Abstract
Plasmablastic lymphoma (PBL) is a rare acquired immunodeficiency syndrome-associated non-Hodgkin’s lymphoma (AIDS-NHL), with predilection for the mucosa of oral cavity. It usually has a plasmablastic morphology, expressing plasma cell-associated antigens with weak or absent expression of B-cell-associated markers. To further define the immunophenotypic and molecular genetics of these tumors, we investigated two cases of plasmablastic lymphomas of the head and neck for c-myc gene rearrangement and immunoglobulin heavy chain (IgVH) hypermutation status. For the first time we report a case of AIDS-related PBL that, by fluorescence in situ hybridization (FISH), shows a c-myc gene rearrangement. Although current literature suggests that most cases of c-myc gene rearranged AIDS-NHL are Burkitt’s lymphoma, our case has an immunophenotype characteristic for PBL. In this case, IgVH hypermutation analysis showed a somatic hypermutation, indicative of germinal center transit. The concurrent B-cell immunophenotype of BCL-6−/CD138+/MUM-1+ also suggests a post-germinal center B-cell origin of this lymphoma. The immunophenotype of our second case (BCL-6−/CD138+/MUM-1+) also suggests a post-germinal center B-cell origin. However, IgVH hypermutation analysis was not possible in this case.
Keywords: Plasmablastic lymphoma, Acquired immunodeficiency syndrome-associated non-Hodgkin’s lymphoma, c-myc gene rearrangement, Immunoglobulin variable heavy chain hypermutation status
Introduction
PBL is a high-grade AIDS-NHL, initially described by Delecluse et al. in 1997 [1]. It is clinically characterized by frequent extranodal occurrence with particular predilection for oral cavity [1, 2].
The neoplastic cells in PBL are generally blastic in appearance and show strong expression of plasma cell-associated antigens such as CD38 and CD138 and weak to absent expression of B-cell-associated markers CD20 and CD79a [1, 3–6]. The high-grade nature of this lymphoma is also represented by numerous interspersed “tingible-body macrophages”, imparting a starry-sky appearance, and brisk mitotic activity [7]. Although immunohistochemistry for Epstein-Barr virus latent membrane protein 1 (EBV-LMP1) is negative in the majority of cases, in situ hybridization for Epstein-Barr virus encoded small RNA (EBER) is consistently positive [1, 3].
We report two additional cases of AIDS-associated EBER positive PBL of the head and neck, one with previously undiagnosed HIV-status. In addition, we investigated both cases for the c-myc gene rearrangement and immunoglobulin heavy chain hypermutation status to further delineate the genetic characteristics of these tumors. We report for the first time a c-myc gene rearrangement in one of the two cases. Although c-myc gene rearrangement in AIDS-NHL is usually seen in the setting of Burkitt’s lymphoma, this report shows that a lymphoma with typical morphology and immunophenotype of plasmablastic lymphoma may show c-myc gene rearrangements. This finding may have significant implications in the therapeutic management of some cases of plasmablastic lymphomas.
Case Reports
Case 1
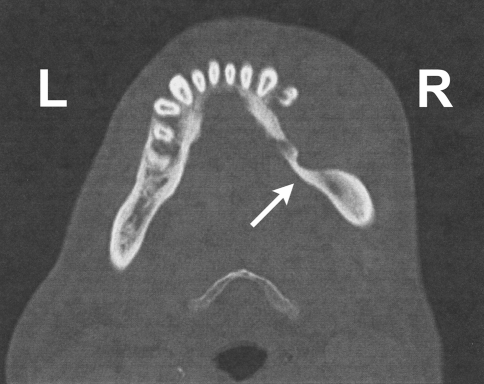
This is a case of a 49-year-old man who presented to the emergency room with an enlarging right lower jaw mass and associated facial swelling of ∼2 months duration. He reported frequent sharp pain in this area but denied dysphagia, weight loss, otalgia, or difficulty breathing or swallowing. Physical exam revealed a 5 × 4 cm oral mass with obvious ulceration. A maxillofacial computed tomography (CT) scan showed a 5.5 × 3.3 cm homogenous mass in the right lower gingiva and buccal mucosa with significant bone destruction of the mandibular body (Fig. 1). A complete blood count and comprehensive metabolic panel were within normal limits except for mild normochromic anemia. Lactate dehydrogenase was normal at 164 IntUnits/L (normal range: 100–250 IntUnits/L). A right buccal biopsy was obtained showing a high-grade lymphoma. Based on this diagnosis, testing for human immunodeficiency virus (HIV) antibodies was suggested and was found to be positive for p24, p31, gp41, p51/55, p66 and gp120/160 by enzyme-linked immunosorbent assay (ELISA) and Western Blot. A staging bone marrow was negative for involvement by lymphoma.
Fig. 1.
Maxillofacial computed tomography scan of case 1 showing a 5.5 × 3.3 cm homogenous mass in the right lower jaw with bone destruction of the mandibular body [white arrow]
Case 2
A 42-year-old man with known HIV infection, diagnosed three years earlier, presented to his primary care physician with a 3 cm cutaneous lesion on his left neck, erythematous and flat in appearance, as well as several ipsilaterally enlarged lymph nodes. Laboratory blood testing revealed a normal complete blood count except for mild normochromic anemia. The total CD4+ T-cell count was mildly decreased. Excisional biopsies of the skin lesion and neck lymph nodes were performed, showing a high-grade lymphoma. A staging bone marrow and cerebrospinal fluid examination showed no involvement by lymphoma. Despite chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), the patient developed progressive lymphadenopathy and died of progressive disease 8 months after diagnosis.
Materials and Methods
The tissues were routinely fixed in 10% buffered formalin, embedded in paraffin, and serially sectioned into 4-μm-thick sections for routine hematoxylin and eosin staining and immunohistochemistry.
Immunoperoxidase studies were performed with use of an autostainer (Benchmark XT System, Ventana Medical Systems, Tucson, AZ), as per the manufacturer’s instructions, with antibodies listed in Table 1. Positive and negative controls were run simultaneously.
Table 1.
Antibodies and sources
| Antibody | Dilution | Source |
|---|---|---|
| ALK1 | 1:50 | Dako M0630 |
| CD2 | 1:200 | Biogenex M4438 |
| CD3 | 1:100 | Dako M7254 |
| CD4 | 1:10 | Vector VPC318 |
| CD8 | 1:100 | Dako M7103 |
| CD20 | 1:2000 | Dako M0755 |
| CD30 | 1:100 | Dako M0751 |
| CD45 | 1:200 | Dako M0701 |
| CD45RO | 1:400 | Dako M0742 |
| CD56 | 1:50 | Cell marquee |
| CD79a | 1:40 | Dako M7054 |
| CD138 | 1:200 | Dako |
| CK | 1:1000 | Dako M3513 |
| EBER | Proprietary | Ventana |
| EMA | 1:1000 | Dako M0613 |
| Kappa ISH | Proprietary | Ventana |
| Lambda ISH | Proprietary | Ventana |
| Myeloperoxidase | 1:10,000 | Dako A0398 |
| S100 | 1:3000 | Dako 20311 |
| Tdt | 1:50 | Dako A3524 |
EBER in situ hybridization (ISH) was performed on an autostainer (Benchmark XT System, Ventana Medical Systems, Tucson, AZ) utilizing the ISH iVIEW Blue Detection Kit (Ventana Medical Systems, Tucson Tuscan, AZ), as per the manufacturer’s instructions. Briefly, fluorescein-labeled EBER probes (Ventana Inform Series probes, Ventana Medical Systems, Tucson, AZ) are directed to early RNA transcripts in the nucleus of actively infected cells. Rabbit anti-fluorescein primary antibody detects the fluorescein-labeled probes bound to the target sequence. This is followed by an amplification reagent (mouse anti-rabbit antibody) and the binding of a biotinylated secondary antibody (goat anti-mouse IgG). Strepavidin conjugated alkaline phosphatase is then used as a chromogenic enzyme, generating a visible blue nuclear signal.
In situ hybridization for kappa and lambda immunoglobulin light chains was performed in a similar fashion utilizing fluorescein-labeled lambda and kappa probes directed towards lambda or kappa light chain immunoglobulin messenger RNA (mRNA).
FISH was performed on paraffin embedded tissue sections utilizing a commercial c-myc (8q24) breakapart probe set (Vysis Inc., Downers Grove, IL). Assay and scoring were strictly performed according to manufacturer’s protocol and standardized methods. This assay provides no specific information regarding the translocation partner.
For IgVH hypermutation analysis, genomic DNA was isolated from paraffin embedded tissue utilizing the PURGENE DNA Purification kit (Gentra Systems Inc., Minneapolis, MN), according to the manufacturer’s instructions. A commercially available PCR kit (IGH BIOMED2 Clonality Assay, In VivoScribe Technologies, San Diego, CA) was used to amplify clonal rearrangement of the IGH gene locus. Clonally rearranged DNA configurations were determined by capillary electrophoresis (2100 Bioanalyzer, Agilent Technologies, Santa Clara, CA). PCR products were sequenced using consensus J primers (JH Consensus, In VivoScribe Technologies, San Diego, CA) and the BigDye Terminator v3.1 cycle sequencing kit (ABI 3130XL, Applied Biosystems, Foster City, CA). The IgVH sequence was compared to known genomic VH gene segment sequences using both VBASE9 (http://vbase.mrc-cpe.cam.ac.uk/) and IGBLAST (http://www.ncbi.nlm.nih.gov/projects/igblast/). The total of mismatched bases was expressed as a percentage of the total sequenced region. Greater than 2% mismatch was scored as positive for the presence of IgVH hypermutation.
Results
Case 1: Pathology
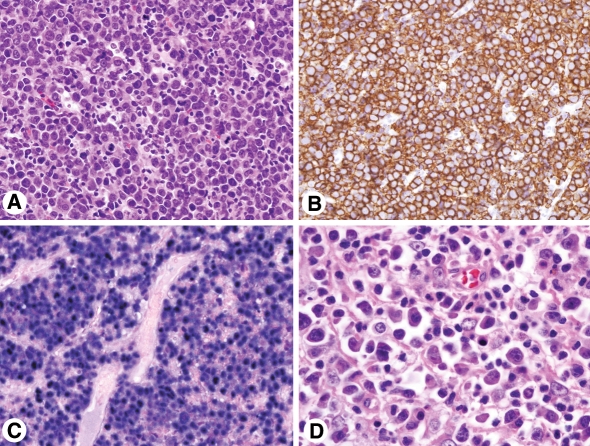
Microscopic evaluation of the right buccal mass showed a diffuse infiltrate of large cells with moderate amounts of cytoplasm, round and oval, mostly eccentrically located nuclei with mature chromatin, and occasional prominent nucleoli. Cytologically, the neoplastic cells were consistent with immunoblasts/plasmablasts. Interspersed mature plasma cells were also present. The mitotic activity was brisk (Fig. 2A).
Fig. 2.
AIDS-associated plasmablastic lymphoma. (A) Representative section of right buccal mass (case 1) with diffuse infiltrate of immunoblasts/plasmablasts and interspersed mature plasma cells. The mitotic activity is brisk (hematoxylin and eosin, original magnification 50×). (B) Neoplastic cells are immunoreactive for CD138/syndecan-1 (original magnification 50×). (C) In situ hybridization for EBER shows strong nuclear staining (original magnification 50×). (D) Representative section of the cervical lymph node (case 2) with diffuse infiltrate of immunoblasts/plasmablasts and interspersed mature plasma cells (original magnification 100×)
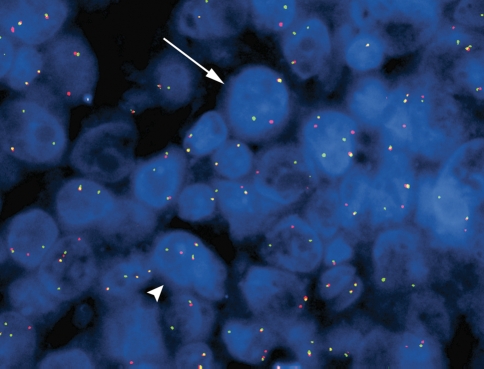
Immunohistochemical features of the neoplastic lymphoid cells are listed in Table 2. Briefly, these were weakly immunoreactive for CD45 (LCA). Strong expression for CD138 and MUM-1 (CD138, Fig. 2B) was detected. The Ki-67 proliferation index was estimated at 90–95%. In situ hybridization for EBER showed strong nuclear staining in neoplastic cells (Fig. 2C) and in situ hybridization for kappa and lambda light chains was negative. Fluorescence in situ hybridization with the c-myc break apart rearrangement probe showed 28% of analysed interphases to have one fusion signal and one split of a red and a green signal consistent with a c-myc-containing chromosomal rearrangement (Fig. 3). In addition, 13% of interphases revealed an extra copy of chromosome 8, with one copy showing a c-myc rearrangement (Fig. 3).
Table 2.
Summary of immunohistochemical stains with results on Case 1 and Case 2
| Immunohistochemical stain | Case 1 | Case 2 |
|---|---|---|
| ALK-1 | Negative | Negative |
| BCL-6 | Negative | Negative |
| CD3 | Negative | Negative |
| CD20 | Negative | Negative |
| CD30 | Negative | Positive |
| CD79a | Negative | Negative |
| CD138 | Positive | Positive |
| Cytokeratin | Negative | Negative |
| EBER-ISH | Positive | Positive |
| EBV-LMP | Negative | Negative |
| MUM-1 | Positive | Positive |
Fig. 3.
Fluorescence in situ hybridization with c-myc (8q24) breakapart probe on paraffin embedded tissue section of case 1 showing a fusion signal and a split of a red and a green signal in a subset of interphase cells consistent with a c-myc rearrangement (arrow). In addition, a smaller subset revealed an extra copy of chromosome 8, with one copy showing a c-myc rearrangement (arrow head)
Analysis for IgVH hypermutation status showed increased levels of base variation (4%) in VH 1-46 region.
Case 2: Pathology
Microscopic examination of the left cervical lymph node showed complete effacement of normal architecture by intermediate to large lymphoid cells with abundant cytoplasm, eccentric nuclei and prominent nucleoli. Numerous smaller cells showed plasmacytic differentiation with eccentrically placed nucleus and wheel-like chromatin pattern. Occasional binucleated forms were also seen (Fig. 2D). Immunoperoxidase profile is summarized in Table 2. In situ hybridization for EBER showed strong nuclear reactivity. Fluorescence in situ hybridization did not detect a split of c-myc signal. Analysis for hypermutation status yielded insufficient cellular material for evaluation.
Discussion
The designation “plasmablastic lymphoma” was first proposed in 1997 by Delecluse et al. for a new entity of HIV-related high-grade lymphoma, based on blastic morphology and an antigen profile similar to plasma cells [1]. In their reported series of sixteen cases, fifteen were found to be in patients with HIV infection. All cases were localized in the mucosa of the oral cavity. The immunophenotype was that of plasma cell type with expression of VS38c and variable weak expression of CD79a, and weak to absent expression of B-cell markers. Despite EBV-LMP negativity in nearly all cases, in situ hybridization for EBER was positive in 9 of 16 cases. Subsequent publications with larger case studies have shown that PBL is not restricted to the oral cavity, but may also present at other mucosal sites in the head and neck, bone, soft tissue, gastrointestinal tract, and spermatic cord [3, 8]. Although the majority of these cases occur in association with HIV-infection, a small subset is associated with immunosuppression after solid organ transplantation or steroid therapy and very rare cases occur in immunocompetent patients [1, 6, 9]. Morphology of these subsequently reported cases vary from a monotonous proliferation of lymphoid cells with immunoblastic features and minimal plasmacytic differentiation to a variable mixture of large immunoblasts and plasmablasts with cells showing differentiation to mature plasma cells [1, 9]. The two cases presented here display some degree of plasma cell differentiation.
Immunophenotyping consistently shows strong CD138 and VS38c expression, variable expression of CD79a, EBER reactivity in the majority of cases, and absent or very weak expression of CD20 [1–6]. The clinical behavior has been reported as highly aggressive with generally poor response to therapy and short survival ranging between 1 and 28 months after diagnosis [1, 4, 9].
The Word Health Organization (WHO) has classified PBL as a subtype of diffuse large B-cell lymphoma occurring predominantly in HIV-positive patients [10]. The most common types of AIDS-related NHL include diffuse large B-cell lymphoma, immunoblastic type and Burkitt’s lymphoma [3]. Rare types occurring predominantly in HIV-positive patients are primary effusion lymphoma, plasmablastic lymphoma, and plasmablastic lymphoma associated with multicentric Castleman’s disease [3]. A common histopathologic feature shared by AIDS-NHL is the variable presence of plasmacytoid differentiation. Despite the relatively low incidence of plasma cell malignancies in HIV+ patients, plasmablastic plasmacytoma or myeloma, should be included in the differential diagnosis. A study published by Vega et al. was not able to reliably distinguish between PBL and plasmablastic plasma cell myeloma by morphology and immunohistochemistry alone, since no statistically significant difference in frequency of lymphoid and plasma cell-related markers was seen between these malignancies [11]. All cases showed strong uniform expression of the post-germinal center associated B-cell- and plasma cell-associated markers MUM-1, CD38 and CD138. The main difference between the two entities was the association of PBL with EBV infection, as demonstrated by EBER in situ hybridization. Furthermore, distinction between these two malignancies heavily depended predominantly on clinical correlation, such as the presence of a serum paraprotein or radiographically evident lytic lesions.
Delineation of plasmablastic lymphoma from diffuse large B-cell lymphoma or Burkitt’s lymphoma can easily be accomplished by immunophenotyping. The latter two lymphomas strongly express B-cell-associated markers CD20 and CD79a along with strong expression of CD45 (LCA). Whereas BCL-6 and CD10 are expressed in Burkitt’s lymphoma, they are positive in only 30–50% of diffuse large B-cell lymphoma and largely absent in PBL. EBER in situ hybridization does not aide in delineating PBL from other HIV-associated lymphomas, since EBV-infection is associated with diffuse large B-cell lymphoma in ∼80–100% of cases and with Burkitt’s lymphoma in ∼30–40% of cases [3, 10].
Although, the presence of EBV-encoded RNA has been documented in the majority of PBL, its absence in a small number of cases suggests a lack of direct etiologic relationship and suggests that EBV is not the sole factor involved in the pathogenesis of immunodeficiency-related lymphomas. Other genetic mutations commonly seen in AIDS-NHL are c-myc gene rearrangements, bcl-6 gene rearrangements, ras gene mutations, and p53 gene mutations/deletions. Virtually all cases of AIDS-related Burkitt’s lymphoma exhibit c-myc gene rearrangements. In contrast, only ∼25% of AIDS-related immunoblastic lymphomas are reported to harbor c-myc gene rearrangements [12–14]. Delecluse et al. proposed in their study of 14 cases of AIDS-NHL that the cases of immunoblastic lymphomas with a c-myc gene rearrangement in actuality represent Burkitt’s lymphoma cases that have adopted an immunoblastic morphology in the context of severe immunosupression by AIDS [15]. The authors base their hypothesis on in vitro studies of Burkitt’s lymphoma cells that give rise to a different, in some cases immunoblastic morphology, when passaged in culture. One of our two cases showed a c-myc gene rearrangement. To our knowledge, the presence of a c-myc gene rearrangement has not been documented before in a plasmablastic lymphoma. As mentioned above, c-myc gene rearrangement has been reported in a subset of AIDS-related diffuse large B-cell lymphomas. However, numerous papers suggest that these presumably are cases of AIDS-related Burkitt’s lymphoma that were misdiagnosed because of the histologic pleomorphism characteristic of AIDS-NHL [15, 16]. Despite the very high Ki-67 index in our case which approaches that of Burkitt’s lymphoma, the negative reactivity for CD20, CD79a, and BCL-6 is not typical for Burkitt’s lymphoma and argues against this diagnosis.
Recent studies have also shown that despite a common phenotype, plasmablastic lymphomas of the oral cavity show heterogeneity with regard to mutations of the IgVH. The analysis for the IgVH hypermutation status in our two cases showed the presence of somatic hypermutations in one case. The second case yielded insufficient cellular material with inconclusive results. Gaidano et al. investigated 12 cases of PBL of the oral cavity for somatic IgVH hypermutations and found 4 of 10 cases to be positive for this mutation while six cases displayed germline IgVH genes. Whereas all of their cases, regardless the IgVH mutational state were BCL-6 negative, indicating a lack of transit through the germinal center, all of the cases consistently revealed strong expression of CD138 and MUM-1, markers of preterminal plasma-cell differentiation [17]. From these studies the authors concluded that the IgVH hypermutated subset carried the molecular clues of germinal center transit and conceivably originated from a post-germinal center B-cell, while the IgVH unmutated group appeared to originate from a naïve B-cell that had undergone post-germinal differentiation independent of germinal center transit. Our IgVH hypermutated case was negative for BCL-6 and positive for CD138 and MUM-1 and likely originated from a post-germinal center B-cell. The immunophenotype of our second case (BCL-6-, CD138+ and MUM-1+) also suggests a post-germinal center B-cell origin. However, we cannot comment on whether cells passed through the germinal center, since IgVH hypermutation analysis was not possible in this case. The clinical significance of IgVH hypermutation in PBL is not known. It has, however, been shown to be of prognostic relevance in cases of small lymphocytic lymphoma/chronic lymphocytic leukemia [18, 19].
In conclusion, we present two cases of AIDS-related PBL that are in accordance with the previously delineated morphology and immunophenotype. In addition, studies for the c-myc gene rearrangement and somatic IgVH hypermutation revealed the presence of both the IgVH hypermutation and c-myc gene rearrangement in one of the two cases. Although current literature suggests that all cases of c-myc gene rearranged AIDS-related NHL likely represent AIDS-related Burkitt’a lymphoma, the morphology and immunophenotype of our c-myc gene rearranged case strongly suggests the diagnosis plasmablastic lymphoma. This finding may have significant implications in the therapeutic approach of some PBL with c-myc gene rearrangements.
References
- 1.Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, Huhn D, Schmidt-Westhausen A, Reichart PA, Gross U, Stein H. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;4:1413–20. [PubMed] [Google Scholar]
- 2.Cattaneo C, Facchetti F, Re A, Borlenghi E, Majorana A, Bardellini E, Cassari S, Tucci A, Conti G, Rossi G. Oral cavity lymphomas in immunocompetent and human immunodeficiency virus infected patients. Leuk Lymphoma. 2005;46:77–81. doi: 10.1080/10428190400007789. [DOI] [PubMed] [Google Scholar]
- 3.Dong HY, Scadden DT, Leval L, Tang Z, Isaacson PG, Harris NL. Plasmablastic lymphoma in HIV-positive patients. An aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. Am J Surg Pathol. 2005;29:1633–41. doi: 10.1097/01.pas.0000173023.02724.1f. [DOI] [PubMed] [Google Scholar]
- 4.Flaitz CM, Nichols CM, Walling DM, Hicks MJ. Plasmablastic lymphoma: an HIV-associated entity with primary oral manifestations. Oral Oncol. 2002;38:96–102. doi: 10.1016/S1368-8375(01)00018-5. [DOI] [PubMed] [Google Scholar]
- 5.Lin O, Gerhard R, Zerbini MCN, Teruya-Feldstein J. Cytologic features of plasmablastic lymphoma. Cancer Cytopathol. 2005;105:139–44. doi: 10.1002/cncr.21036. [DOI] [PubMed] [Google Scholar]
- 6.Scheper MA, Nikitakis NG, Fernandes R, Gocke CK, Ord RA, Sauk JJ. Oral plasmablastic lymphoma in an HIV-negative patient: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:198–206. doi: 10.1016/j.tripleo.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Folk GS, Abbondanzo SL, Childers EL, Foss RD. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:608–9. doi: 10.1016/j.anndiagpath.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Chetty R, Hlatswayo N, Muc R, Sabaratnam R, Gatter K. Plasmablastic lymphoma in HIV+ patients: an expanding spectrum. Histopathology. 2003;42:605–9. doi: 10.1046/j.1365-2559.2003.01636.x. [DOI] [PubMed] [Google Scholar]
- 9.Colomo L, Loong F, Rives S, Pittaluga S, Martinez A, Lopez-Guillermo A, Ojanguren J, Romagosa V, Jaffe ES, Campo E. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004;28:736–47. doi: 10.1097/01.pas.0000126781.87158.e3. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe ES, Harris NL, Stein H. World Health Organization classification of tumor: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 11.Vega F, Chang CC, Medeiros LJ, Udden MM, Cho-Vega JH, Lau C-C, Finch CJ, Vilchez RA, McGregor D, Jorgensen JL. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotyic profiles. Mod Pathol. 2005;18:806–15. doi: 10.1038/modpathol.3800355. [DOI] [PubMed] [Google Scholar]
- 12.Ballerini P, Gaidano G, Gong JZ, Tassi Vittorio SG, Knowles DM, Dalla-Favera R. Multiple genetic lesions in acquired immunodeficiency syndromes-related non-Hodgkin’s lymphoma. Blood. 1993;81:166–76. [PubMed] [Google Scholar]
- 13.Carbone A. AIDS-related non-Hodgkin’s lymphomas: from pathology and molecular pathogenesis to treatment. Hum Pathol. 2002;33:392–404. doi: 10.1053/hupa.2002.124723. [DOI] [PubMed] [Google Scholar]
- 14.Knowles DM. Molecular pathology of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphoma. Semin Diagn Pathol. 2002;14:67–82. [PubMed] [Google Scholar]
- 15.Delecluse HJ, Raphael M, Magaud JP, Felman P, the French Study Group of Pathology for Human Immunodeficiency Virus-Associated Tumors, Alsamad IA, Bornkamm GW, Lenoir GM. Variable morphology of human immunodeficiency virus-associated lymphomas with C-MYC rearrangements. Blood 1993;82:552–63. [PubMed]
- 16.Gaidano G, Carbone A, Dalla-Favera R. Genetic basis of acquired immunodeficiency syndrome-related lymphomagenesis. J Natl Cancer Inst Monogr. 1998;23:95–100. doi: 10.1093/oxfordjournals.jncimonographs.a024181. [DOI] [PubMed] [Google Scholar]
- 17.Gaidano G, Cerri M, Capello D, Berra E, Deambrogi C, Rossi D, Larocca LM, Dampo E, Gloghini A, Tirelli U, Carbone A. Molecular histogenesis of plasmablastic lymphoma of the oral cavity. Br J Haematol. 2002;119:622–8. doi: 10.1046/j.1365-2141.2002.03872.x. [DOI] [PubMed] [Google Scholar]
- 18.Vasconcelos Y, Davi F, Levy V, Oppezzo P, Magnac C, Michel A, Yamamoto M, Pritsch O, Merle-Béral M, Maloum K, Ajchenbaum-Cymbalista F, Dighiero G. Binet’s staging system and VH genes are independent but complementary prognostic indicators in chronic lymphocytic leukemia. J Clin Oncol. 2003;21:3928–32. doi: 10.1200/JCO.2003.02.134. [DOI] [PubMed] [Google Scholar]
- 19.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig VH genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–54. [PubMed] [Google Scholar]