Abstract
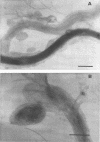
A 53-kDa protein from the outer sheath of the oral spirochete Treponema denticola was purified to homogeneity and shown to reconstitute channels in black lipid bilayer model membranes. The channel had a single-channel conductance of 1.8 nS in 0.1 M KCl, making this the largest porin channel observed to date (estimated diameter, 3.4 nm). Electron micrographs of 53-kDa-protein-containing outer sheaths of T. denticola showed a regular hexagonal array of darker staining pits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Schmid A., Hancock R. E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985 May;162(2):722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Schmid A., Wagner W., Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989 Mar;57(3):887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer H., Berthold P. H., Taichman N. S. Studies on the interaction of human neutrophils with plaque spirochetes. J Periodontal Res. 1986 May;21(3):195–209. doi: 10.1111/j.1600-0765.1986.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Grenier D., Uitto V. J., McBride B. C. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990 Feb;58(2):347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M., Müller K. H., Uitto V. J., Leung W. K., McBride B. C. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect Immun. 1992 May;60(5):2058–2065. doi: 10.1128/iai.60.5.2058-2065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Benz R. Demonstration and chemical modification of a specific phosphate binding site in the phosphate-starvation-inducible outer membrane porin protein P of Pseudomonas aeruginosa. Biochim Biophys Acta. 1986 Sep 11;860(3):699–707. doi: 10.1016/0005-2736(86)90569-9. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Role of porins in outer membrane permeability. J Bacteriol. 1987 Mar;169(3):929–933. doi: 10.1128/jb.169.3.929-933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Brennan M. J., Trus B. L., Bisher M. E., Steven A. C. Naturally crystalline porin in the outer membrane of Bordetella pertussis. J Mol Biol. 1988 Sep 5;203(1):275–278. doi: 10.1016/0022-2836(88)90108-8. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Parr T. R., Jr, Angus B. L., Hancock R. E., Ghiorse W. C., Greenberg E. P. Isolation of the outer membrane and characterization of the major outer membrane protein from Spirochaeta aurantia. J Bacteriol. 1987 Jan;169(1):172–179. doi: 10.1128/jb.169.1.172-179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. D., Federman D., Flora J. L., Beck B. L. Computer-assisted determination of protein concentrations from dye-binding and bicinchoninic acid protein assays performed in microtiter plates. J Immunol Methods. 1986 Sep 27;92(2):261–270. doi: 10.1016/0022-1759(86)90174-2. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Hancock R. E., Ingraham J. L. Properties of a Pseudomonas stutzeri outer membrane channel-forming protein (NosA) required for production of copper-containing N2O reductase. J Bacteriol. 1989 Apr;171(4):2096–2100. doi: 10.1128/jb.171.4.2096-2100.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K., Kawata T. Isolation, properties, and reassembly of outer sheath carrying a polygonal array from an oral treponeme. J Bacteriol. 1982 Jun;150(3):1405–1413. doi: 10.1128/jb.150.3.1405-1413.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Sleytr U. B. Crystalline bacterial cell-surface layers. Adv Microb Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- Nemoto T., Suzuki M., Watanabe T. [Electron microscopic studies of fine structure on the oral spirochetes]. Shigaku. 1989 Jun;77(1):2–79. [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. Attachment of Treponema denticola to cultured human epithelial cells. Scand J Dent Res. 1984 Feb;92(1):55–63. doi: 10.1111/j.1600-0722.1984.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson L. G., Goodman C. H., Bial J. J., Morton H. E. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988 Apr;56(4):726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Zhang Y. X., Barrera O., Watkins N. G., Caldwell H. D. Differential effect of trypsin on infectivity of Chlamydia trachomatis: loss of infectivity requires cleavage of major outer membrane protein variable domains II and IV. Infect Immun. 1988 Aug;56(8):2094–2100. doi: 10.1128/iai.56.8.2094-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M., Hancock R. E. Fluoroquinolone supersusceptibility mediated by outer membrane protein OprH overexpression in Pseudomonas aeruginosa: evidence for involvement of a nonporin pathway. Antimicrob Agents Chemother. 1992 Nov;36(11):2365–2369. doi: 10.1128/aac.36.11.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]