Abstract
Metastatic renal cell carcinoma (RCC) can pose diagnostic challenges in the head and neck often resembling benign and malignant oncocytic lesions. Immunohistochemical panels have been reported to help with this differential but are not entirely specific or sensitive. We have noticed that p63 routinely stains salivary gland oncocytomas but not metastatic RCC. Nineteen oncocytomas, 9 cases of oncocytosis, 9 oncocytic carcinomas and 16 head and neck metastatic RCC were studied. Morphologic features evaluated were cytoplasmic character (clear versus oncocytic), Fuhrman nuclear grade, mitotic rate, growth pattern, presence of lumens/blood lakes and stromal characteristics. Tumors were stained with antibodies to p63, renal cell carcinoma marker (RCCm), CD10, and vimentin. Eight benign oncocytic tumors (29%) had clear cell features while 6 metastatic RCC (37%) had oncocytic features. Median Fuhrman nuclear grade was 2 in oncocytoma and oncocytosis and 3 both oncocytic carcinoma and metastatic RCC. Mitotic rates were only significantly different between benign oncocytic tumors and metastatic RCC. All oncocytomas had lumina compared to half of metastatic RCC, all of which also demonstrated blood lakes. Seven benign oncocytic tumors (25%) and 5 oncocytic carcinomas (56%) had RCC-like vascular stroma. All primary salivary gland tumors were positive for p63, predominately in basal cell-type distribution. None of the metastatic RCC was positive. RCCm was entirely specific but lacked sensitivity for metastatic RCC while CD10 and vimentin showed variable sensitivity and specificity. While clinical history and morphology usually are adequate, demonstration of p63 staining can definitively exclude metastatic RCC from the differential diagnosis of similar appearing tumors in salivary glands, namely oncocytoma and oncocytic carcinoma, with 100% specificity and sensitivity. While RCCm, CD10, and vimentin performed adequately, they were significantly less reliable than p63 with both false positives and false negatives.
Keywords: p63, Oncocytoma, Oncocytic carcinoma, Metastatic renal cell carcinoma
Introduction
Salivary gland oncocytes, as in other anatomic sites, are found after gland maturity and demonstrate a high level of oxidative activity due to increased numbers of atypical mitochondria as seen ultrastructurally [1]. Furthermore, these cells lack features of normal salivary gland epithelial cells, specifically, the basal membrane infolding that is characteristic of striated ductal cells. On hematoxylin and eosin-stained sections, the characteristic ultrastructural changes manifest as large polygonal cells with abundant granular eosinophilic cytoplasm [2].
Primary oncocytic lesions are rare in the salivary gland and account for approximately 1% of primary salivary gland tumors. They can be classified as: diffuse oncocytosis, nodular oncocytic hyperplasia (nodular oncocytosis), oncocytoma (oncocytic adenoma) and oncocytic carcinoma [3]. The majority occurs in the parotid gland with the remainder occurring in the submandibular gland and only rare reports in the minor salivary glands [3]. The benign lesions are typically well circumscribed, and are multinodular in the case of oncocytosis. They are comprised of solid to trabecular arrangements of oncocytes with a delicate vascular stroma and, similar to their renal counterparts, may manifest a central scar [4]. In some tumors, the cytoplasm is clear secondary to glycogen accumulation and/or fixation artifact [5]. The clear cell change may range from focal to diffuse, and in the latter cases, the designation, “clear cell oncocytoma/oncocytosis” may be appropriate.
Occasionally, separation of primary salivary oncocytic lesions can be problematic as other salivary gland tumors can demonstrate both oncocytic and clear cell change. Clear cell and/or oncocytic change is either characteristic or present as a common variant in clear cell carcinoma, sebaceous adenoma/carcinoma, pleomorphic adenoma, myoepithelioma, myoepithelial carcinoma, acinic cell carcinoma, epithelial-myoepithelial carcinoma and mucoepidermoid carcinoma [6–8].
To add to the diagnostic dilemma, metastases to the head and neck region can manifest as oncocytic and/or clear cell lesions. The prototypical metastasis that can masquerade as oncocytoma/oncocytosis is metastatic renal cell carcinoma (RCC). Both lesions can be composed of oncocytic and/or clear cells and both can have similar architectural grown patterns, lumen formation, and vascularized stroma. Although immunohistochemistry panels have been used to aid with this differential, significant overlap in staining limits their usefulness [9]. We have anecdotally noted that p63 immunohistochemical expression occurs in oncocytomas but not in metastatic RCC to the head and neck. Therefore, we studied the utility of this marker in this differential diagnosis as well as its use in salivary gland oncocytic carcinoma.
Materials and Methods
Case Selection
This study was approved by the University of Pittsburgh Institutional Review Board (protocol #0601084). Twenty-eight benign oncocytic tumors with adequate paraffin-embedded tissue for immunohistochemical study were identified for analysis between 1977 and 2006. These included 19 oncocytomas and 9 cases of nodular oncocytosis. With the exception of one submandibular oncocytoma, all tumors were from the parotid gland. Oncocytomas were defined as solitary circumscribed masses of oncocytes demarcated from adjacent salivary gland parenchyma. In contrast, oncocytosis was defined as multiple, variably sized, oncocytic nodules generally imparting a less organized or lobular pattern than the circumscribed and encapsulated oncocytomas [2].
Nine oncocytic carcinomas with adequate paraffin-embedded tumor tissue were identified between 1996 and 2006 and included 7 parotid tumors and one tumor each from the lip and oral cavity. The primary diagnostic criterion for oncocytic carcinoma used in this series was the presence of destructive invasive growth of oncocytic tumor cells into adjacent salivary gland parenchyma and/or soft tissue. Other accepted criteria for malignancy included metastases, perineural invasion and vascular invasion. “High grade features” such and uniform nuclear anaplasia, necrosis, and high mitotic activity were not noted in our series. In addition, oncocytic carcinomas did not demonstrate histologic or immunophenotypic features of other salivary gland malignancies with oncocytic features (i.e. salivary duct carcinoma, oncocytic mucoepidermoid carcinoma, etc.) Three other cases that were initially diagnosed as oncocytic carcinoma were reviewed and reclassified as salivary duct carcinomas and subsequently excluded from our analysis.
These primary salivary gland oncocytic lesions were compared with 16 RCCs metastatic to head and neck sites from patients with documented primaries in the kidney and with adequate paraffin-embedded tumor tissue for immunohistochemical analysis. The metastatic RCC cases were identified between 1983 and 2006. The most common location for metastatic RCC was the parotid gland (7 cases) followed by the nasal cavity (3 cases) and thyroid gland (2 cases). One case each involved the submandibular gland, maxilla, oral cavity and paratracheal soft tissues.
Ten of the oncocytomas and five of the metastatic RCC (both thyroid RCCs and one each to the nasal cavity, maxilla and parotid gland) reported in this study have been previously published [9].
Histopathologic Assessment
For each case several architectural and cytologic features were evaluated. Architectural features analyzed included growth pattern (infiltrative, circumscribed or multinodular), presence/absence of lumina or pseudolumina, presence/absence of blood lakes and quality of stromal vasculature. Cytologic features analyzed included cytoplasmic tinctorial quality (clear versus oncocytic), distribution of clear and oncocytic cells, mitotic rate/10 high powered fields (hpf; 40X) and Fuhrman nuclear grade.
Blood lakes were defined as pseudolumina filled with “fresh” red blood cells. For purposes of this study, pseudolumina were considered as irregular gland-like spaces lacking intraluminal secretion and lined by epithelial cells that lacked meaningful orientation towards the luminal surface. The stromal vasculature was characterized as delicate or RCC-like. Delicate vasculature was characterized by small, thin-walled, almost inconspicuous vascular channels filled with red blood cells and separating tumor nests and trabeculae. In contrast, RCC-like vasculature was characterized by the presence of more prominent and thicker-walled, small, muscular arteries distributed in a branching fashion separating the tumor nests and trabeculae identical to that seen in kidney RCCs.
Immunohistochemistry
Immunohistochemical analysis was performed on 4 μm-thick sections obtained from representative archived paraffin-embedded tumor tissue blocks. The antibodies, manufacturers, clones, dilutions, and retrieval methods are listed in Table 1. For all antibodies, labeling was performed using the I-view 2′-diaminobenzamide (DAB) detection kit (Ventana systems, Tucson, AZ) as the brown chromogen substrate. For p63 staining, only nuclear reactivity was considered positive. Faint granular staining of oncocytes was considered non-specific for all antibodies. For sensitivity and specificity calculations, p63 was considered a positive marker for salivary gland oncocytic lesions while the other markers were considered positive markers for RCC.
Table 1.
Antibodies used for immunohistochemistry
| Antibody | Manufacturer | Clone | Dilution | Retrieval |
|---|---|---|---|---|
| p63 | Dako, Carpinteria, CA | 4A4 | 1:500 | |
| RCCm* | Novocastra, Burlingame, CA | – | 1:100 | Trypsin digestion |
| Vimentin | Dako, Carpinteria, CA | V9 | 1:50 | Microwave in citrate buffer |
| CD10 | Novocastra, Burlingame, CA | – | 1:10 | Microwave in citrate buffer |
*RCCm = renal cell carcinoma marker
As a presumed biologically negative control, we also stained a tissue microarray (TMA) containing 60 renal oncocytomas and 12 chromophobe renal cell carcinomas, various adjacent normal renal tissue controls, and other control tissue types (brain, myocardium, lung, breast ductal adenocarcinoma, head and neck squamous cell carcinoma, liver, spleen, colon, adrenal, urinary bladder, ovary, prostate and testis) constructed from archival material from University of Pittsburgh Medical Center. The array was constructed on a recipient paraffin block (35 × 20 mm) using a manual tissue arrayer (Beecher Instruments, Sun Prairie, WI). Each case on the array was represented by two 0.6 mm tissue cores. Serial sections (5 μm thick) were transferred to an adhesive-coated glass slide system for immunohistochemical staining (Instrumedics, Hackensack, NJ).
Statistical Analysis
Statistical analysis was performed using SPSS software (SPSS version 14.0.0). For Fuhrman nuclear grade, the non-parametric method Kruskal–Wallis test was performed with post hoc Dunn method comparison for pairs. For mitotic rate, one way ANOVA comparing means was performed with post hoc Bonferroni comparison for pairs.
Results
Histopathologic Findings
The architectural and cytologic findings for each tumor group are summarized in Fig. 1 and Table 2. Two oncocytomas were removed in a piecemeal fashion and growth pattern could not be reliably determined, however, neither was infiltrative. Fifteen oncocytomas (88%) were well-circumscribed whereas 2 (12%) had a multinodular growth pattern. By definition, all cases of oncocytosis were multinodular. All oncocytic carcinomas were infiltrative. Two metastatic RCC were biopsied and, therefore, growth pattern could not be reliably determined. Regarding metastatic RCC, 2 (14%) were circumscribed, 7 (50%) were multinodular and 5 (36%) were infiltrative.
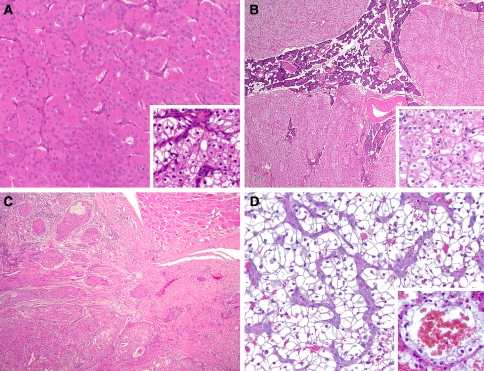
Fig. 1.
(a) Typical oncocytoma composed of nests and trabeculae of granular eosinophilic cells with scattered lumina and separated by a delicate vascular stroma. Many examples demonstrated clear cells and about one-fourth had a prominent RCC-like vascular stroma (inset). (b) Typical case of oncocytosis with multiple unencapsulated nodules of oncocytes growing in a lobular configuration. Like this example, one-third of the cases demonstrated a predominance of clears cells (inset). (c) Oncocytic carcinomas typically demonstrated bland oncocytic cytology, however, unequivocal invasion was identified in all cases and features of other salivary gland carcinomas with oncocytic cytoplasm were not present. (d) Metastatic conventional (clear cell) RCC with characteristic prominent vascular stroma. Nearly two-thirds of cases were composed of clear cells but over one-third were of oncocytic type. Blood lakes (inset) were found in half of the metastatic RCC and were specific for RCC as none of the primary salivary gland tumors had them
Table 2.
Architectural and cytologic features
| Parameter | Oncocytoma | Oncocytosis | Oncocytic CA* | Metastatic RCC |
|---|---|---|---|---|
| Growth pattern | ||||
| Circumscribed | 15/17 (88%) | 0/9 | 0/9 | 2/14 (14%) |
| Multinodular | 2/17 (12%) | 9/9 | 0/9 | 7/14 (50%) |
| Infiltrative | 0/17 | 0/9 | 9/9 | 5/14 (36%) |
| Lumina/pseudolumina | 19/19 | 9/9 | 9/9 | 8/16 (50%) |
| Blood lakes | 0/19 | 0/9 | 0/9 | 8/16 (50%) |
| Stroma quality | ||||
| RCC-like | 5/19 (26%) | 2/9 (22%) | 5/9 (56%) | 16/16 |
| Delicate | 14/19 (74%) | 7/9 (78%) | 4/9 (44%) | 0/16 |
| Predominant cell type** | ||||
| Oncocytic | 14/19 (74%) | 6/9 (67%) | 9/9 | 6/16 (37%) |
| Clear | 5/19 (26%) | 3/9 (33%) | 0/9 | 10/16 (63%) |
| Median Fuhrman grade | 2 | 2 | 3 | 3 |
| Mean mitotic rate/10 hpf | 0.04 | 0.04 | 1.1 | 2.2 |
*Oncocytic CA = oncocytic carcinoma
**Predominant = > 75% of tumor cells
The majority of the benign oncocytic lesions were entirely or predominantly (>75% of tumor cells) composed of classical eosinophilic oncocytes (20/28, 71%). The rest (8/28, 29%) were predominantly composed of clear cells, however, all had at least focal areas of classical oncocytes. Specifically, 5 (26%) oncocytomas and 3 (33%) cases of oncocytosis were composed predominantly of clear cells. All oncocytic carcinomas were composed entirely or predominantly of oncocytic cells with only one case (11%) showing focal clear cell change. In contrast, 7 (44%) metastatic RCC contained oncocytic cells and, in 6 (37%) of these, the oncocytic cells were the predominant cell type. The remaining metastatic RCC were composed predominantly of clear cells (10/16, 63%).
Median Fuhrman nuclear grade was 2 in oncocytoma and oncocytosis (range 1–3), 3 in oncocytic carcinoma (range 2–3) and 3 in metastatic RCC (range 2–4). Grade was significantly different between benign oncocytic tumors and oncocytic carcinomas (p = 0.001) as well as between benign oncocytic tumors and metastatic RCC (p < 0.001) but not between oncocytic carcinoma and metastatic RCC (p = 0.641). Mean mitotic rates were 0.04/10 hpf for oncocytoma and oncocytosis (range 0–1), 1.1/10 hpf for oncocytic carcinoma (range 0–5) and 2.2/10 hpf for metastatic RCC (range 0–8). Mitotic rates did not differ significantly comparing benign oncocytic tumors and oncocytic carcinoma (p = 0.2) and comparing oncocytic carcinoma and metastatic RCC (p = 0.3). However, benign oncocytic tumors had significantly lower mitotic rates compared to metastatic RCC (p < 0.001).
All oncocytomas, oncocytoses and oncocytic carcinomas had at least scattered true lumina of variable size including some with associated eosinophilic intraluminal secretions. In contrast, 8 (50%) of the metastatic RCC had scattered pseudolumina and were accompanied by characteristic blood lakes. None of the primary salivary gland oncocytic lesions demonstrated blood lakes. All metastatic RCC demonstrated the characteristic RCC vascular stroma as typically seen in the primary kidney tumors. In contrast, 7 (25%) of the benign oncocytic lesions and 5 (56%) of the oncocytic carcinomas had a prominent RCC-like vascular stroma with the remainder having a more subtle delicate vascular stroma.
Immunohistochemistry Findings
Immunohistochemical staining results are summarized in Tables 3 and 4 and Fig. 2. Immunohistochemical p63 staining demonstrated 100% sensitivity and specificity for both benign and malignant primary salivary gland oncocytic tumors. Specifically, all cases demonstrated positive nuclear staining compared to none of the metastatic RCC. The positively stained nuclei tended to be located towards the periphery of the tumor nests reminiscent of a basal cell-type distribution. None of the renal oncocytomas or chromophobe renal cell carcinomas on the TMA showed p63 reactivity.
Table 3.
Immunohistochemistry results
| Stain | Benign Oncocytic | Oncocytic CA | Metastatic RCC |
|---|---|---|---|
| p63 | 28/28 | 9/9 | 0/16 |
| RCCm | 0/28 | 0/8 | 7/16 (44%) |
| CD10 | 10/27 (37%) | 4/8 (50%) | 11/15 (73%) |
| Vimentin | 5/26 (19%) | 3/8 (38%) | 11/15 (73%) |
Table 4.
Sensitivities and specificities of immunohistochemical markers (numerical values = sensitivity/specificity)
| Marker | Oncocytic tumors | Metastatic RCC |
|---|---|---|
| p63 | 100/100 | – |
| RCCm | – | 44/100 |
| CD10 | – | 73/60 |
| Vimentin | – | 73/76 |
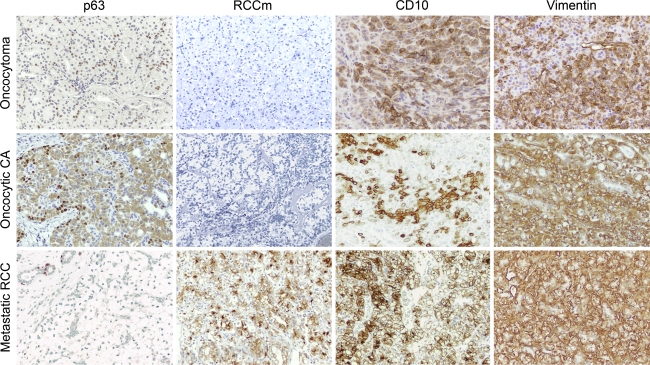
Fig. 2.
Panel comparing immunohistochemical staining results of the different tumor types studied. Nuclear p63 staining was identified in all benign and malignant primary salivary gland oncocytic tumors and was negative in all metastatic RCC tested [note positive internal control (upper left) in metastatic RCC]. Staining for p63 showed a characteristic basal cell-type distribution. While RCCm demonstrated perfect specificity for metastatic RCC, the sensitivity was only 44%. CD10 and vimentin had only marginal sensitivity and specificity. While most primary salivary gland oncocytic lesions were negative for these two markers, a significant number demonstrated unequivocal staining as shown in this panel. When CD10 positivity was identified in the primary salivary gland tumors, it tended to be more focal than the diffuse staining seen in metastatic RCC
Similarly, RCC marker (RCCm) was 100% specific for metastatic RCC, however, the sensitivity was only 44%. CD10 and vimentin each demonstrated sensitivities for metastatic RCC of 73% (11/15 positive cases each). However, the specificities for these two markers were only 60% and 76%, respectively. When positive membranous CD10 staining was identified in benign and malignant primary salivary gland oncocytic lesions, the staining tended to be focal compared to the more diffuse staining seen in metastatic RCC when present.
Discussion
Primary salivary gland oncocytic lesions are traditionally classified as diffuse oncocytosis, nodular oncocytic hyperplasia (nodular oncocytosis), oncocytoma (oncocytic adenoma) and oncocytic carcinoma [3]. These are uncommon and, according to data from the Armed Forces Institute of Pathology (AFIP), oncocytomas accounted for only 1.4% of 13,749 salivary gland tumors on file [10]. Among this same group, only 27 cases of oncocytosis were identified. These data are in accordance with those from other groups who found the incidence of oncocytoma to be in the range of 0.1–1.0% [2, 11].
Approximately 85–90% occur in the parotid gland [2, 4] and, accordingly, oncocytic tumors account for a relatively larger percentage of parotid gland tumors. At the AFIP, oncocytomas accounted for about 3.5% of parotid gland tumors [3]. Similarly, Capone et al. found 21 oncocytic tumors among 561 parotidectomy specimens (3.7%) at The Johns Hopkins University Hospital, which included 13 (2.3%) oncocytomas, 6 (1.1%) cases of oncocytosis and only 2 (0.4%) oncocytic carcinomas [11].
While the diagnosis of these lesions is usually straightforward, the histologic distinction between nodular oncocytosis and oncocytoma is admittedly rather arbitrary in certain cases. Many pathologists believe the presence of a single, well-circumscribed and at least partially encapsulated nodule favors the diagnosis of oncocytoma while multiple, unencapsulated nodules distributed in a lobular configuration favors nodular oncocytosis [2, 12]. Even more confusing is the designation of oncocytoma arising in oncocytosis to describe a dominant often encapsulated nodule in the background of oncocytosis. This distinction, however, is academic and of little to no clinical or prognostic significance.
More important is the distinction of these lesions from their malignant counterparts, oncocytic carcinomas, as well as the oncocytic variants of other salivary gland carcinomas and metastatic lesions with oncocytic morphology. Oncocytic salivary gland carcinoma is uncommon representing only 0.05–0.4% of salivary gland neoplasms and about 5% of oncocytic neoplasms [11, 13]. Similar to their benign counterparts, nearly 80% occur in the parotid gland. Interestingly, the majority is presumed to arise in a pre-existing oncocytoma but they also may occur de novo [14]. Diagnostic criteria for salivary gland oncocytic carcinoma include destructive invasion of adjacent salivary or non-salivary tissue, perineural and/or vascular invasion, and metastases.
Renal cell carcinoma metastatic to the major salivary glands is also notoriously difficult to separate histologically from benign and malignant oncocytic salivary gland tumors due in large part to significant morphologic overlap. Furthermore, about 8% of RCC patients initially present with head and neck metastases prior to identification of the primary kidney lesion [15]. In addition, pathologists are occasionally not given complete historical information regarding prior diagnoses and/or metastatic RCC may not present until many years after nephrectomy. Pathologists increasingly need to maintain a high index of suspicion for the possibility of metastasis when confronted with oncocytic or clear cell salivary gland neoplasms.
Following lung and breast carcinoma, RCC is the most common infraclavicular malignancy to metastasize to the head and neck [16] with some studies placing it second after lung [10]. Approximately 15% of patients with RCC will develop non-CNS head and neck metastases [17] with the thyroid gland being the most common site [18]. In 1% of patients with RCC, the only documented site of metastasis is to the head and neck [15].
Although the incidence varies based on the study evaluated, metastases account for about 3–25% of parotid and submandibular malignancies with the average reported in the literature around 16% [10]. The vast majority are head and neck squamous cell carcinomas followed in frequency by cutaneous and mucosal head and neck melanomas [19]. These two account for about 80% of major salivary gland metastases. In contrast, metastases from more distant sites account for only 2–4% of major salivary gland malignancies [20, 21] and, similar to other head and neck sites, usually are from breast, lung and kidney primaries. In a study of 108 metastases identified from 11,000 salivary gland pathology specimens (9.8%), Seifert et al. found that 6 (5.6%) were metastatic RCC [22]. Therefore, both locoregional and distant metastatic disease should always be considered in the workup of salivary gland tumors that are not easy to classify histologically. Specifically, metastatic RCC should be considered in tumors morphologically resembling oncocytoma, oncocytosis or oncocytic carcinoma.
While clinical history and histology are typically adequate in diagnosing metastatic RCC, for reasons mentioned above and below, sometimes distinguishing this from primary oncocytic lesions can be challenging. Metastatic RCC typically demonstrates clear cells with more cellularity and cytologic pleomorphism than benign oncocytic lesions and often has a characteristic prominent vascular stroma that is not usually seen in salivary gland oncocytic lesions [5]. Metastatic RCC can grow as solid sheets, nests and/or trabeculae and may also contain lumina with or without blood (i.e. blood lakes). However, metastatic RCC can exhibit bland cytologic features among a well-circumscribed nodule with or without the characteristic vascular stroma and may contain cells with oncocytic cytoplasm. Furthermore, primary salivary gland oncocytic lesions, with or without clear cell change, usually demonstrate lumina and, in our experience, occasionally have a prominent vascular stroma reminiscent of that seen in RCC (i.e. RCC-like). Thus, the diagnostic morphologic features of these two tumors can overlap significantly and, especially without a complete clinical history, they may be impossible to separate on routine hematoxylin and eosin-stained sections.
Our findings confirm the significant morphologic overlap frequently noted between salivary gland oncocytic tumors and metastatic RCC. While most RCC are of the conventional (clear cell) type, in the current study, 37% showed a predominance of oncocytic cells. Similarly, while oncocytic tumors typically are predominantly eosinophilic, in this series, 26% of oncocytomas and 33% of oncocytoses were predominantly composed of clear cells while 1 (11%) oncocytic carcinoma showed the presence of only focal clear cells. While Fuhrman nuclear grade was significantly different between the benign and malignant lesions evaluated, there was overlap. Benign oncocytic tumors had significantly lower mitotic rates than metastatic RCC suggesting this feature may be helpful in discriminating these tumors. However, there was no difference benign and malignant oncocytic tumors or between oncocytic carcinoma and metastatic RCC.
Various architectural features also showed overlap among the different tumor types. While an infiltrative growth pattern was unique to oncocytic carcinoma and metastatic RCC, a circumscribed growth pattern (88% vs. 14%) or multinodular growth pattern (12% vs. 50%) could be seen in both oncocytomas and metastatic RCC, respectively, limiting the utility of growth pattern in the differential. The presence of lumina (or pseudolumina) was demonstrated in all primary salivary gland oncocytic tumors and in half of metastatic RCC, however, blood lakes were only identified in cases of metastatic RCC. Therefore when lumina/pseudolumina are accompanied by blood lakes, metastatic RCC should be suspected. Finally, while RCC notoriously demonstrates a unique prominent stromal vascularity, identified in all 16 cases in the current study, a morphologically similar stroma can be identified in salivary gland oncocytic tumors. In fact, we identified this RCC-like vascular stroma in 26, 22, and 56% of oncocytomas, oncocytoses and oncocytic carcinomas, respectively.
For these reasons, immunohistochemical stains are often used to help sort out this differential diagnosis. However, there is only one study comparing the immunohistochemical profile of metastatic RCC to that of salivary gland oncocytomas [9] and there are no studies comparing them to salivary gland oncocytic carcinomas. Ozolek et al. compared the expression of cytokeratin 7 (CK7), cytokeratin 20 (CK20), epithelial membrane antigen (EMA), vimentin, CD10 and RCCm between ten oncocytomas and ten RCCs metastatic to various head and neck sites (including one parotid gland tumor).
In that study, CD10 was identified as the best single marker to aid in this differential diagnosis, being diffusely and strongly positive in 90% of metastatic RCC and in none of the oncocytomas. In a larger group of tumors, we were unable to confirm this finding. Specifically, 11/15 (73%) metastatic RCCs demonstrated strong diffuse membranous and cytoplasmic positivity for CD10. However, 10/27 (37%) benign oncocytic tumors and 4/8 (50%) oncocytic carcinomas showed strong membranous and cytoplasmic positivity, but the staining was focal in all of these cases. Therefore, in the current study, CD10 yielded a sensitivity of 73% but a specificity of only 60% for RCC when considering positive versus negative tumor staining in salivary gland oncocytic tumors and metastatic RCC.
Vimentin and EMA were not helpful markers in separating these two tumor groups in the Ozolek study. Our experience with vimentin was similar to their findings in that 73% of metastatic RCC (vs. 70% in their study) were positive while 19% of oncocytomas (vs. 40% in their study) were positive. Furthermore, 38% of oncocytic carcinomas were positive limiting the utility of this marker in differentiating it from both oncocytomas and metastatic RCC. In both studies, RCCm was negative in all salivary gland oncocytic lesions, however, only 44% of metastatic RCC were positive in the current study, similar to the 40% found in their study, limiting the utility of this marker based on the low sensitivity.
In the current study, immunohistochemical staining for p63 proved to be the most reliable marker for differentiating salivary gland oncocytic tumors from metastatic RCC. The p63 gene is a recently recognized member of the p53 tumor suppressor gene family [23]. It has been shown that p63 plays a major role in epithelial development [24, 25] and is an integral gene whose protein is expressed in basal and myoepithelial cells in various tissues including salivary glands [26, 27]. The gene gives rise to six different major transcripts that segregate into two functional protein classes. Three isoforms (referred to as TAp63) function like p53 as inducers of apoptosis, a function mediated by a transactivating (N)-terminal domain. The other three (referred to as ΔNp63) lack the (N)-terminal transactivation domain and therefore inhibit p53 activity. It is this latter group of isoforms that is expressed in basal cells of multilayered epithelia and in myoepithelial cells [28]. The major theory for its function in these epithelia is to maintain the proliferative capacity of these important progenitor cells.
Few studies have evaluated the expression of p63 in salivary gland oncocytic tumors. Using TMA methodology, Weber et al. found rare positive cells in five of five oncocytomas with the positive cells being distributed in a basal cell-type distribution [29]. Similarly, Bilal et al. demonstrated scattered positive cells in four oncocytomas with the positive cells being present at the tumor-stromal interface in a basal cell-type distribution [30]. Finally, Foschini et al. demonstrated positive p63 staining in one oncocytoma studied [31] while Seethala et al. found no p63 staining in the evaluation of one oncocytoma [32].
All benign oncocytic tumors and oncocytic carcinomas in the current study demonstrated diffuse p63 nuclear-positive cells distributed in a basal cell-type pattern with positive cells being located predominantly towards the periphery of the tumor cell nests. Some tumor nests showed extension of p63 reactivity towards the center of tumor nests, but with decreased intensity. This staining pattern was in distinct contrast to metastatic RCC in which no tumor stained positive for p63. Therefore, p63 immunohistochemical staining, in our experience, demonstrates 100% sensitivity and 100% specificity in distinguishing both primary benign and malignant salivary gland oncocytic tumors from metastatic RCC. Furthermore, the expression of p63 in oncocytic carcinomas was maintained in the invasive component in tumors arising in an underlying oncocytoma.
The exact nature of these p63-positive cells is not clear but they may represent basal cells/tumor stem cells with oncocytic cytoplasm. They do not appear to be myoepithelial cells as they are typically negative on immunohistochemical staining for other myoepithelial markers in our experience (data not shown) as well as that of other investigators [33]. While the majority of p63 positive cells were present at the periphery of tumor nests, some foci did show a diminishing gradient of staining towards the center of the nests. This supports a maturation of phenotype with gradual loss of p63 in primary oncocytic lesions, rather than a truly biphenotypic differentiation profile seen in tumors such as pleomorphic adenomas, or epithelial-myoepithelial carcinomas.
To our knowledge, p63 expression in salivary gland oncocytic carcinomas has not been previously reported. Our findings are the first to demonstrate the frequency and pattern of staining of this marker in this rare malignant salivary gland tumor. The utility of immunohistochemical p63 staining in differentiating between oncocytic carcinoma and metastatic RCC has been demonstrated herein, however, its use in separating it from other malignant oncocytic salivary gland tumors, such as oncocytic mucoepidermoid carcinoma, remains to be proven. Similarly, p63 expression in primary renal tumors has not been studied extensively. In accordance with our findings, Langner et al., using a TMA, found this marker to be negative in all 188 RCC, which included 133 conventional (clear cell) subtypes [34]. Two smaller studies evaluating p63 expression found no staining in four conventional (clear cell) RCC [35] and in one of thirteen RCC [36], however, the histologic types were not provided in the later study. We additionally demonstrate that p63 is negative in all 60 renal oncocytomas and 12 chromophobe renal cell carcinomas tested on our TMA. While this finding is not of diagnostic importance, since essentially all RCCs that have metastasized above the clavicle are of the conventional type, it is of biologic significance in that it demonstrates that p63 reactivity is more closely linked to the tissue of origin rather than the oncocytic phenotype.
In summary, primary oncocytic salivary gland lesions and metastatic RCC show considerable morphologic overlap. In most cases, a careful consideration of morphologic features along with a clinical history is sufficient to separate these tumors. We have shown that immunohistochemical p63 positivity effectively distinguishes primary salivary oncocytic lesions from metastatic RCC in problematic cases with 100% sensitivity and specificity.
Acknowledgment
The authors would like to thank Ms. Kim Adams for help in preparation of this manuscript.
Footnotes
The results of this study were presented at the 96th Annual Meeting of the United States and Canadian Academy of Pathologists in San Diego, CA, March 2007.
References
- 1.Tandler B, Hutter RV, Erlandson RA. Ultrastructure of oncocytoma of the parotid gland. Lab Invest. 1970;23:567–80. [PubMed] [Google Scholar]
- 2.Palmer TJ, Gleeson MJ, Eveson JW, et al. Oncocytic adenomas and oncocytic hyperplasia of salivary glands: a clinicopathological study of 26 cases. Histopathology. 1990;16:487–93. doi: 10.1111/j.1365-2559.1990.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 3.Ellis G, Auclair P. Atlas of tumor pathology. Tumors of the salivary glands. 3rd ed. Washington DC: Armed Forces Institute of Pathology; 1996
- 4.Brandwein MS, Huvos AG. Oncocytic tumors of major salivary glands. A study of 68 cases with follow-up of 44 patients. Am J Surg Pathol. 1991;15:514–28. doi: 10.1097/00000478-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ellis GL. “Clear cell” oncocytoma of salivary gland. Hum Pathol. 1988;19:862–7. doi: 10.1016/S0046-8177(88)80271-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Brandwein M, Gordon R, et al. Primary salivary clear cell tumors—a diagnostic approach: a clinicopathologic and immunohistochemical study of 20 patients with clear cell carcinoma, clear cell myoepithelial carcinoma, and epithelial-myoepithelial carcinoma. Arch Pathol Lab Med. 2002;126:676–85. doi: 10.5858/2002-126-0676-PSCCTA. [DOI] [PubMed] [Google Scholar]
- 7.Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007;31:44–57. doi: 10.1097/01.pas.0000213314.74423.d8. [DOI] [PubMed] [Google Scholar]
- 8.Maiorano E, Altini M, Favia G. Clear cell tumors of the salivary glands, jaws, and oral mucosa. Semin. 1997;14:203–12. [PubMed] [Google Scholar]
- 9.Ozolek JA, Bastacky SI, Myers EN, et al. Immunophenotypic comparison of salivary gland oncocytoma and metastatic renal cell carcinoma. Laryngoscope. 2005;115:1097–100. doi: 10.1097/01.MLG.0000163497.61332.77. [DOI] [PubMed] [Google Scholar]
- 10.Ellis G, Auclair P, Gnepp D, et al. Surgical pathology of the salivary glands. Philadelphia: W.B. Sanders; 1991. [Google Scholar]
- 11.Capone RB, Ha PK, Westra WH, et al. Oncocytic neoplasms of the parotid gland: a 16-year institutional review. Otolaryngol Head Neck Surg. 2002;126:657–62. doi: 10.1067/mhn.2002.124437. [DOI] [PubMed] [Google Scholar]
- 12.Chang A, Harawi SJ. Oncocytes, oncocytosis, and oncocytic tumors. Pathol Annu. 1992;27 Pt 1:263–304. [PubMed] [Google Scholar]
- 13.Goode RK, Corio RL. Oncocytic adenocarcinoma of salivary glands. Oral Surg Oral Med Oral Pathol. 1988;65:61–6. doi: 10.1016/0030-4220(88)90193-4. [DOI] [PubMed] [Google Scholar]
- 14.Nakada M, Nishizaki K, Akagi H, et al. Oncocytic carcinoma of the submandibular gland: a case report and literature review. J Oral Pathol Med. 1998;27:225–8. doi: 10.1111/j.1600-0714.1998.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 15.Boles R, Cerny J. Head and neck metastases from renal carcinomas. Mich Med. 1971;70:616–8. [PubMed] [Google Scholar]
- 16.Pritchyk KM, Schiff BA, Newkirk KA, et al. Metastatic renal cell carcinoma to the head and neck. Laryngoscope. 2002;112:1598–602. doi: 10.1097/00005537-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Cheng ET, Greene D, Koch RJ. Metastatic renal cell carcinoma to the nose. Otolaryngol Head Neck Surg. 2000;122:464. doi: 10.1016/S0194-5998(00)70069-6. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto R, Helmus C. Hypernephroma metastatic to the head and neck. Laryngoscope. 1973;83:898–905. doi: 10.1288/00005537-197306000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Owens RM, Friedman CD, Becker SP. Renal cell carcinoma with metastasis to the parotid gland: case reports and review of the literature. Head Neck. 1989;11:174–8. doi: 10.1002/hed.2880110212. [DOI] [PubMed] [Google Scholar]
- 20.Rees R, Maples M, Lynch JB, et al. Malignant secondary parotid tumors. South Med J. 1981;74:1050–2. doi: 10.1097/00007611-198109000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Yarington CT., Jr Metastatic malignant disease to the parotid gland. Laryngoscope. 1981;91:517–9. doi: 10.1288/00005537-198104000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Seifert G, Hennings K, Caselitz J. Metastatic tumors to the parotid and submandibular glands–analysis and differential diagnosis of 108 cases. Pathol Res Pract. 1986;181:684–92. doi: 10.1016/S0344-0338(86)80044-9. [DOI] [PubMed] [Google Scholar]
- 23.Yang A, Kaghad M, Wang Y, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. doi: 10.1016/S1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 24.Parsa R, Yang A, McKeon F, et al. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol. 1999;113:1099–1105. doi: 10.1046/j.1523-1747.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–8. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 26.Barbareschi M, Pecciarini L, Cangi MG. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001;25:1054–60. doi: 10.1097/00000478-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Reis-Filho JS, Schmitt FC. Taking advantage of basic research: p63 is a reliable myoepithelial and stem cell marker. Adv Anat Pathol. 2002;9:280–9. doi: 10.1097/00125480-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Yang A, Kaghad M, Caput D, et al. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–5. doi: 10.1016/S0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 29.Weber A, Langhanki L, Schutz A, et al. Expression profiles of p53, p63, and p73 in benign salivary gland tumors. Virchows Arch. 2002;441:428–36. doi: 10.1007/s00428-002-0705-y. [DOI] [PubMed] [Google Scholar]
- 30.Bilal H, Handra-Luca A, Bertrand J-C, et al. p63 is expressed in basal and myoepithelial cells of human normal and tumor salivary gland tissues. J Histochem Cytochem. 2003;51:133–9. doi: 10.1177/002215540305100201. [DOI] [PubMed] [Google Scholar]
- 31.Foschini MP, Gaiba A, Cocchi R, et al. p63 expression in salivary gland tumors: role of DeltaNp73L in neoplastic transformation. Int J Surg Pathol. 2005;13:329–5. doi: 10.1177/106689690501300404. [DOI] [PubMed] [Google Scholar]
- 32.Seethala RR, LiVolsi VA, Zhang PJ, et al. Comparison of p63 and p73 expression in benign and malignant salivary gland lesions. Head Neck. 2005;27:696–702. doi: 10.1002/hed.20227. [DOI] [PubMed] [Google Scholar]
- 33.Thompson LD, Wenig BM, Ellis GL. Oncocytomas of the submandibular gland. A series of 22 cases and a review of the literature. Cancer. 1996;78:2281–7. doi: 10.1002/(SICI)1097-0142(19961201)78:11<2281::AID-CNCR3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 34.Langner C, Ratschek M, Tsybrovskyy O, et al. p63 immunoreactivity distinguishes upper urinary tract transitional-cell carcinoma and renal-cell carcinoma even in poorly differentiated tumors. J Histochem Cytochem. 2003;51:1097–9. doi: 10.1177/002215540305100813. [DOI] [PubMed] [Google Scholar]
- 35.Di Como CJ, Urist MJ, Babayan I, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- 36.Kaufmann O, Fietze E, Mengs J, et al. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol. 2001;116:823–30. doi: 10.1309/21TW-2NDG-JRK4-PFJX. [DOI] [PubMed] [Google Scholar]