Abstract
Epidemiologic, clinical, morphologic and molecular evidence show that high risk HPV, particularly type 16, is a prerequisite for some carcinomas of the upper aerodigestive tract (UADT), particularly tonsil and base of tongue. Sexual transmission is an important mode of infection while tobacco use and excessive drinking are not considered risk factors. HPV + tumors are distinct clinically and pathologically. They are more common in young patients (<40 years) with a male to female ratio of 4:1. They usually present as a small or occult primary tumor with advanced neck disease. Microscopically they are non-keratinizing squamous cell carcinomas with basaloid features, excessive mitosis and comedo type necrosis. The tumors have a distinct immunohistochemical profile characterized by strong and diffuse p16 reactivity, low or negative p53 staining and high Ki67 labeling scores. HPV + carcinomas are more radio-sensitive and have a better prognosis than the classical keratinizing SCC of the UADT. An anti-HPV vaccine has recently been made available for prevention of cervical cancer. The impact of the vaccine on the prevalence of HPV related carcinomas of the UADT is currently not known but likely beneficial.
Keywords: Human papillomavirus, HPV16, Upper aerodigestive tract, Oropharynx, Tonsils, Base of tongue, Non-keratinizing squamous cell carcinoma, p16, Sexual transmission, Radiosensitivity, Vaccine
Background
The role of human Papillomavirus (HPV) as a prerequisite for the development of cancer of the uterine cervix has been established for many years. The prevalence of high risk (oncogenic) HPV infection in cervical cancer tissue is estimated to be in the range of 90–99%. According to the WHO, about 500,000 new cases of cervical cancer occur globally each year. The majority is identified in developing countries [1–3]. According to CDC epidemiologic studies, 75% of the 15–75 year-old population in the United States acquire genital HPV infection at some point in their lives. By the age of 50 at least 80% of women will have acquired genital HPV infection [1–4].
More recently epidemiologic, clinical and molecular evidence have implicated HPV particularly type 16 in the causation of some upper aerodigestive tract (UADT) carcinomas, particularly in the oropharynx and notably the tonsils and base of tongue [5–14].
The Virus
Human papillomaviruses belong to the family of DNA Papovaviridae. They are small non-enveloped icosahedral viruses with an 8 Kb-long double-stranded circular DNA genome. They include more than a 100 different strains or genotypes, more than 30 of which are sexually transmitted, infecting the genital areas of men and women. Some of these viruses can cause premalignant lesions and carcinomas in the affected areas, and are called “high-risk” types. Others called “low-risk” types may cause mild cytologic abnormalities and genital warts as well as laryngeal papillomatosis and oral condyloma accuminata. The most common high-risk types are types 16 and 18, while the most common of the low risk ones are types 6 and 11.
Human papillomavirus genome is made up of 7 early (E) genes and 2 late (L) genes that encode the early proteins E1–E7 and late proteins L1–L2 respectively (Fig. 1). The E proteins are nonstructural and are involved in replication and transcription of the genome, while the L proteins are the structural capsid proteins of the intact virion [15, 16, 3]. Molecular evidence in cervical as well as oropharyngeal carcinomas show that the HPV oncogenes E6 and E7 act through inactivation of p53 and retinoblastoma (Rb) tumor suppressor genes, respectively, inducing cell cycle deregulation and genomic instability. In addition E6 can directly activate telomerase and E7 induces abnormal centrosome duplication [3, 15, 16].
Fig. 1.
Diagrammatic illustration of Human papilloma virus type 16 genome; E, early genes; L, late genes; LCR, long control region
HPV-related Carcinomas of the Head and Neck
During the last few decades there has been an increase in incidence of oropharyngeal carcinoma in young patients under 45 years of age. Using the SEER data base a statistically significant increase in incidence of carcinomas of the tonsils and base of tongue was documented during the period 1973–2001 in the US population aged 20–44 years. No similar increase occurred in other oral sites outside the oropharynx. Many of these patients have little or no exposure to known risk factors such as smoking or excessive drinking [17, 18].
About 20 years ago high risk HPV was identified in squamous cell carcinoma of the head and neck [11]. A multitude of studies using a variety of techniques including in situ hybridization (ISH), immunohistochemistry, and polymerase chain reaction (PCR) have since been able to demonstrate the presence of HPV genome in UADT carcinomas, not only in the oropharynx but also in some laryngeal and sinonasal carcinomas. The virus is very rarely identified in squamous cell carcinoma of the anterior oral cavity [5–14].
HPV-Related Oropharyngeal Squamous Cell Carcinoma
Demographic and Clinical Features
The prevalence of HPV DNA in oropharyngeal carcinoma has varied in different studies form 18 to 90% (6–9, 12, 13). In a review of 235 cases of oropharyngeal carcinomas, in all age groups, at our institution we found that 36% of tonsillar and 32% of base of tongue carcinomas were HPV related [7]. Alternatively, 91% of tonsillar carcinomas in young patients, 40 years of age or younger were HPV type 16 positive [6]. The male to female ratio for all age groups was 4:1 [6, 7].
In another large study of 1,670 patients who had oral or oropharyngeal carcinomas and 1,732 healthy volunteers, from nine countries, the International Agency for Cancer found that 18.3% of oropharyngeal carcinomas were HPV 16 positive. Patients with HPV positive tumors were three times as likely to report having had oral sex as those with HPV negative tumors and were also more likely to have had multiple sex partners [19]. An analysis of the Swedish cancer registry data (1958–1996) showed that husbands of women with cervical cancer had significantly increased risk of developing tonsillar carcinoma [20]. HPV is less frequently detected in cancer biopsies from patients who are tobacco smokers or paan chewers [17–19].
Early asymptomatic carcinomas usually develop in the crypts of the palatine and lingual tonsils without apparent clinical manifestations. Because of their deep location neither clinical examination nor cytologic tests, analogous to the Pap smears used for cervical lesions, are useful in early detection. These small, occult tumors are not uncommonly associated with extensive cervical lymph node metastasis [21]. In the more advanced primary tumors, patients may complain of sore throat, dysphagia, otalgia, and sensation of a foreign body in the throat.
Pathologic Features
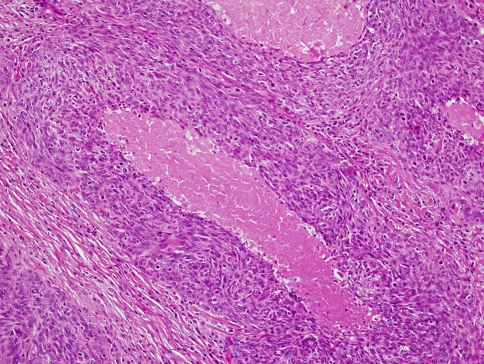
HPV-related oropharyngeal carcinomas are not only distinct clinically but also microscopically and molecularly. The tumors are characterized by non-keratinizing, basaloid cell morphology. Microscopically the neoplastic cells are generally monomorphic, oval or spindle shaped, with hyperchromatic basophilic nuclei, inconspicuous cytoplasm and indistinct cell borders. They form cords, sheets and nests with sharply defined borders. Palisading of the peripheral cells may be present. Excessive mitosis and apoptosis are observed as well as comedo type necrosis (6,7) (Fig. 2). Keratinization and keratin pearl formation are generally absent although some trend towards cell maturation may occasionally be present in focal areas (21).
Fig. 2.
HPV related tonsillar non-keratinizing carcinoma with basaloid cell features. Note comedo type necrosis and marked mitosis and apoptosis
In lymph node metastasis tumor masses commonly show extensive central necrosis leading to a characteristic cystic change (Fig. 3). The lining epithelium of the cystic structures can be so scant and bland appearing that diagnosis of a benign cyst may be erroneously made, particularly in cases in which the primary tumors are occult.
Fig. 3.
Cystic changes in cervical lymph node metastasis of HPV + non-keratinizing carcinoma
Immunohistochemistry
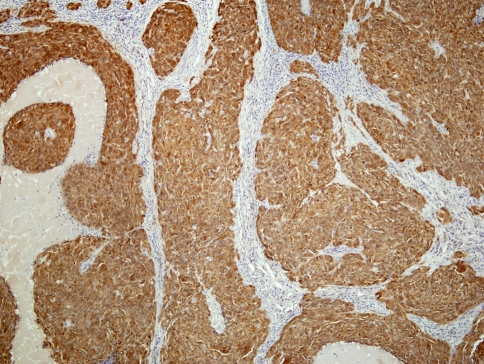
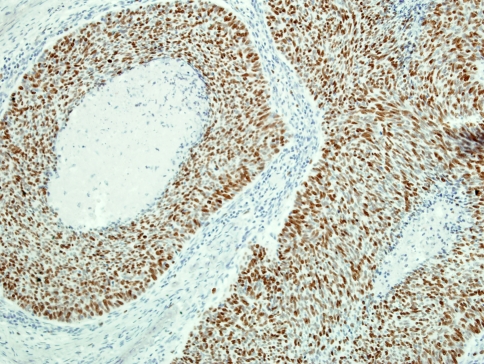
A characteristic and distinct immunophenotype is exhibited by oropharyngeal HPV-related non-keratinizing carcinoma. These tumors are distinguished by a strong and diffuse staining for p16INK4a (p16) antibodies (Fig. 4), a negative or weak reactivity to p53 protein and higher Ki67 staining scores (Fig. 5), as compared to the keratinizing type carcinomas of the same site [6, 7, 22]. This immunohistochemical profile is analogous to that seen in HPV positive anogenital carcinomas [23–25].
Fig. 4.
Strong and diffuse reactivity to p16 antibodies in HPV related non-keratinizing carcinoma
Fig. 5.
High labeling score to Ki67 immunostain in non-keratinizing HPV + carcinoma
Detection
As mentioned above the majority of oropharyngeal HPV-related lesions start at the bottom of the crypts of the palatine and lingual tonsils, and thus are inaccessible to routine cytologic smears. Advanced disease is symptomatic and manifest, clinically and radiographically, as a tumor mass usually with enlarged neck lymph nodes.
Occult Primary Tumors
Not uncommonly small primary tumors, which are undetectable on routine clinical or radiographic examination, are associated with significant neck node metastasis [21]. We have recently shown that more than 90% of HPV positive neck metastasis arise from the oropharynx, mainly the tonsils and base of tongue, while less than 10% of those metastasis originated outside the oropharynx including the oral cavity proper, larynx and hypopharynx. HPV related metastatic carcinomas were identified in FNA biopsies as well as in surgical specimens, by morphologic criteria, p16 reactivity and by ISH for high risk HPV [21, 26, 27].
Treatment and Prognosis
An accumulating body of evidence in the American as well as the international literature confirms that HPV positive carcinomas of the tonsils and base of tongue have a statistically significant better prognosis regarding disease free and overall survival than HPV negative tumors. The favorable outcome for patients with HPV positive tumors is independent of TNM stage, nodal status, age or gender [28–33]. It is suggested that the favorable outcome is attributable to higher sensitivity toward radiotherapy.
Prevention
Because HPV infection of the oropharynx is believed to be sexually transmitted, the practice of protective sexual behavior is likely to have preventive effects. Abstinence, monogamy, and limiting the number of sexual partners have all been advocated for prevention of STD. Unfortunately HPV infection can occur in the genital areas that are covered as well as areas not covered by a latex condom. According to NCI the efficacy of the use of condoms in prevention of HPV infection is not known, although condom use has been associated with a lower rate of cervical cancer.
The HPV Vaccine
In June 8, 2006, the Food and Drug Administration (FDA) licensed the first anti HPV vaccine. The quadrivalent vaccine, Gardasil, immunizes against HPV types 6, 11, 16 and 18. It is made from non-infectious viral-like particles (VLP) [34–37].
On June 29, 2006, the Advisory Committee on Immunization Practice (ACIP) voted to recommend this vaccine for females, ages 9–26. The vaccine has been tested in over 11,000 females of that age group in many countries around the world including the USA. These studies demonstrated 100% efficacy in preventing cervical precancers, and nearly 100% efficacy in preventing vulvar and vaginal precancers, as well as genital warts, caused by the targeted HPV types. These studies also found that the vaccine is safe and is without side effects [35–37].
The impact of wide use of anti HPV vaccines on HPV-related oropharyngeal carcinoma is currently not known. However it is reasonable to conclude that a direct or indirect benefit may be achieved.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics. Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186: 131–56. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]
- 4.Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol. 2006;208:152–64. doi: 10.1002/path.1866. [DOI] [PubMed] [Google Scholar]
- 5.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–72. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 6.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27:1463–70. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 7.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:339–45. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi S, Munoz N, Bosch XF, Snijders PJ, Walboomers JM. Human papillomavirus and cancers of the upper aerodigestive tract: a review of epidemiological and experimental evidence. Cancer Epidemiol Biomarkers Prev. 1996;5:567–75. [PubMed] [Google Scholar]
- 9.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 10.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 11.Loning T, Ikenberg H, Becker J, Gissmann L, Hoepfer I, zur Hausen H. Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papillomavirus type related DNA. J Invest Dermatol. 1985;84:417–20. doi: 10.1111/1523-1747.ep12265517. [DOI] [PubMed] [Google Scholar]
- 12.Mineta H, Ogino T, Amano HM, et al. Human papillomavirus(HPV) type 16and 18 detected in head and neck squamous cell carcinoma. Anticancer Res. 1998;18:4765–8. [PubMed] [Google Scholar]
- 13.Mork J, Lie AK, Glatter E, et al. Human papillomavirus infection as a risk factor for squamous cell carcinoma of the head and neck. N Eng J Med. 2001;344:1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 14.Houten VM, Snijders PJ, Brekel MW, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93:232–5. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 15.Chen XS, Garcea RL, Goldberg I, et al. Structure of small virus-like particles assembled from L1 protein of human papillomavirus 16. Mol Cell. 2000;5:557–67. doi: 10.1016/S1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 16.Syrjanen SM, Syrjanen KJ. New concepts on the role of human papillomavirus in cell cycle regulation. Ann Med. 1999;31:175–87. doi: 10.3109/07853899909115976. [DOI] [PubMed] [Google Scholar]
- 17.Frisch M, Hjalgrim H, Jaeger AB, et al. Changing pattern of tonsillar squamous cell carcinoma in the United States. Cancer Causes Control:CCC. 2000;11:489–495. doi: 10.1023/A:1008918223334. [DOI] [PubMed] [Google Scholar]
- 18.Shiboski CH, Schmidt BL, Jordan RCK. Tongue and tonsil carcinoma: increasing trends in the US population ages 20–44 years. Cancer. 2005;103:1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90:1626–36. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 20.Hemminki K, Dong C, Frisch M. Tonsillar and other upper aerodigestive tract carcers among cervical cancer patients and their husbands. Eur J Cancer Prev. 2000;9:433–7. doi: 10.1097/00008469-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 21.El-Mofty SK, Zhang MQ, Davila RM. Detection of HPV in metastatic squamous cell carcinoma in cervical lymph nodes: a tool for identification of the site of occult head and neck primary. Mod Pathol. 2007;20:1016A. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Naggar AK, Lai S, Clayman GL, et al. Expression of p16, Rb, and cyclin D1 gene products in oral and laryngeal squamous carcinoma: biological and clinical implications. Hum Pathol. 1999;30:1013–8. doi: 10.1016/S0046-8177(99)90217-4. [DOI] [PubMed] [Google Scholar]
- 23.Dray M, Russell P, Dalrymple C, et al. p16INK4a as complimentary marker of high-grade intraepithelial lesions of the uterine cervix: experience with squamous lesions in 189 consecutive cervical biopsies. Pathology. 2005;37:112–4. doi: 10.1080/00313020500058607. [DOI] [PubMed] [Google Scholar]
- 24.Lu DW, El-Mofty SK, Wang HL. Expression of p16, Rb, and p53 proteins in squamous cell carcinomas of the anorectal region harboring human papillomavirus DNA. Mod Pathol. 2003;16:692–9. doi: 10.1097/01.MP.0000077417.08371.CE. [DOI] [PubMed] [Google Scholar]
- 25.Samama B, Lipsker D, Boehm N. p16 expression in relation to human papillomavirus in anogenital lesions. Human Pthol. 2006;37:513–9. doi: 10.1016/j.humpath.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhang MQ, Davila R, El-Mofty SK. Fine needle aspirarion cytology of cervical lymph nodes as an aid for localization of occult primary tumor. IAP abstract, Montreal Canada: 2006.
- 27.Zhang MQ, El-Mofty SK, Davila RM. Detection of HPV by ISH in FNA biopsies of cervical metastasis of head and neck squamous cell carcinoma: a tool for identifying the site of occult primary. Mod Pathol. 2007;20:385A. [Google Scholar]
- 28.Dahlgren L, Dahlstrand HM, Lindquist D, et al. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112:1015–9. doi: 10.1002/ijc.20490. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Thompson CH, O’Brien CJ, et al. Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer. 2003;106:553–8. doi: 10.1002/ijc.11261. [DOI] [PubMed] [Google Scholar]
- 30.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–13. doi: 10.1002/1097-0142(20010815)92:4<805::AID-CNCR1386>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89:300–4. doi: 10.1002/1097-0215(20000520)89:3<300::AID-IJC14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336–44. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 33.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–47. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Public health. High hopes and dilemmas for a cervical cancer vaccine. Science. 2005;308(5722):618–21. doi: 10.1126/science.308.5722.618. [DOI] [PubMed] [Google Scholar]
- 35.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 36.Steinbrook R. The potential of human papillomavirus vaccines. N Engl J Med. 2006;354:1109–12. doi: 10.1056/NEJMp058305. [DOI] [PubMed] [Google Scholar]
- 37.Schiffman M, Castle PE. The promise of global cervical-cancer prevention. N Engl J Med. 2005;353:2101–4. doi: 10.1056/NEJMp058171. [DOI] [PubMed] [Google Scholar]