubMed] [Google Scholar]
- 17.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev 1998–1999;17:411–9. [DOI] [PubMed]
- 18.Lohr M, Schmidt C, Ringel J, et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–5. [PubMed] [Google Scholar]
- 19.Ryu B, Jones J, Hollingsworth MA, et al. Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res. 2001;61:1833–8. [PubMed] [Google Scholar]
- 20.Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol Res Pract. 1996;192:712–7. doi: 10.1016/S0344-0338(96)80093-8. [DOI] [PubMed] [Google Scholar]
- 21.Araújo VC, Furuse C, Cury PR, et al. Tenascin and fibronectin expression in carcinoma ex pleomorphic adenoma. Appl Immunohistochem Mol Morphol (in press). [DOI] [PubMed]
- 22.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 23.Sakko AJ, Ricciardelli C, Mayne K, et al. Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor beta1. Cancer Res. 2001;61:926–30. [PubMed] [Google Scholar]
- 24.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–62. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 25.Caporale A, Vestri AR, Benvenuto E, et al. Is desmoplasia a protective factor for survival in patients with colorectal carcinoma? Clin Gastroenterol Hepatol. 2005;3:370–5. doi: 10.1016/S1542-3565(04)00674-3. [DOI] [PubMed] [Google Scholar]
- 26.Sobral AP, Loducca SV, Nunes FD, et al. Relationship between major and minor salivary gland mucoepidermoid carcinoma malignancy grading and presence of stromal myofibroblasts: immunohistochemical study. J Oral Pathol Med. 2004;33:335–9. doi: 10.1111/j.1600-0714.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 27.Vasudev KS, Harris M. A sarcoma of myofibroblasts: an ultrastructural study. Arch Pathol Lab Med. 1978;102:185–8. [PubMed] [Google Scholar]
- 28.Schurch W, Seemayer TA, Lagace R, et al. The intermediate filament cytoskeleton of myofibroblasts: an immunofluorescence and ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1984;403:323–36. doi: 10.1007/BF00737283. [DOI] [PubMed] [Google Scholar]
- 29.Schurch W, Seemayer TA, Gabbiani G. The myofibroblast: a quarter century after its discovery. Am J Surg Pathol. 1998;22:141–7. doi: 10.1097/00000478-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Lieubeau B, Heymann MF, Henry F. Immunomodulatory effects of tumor-associated fibroblasts in colorectal-tumor development. Int J Cancer. 1999;81:629–36. doi: 10.1002/(SICI)1097-0215(19990517)81:4<629::AID-IJC20>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Tsushima H, Kawata S, Tamura S, et al. High levels of transforming growth factor beta 1 in patients with colorectal cancer: association with disease progression. Gastroenterology. 1996;110:375–82. doi: 10.1053/gast.1996.v110.pm8566583. [DOI] [PubMed] [Google Scholar]
- 32.Steiner MS, Barrack ER. Transforming growth factor-beta 1 overproduction in prostate cancer: effects on growth in vivo and in vitro. Mol Endocrinol. 1992;6:15–25. doi: 10.1210/me.6.1.15. [DOI] [PubMed] [Google Scholar]
- 33.Arteaga CL, Hurd SD, Winnier AR, et al. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993;92:2569–76. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui W, Fowlis DJ, Bryson S, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–42. doi: 10.1016/S0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 35.Weeks BH, He W, Olson KL, et al. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001;61:7435–43. [PubMed] [Google Scholar]
- 36.Sternlicht MD, Barsky SH. The myoepithelial defense: a host defense against cancer. Med Hypotheses. 1997;48:37–46. doi: 10.1016/S0306-9877(97)90022-0. [DOI] [PubMed] [Google Scholar]
- 37.Sternlicht MD, Safarians S, Rivera SP, et al. Characterizations of the extracellular matrix and proteinase inhibitor content of human myoepithelial tumors. Lab Invest. 1996;74:781–96. [PubMed] [Google Scholar]
- 38.Martins MT, Altemani A, Freitas L, et al. Maspin expression in carcinoma ex pleomorphic adenoma. J Clin Pathol. 2005;58:1311–4. doi: 10.1136/jcp.2004.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]