Abstract
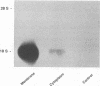
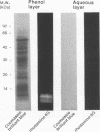
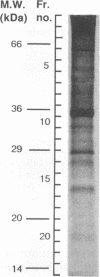
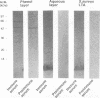
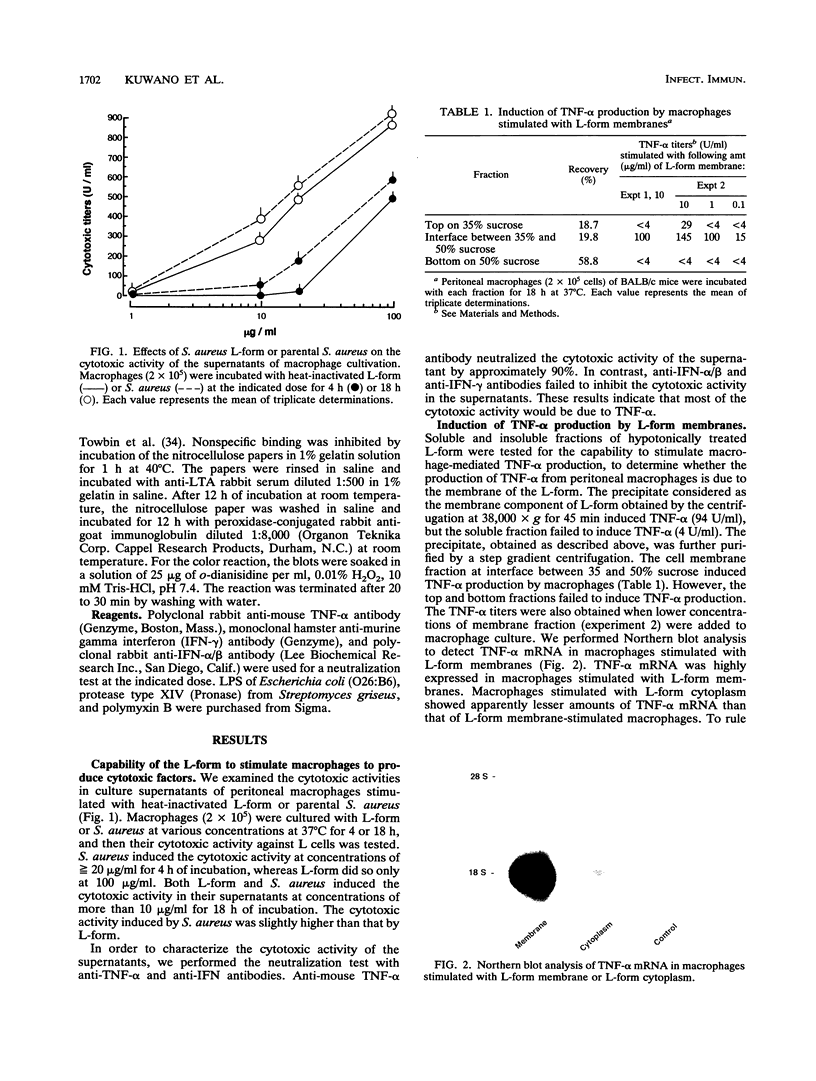
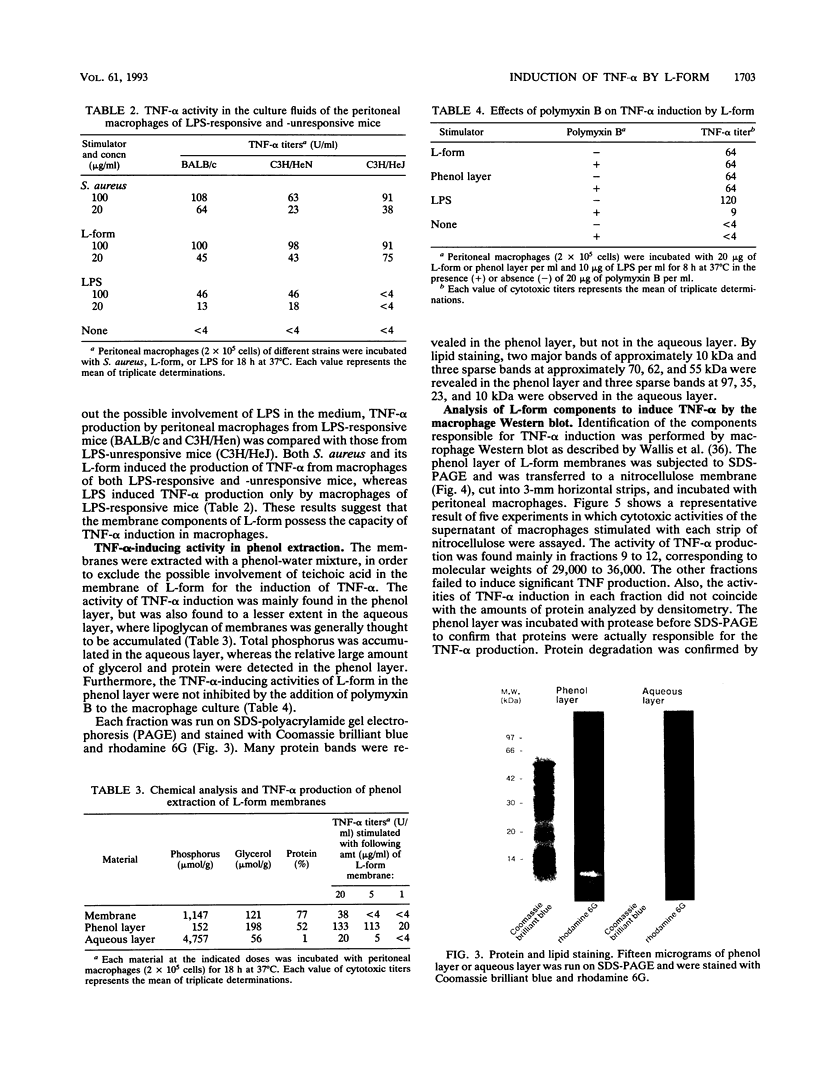
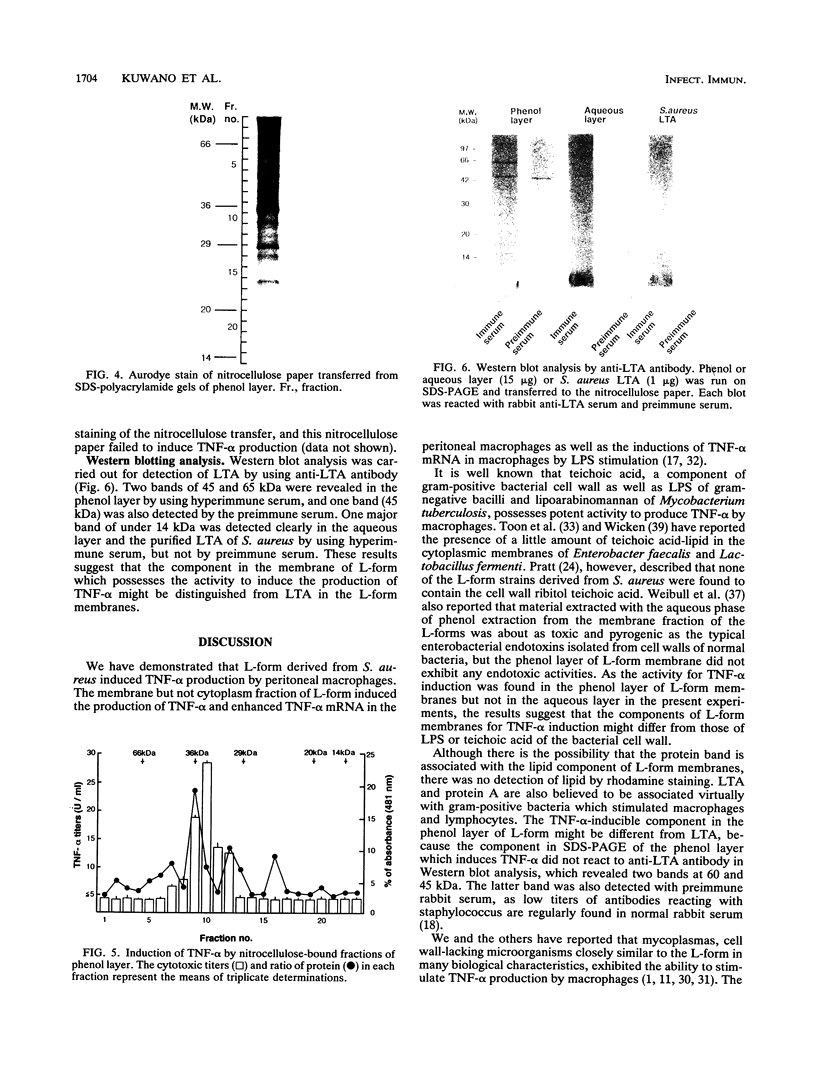
We investigated the capability of an L-form derived from Staphylococcus aureus to induce tumor necrosis factor alpha (TNF-alpha) production in murine peritoneal macrophages. The activity for TNF-alpha induction was found in the membrane fraction of the L-form but not in the cytoplasmal fraction purified by the sucrose step gradient centrifugation. TNF-alpha mRNA was also detected in macrophages stimulated with L-form membranes. L-form induced TNF-alpha production in macrophages from both lipopolysaccharide-responsive and -unresponsive mouse strains. Regardless of the presence of polymyxin B, the activity of TNF-alpha induction of L-form was mostly found in the phenol layer, but not in the aqueous layer, both of which were prepared by phenol extraction method. Fractions of L-form membranes representing molecular masses of approximately between 29 and 36 kDa were primarily responsible for inducing the production of TNF-alpha consistently. Moreover, this stimulatory effect was abolished by digestion with Streptomyces griseus protease. In Western blot (immunoblot) analysis with anti-lipoteichoic acid antibody, two bands (65 and 45 kDa) were observed in the sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the phenol layer, whereas one band (14 kDa) was observed in either the aqueous layer or lipoteichoic acid of S. aureus. These results suggest that the component in the membrane of the L-form, distinct from cell wall components such as teichoic acid or lipopolysaccharide, possesses the capability to stimulate TNF-alpha production by macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai S., Furukawa M., Munakata T., Kuwano K., Inoue H., Miyazaki T. Enhancement of cytotoxicity of active macrophages by mycoplasma: role of mycoplasma-associated induction of tumor necrosis factor-alpha (TNF-alpha) in macrophages. Microbiol Immunol. 1990;34(3):231–243. doi: 10.1111/j.1348-0421.1990.tb01006.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Belsheim M. R., Darwish R. Z., Watson W. C., Schieven B. Bacterial L-form isolation from inflammatory bowel disease patients. Gastroenterology. 1983 Aug;85(2):364–369. [PubMed] [Google Scholar]
- Calandra T., Baumgartner J. D., Grau G. E., Wu M. M., Lambert P. H., Schellekens J., Verhoef J., Glauser M. P. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990 May;161(5):982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- Carro T., Pedersen N. C., Beaman B. L., Munn R. Subcutaneous abscesses and arthritis caused by a probable bacterial L-form in cats. J Am Vet Med Assoc. 1989 Jun 1;194(11):1583–1588. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmel H. Graft infection and bacteremia with a tolerant L-form of Streptococcus sanguis in a patient receiving hemodialysis. J Clin Microbiol. 1986 Aug;24(2):294–295. doi: 10.1128/jcm.24.2.294-295.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Terry T. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. I. Sodium dodecyl sulfate solubilization. Biochim Biophys Acta. 1967 Jul 3;135(3):381–390. doi: 10.1016/0005-2736(67)90028-4. [DOI] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Immunogenicity of a purified and carrier-complexed streptococcal lipoteichoic acid. Infect Immun. 1976 Jun;13(6):1585–1590. doi: 10.1128/iai.13.6.1585-1590.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- GODZESKI C. W., BRIER G., GRIFFITH R. S., BLACK H. R. ASSOCIATION OF BACTERIAL L-PHASE ORGANISMS IN CHRONIC INFECTIONS. Nature. 1965 Mar 27;205:1340–1340. doi: 10.1038/2051340a0. [DOI] [PubMed] [Google Scholar]
- Gallily R., Sher T., Ben-Av P., Loewenstein J. Tumor necrosis factor as a mediator of Mycoplasma orale-induced tumor cell lysis by macrophages. Cell Immunol. 1989 Jun;121(1):146–153. doi: 10.1016/0008-8749(89)90012-9. [DOI] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Chiasson M. A., Carswell E., Schwartz M. K., Old L. J. Corynebacterium parvum as the priming agent in the production of tumor necrosis factor in the mouse. J Natl Cancer Inst. 1977 Nov;59(5):1519–1522. doi: 10.1093/jnci/59.5.1519. [DOI] [PubMed] [Google Scholar]
- Gutman L. T., Turck M., Petersdorf R. G., Wedgwood R. J. Significance of bacterial variants in urine of patients with chronic bacteriuria. J Clin Invest. 1965 Dec;44(12):1945–1952. doi: 10.1172/JCI105300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley S., Paul J., Crook D. W., White S. H. Acinetobacter spp L-form infection of a cemented Charnley total hip replacement. J Clin Pathol. 1990 Sep;43(9):781–781. doi: 10.1136/jcp.43.9.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Ma G. P., Chisolm G. M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990 Mar 15;144(6):2343–2350. [PubMed] [Google Scholar]
- Haukenes G. Serological typing of Staphylococcus aureus. 7. Technical aspects. Acta Pathol Microbiol Scand. 1967;70(4):590–600. doi: 10.1111/j.1699-0463.1967.tb01328.x. [DOI] [PubMed] [Google Scholar]
- Kagan G. Y., Koptelova E. I., Pokrovsky B. M. Isolation of pleuropneumonia-like organisms, L-forms and heteromorphous growth of bacteria from the cerebrospinal fluid of patients with septic meningitis. J Hyg Epidemiol Microbiol Immunol. 1965;9(3):310–317. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapinski E. M., Flakas E. D. Induction of L forms of Haemophilus influenzae in culture and their demonstration in human bronchial secretions. J Bacteriol. 1967 Apr;93(4):1438–1445. doi: 10.1128/jb.93.4.1438-1445.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Shoin S., Koshimura S., Shimizu R. Studies on the anticancer and streptolysin S-forming abilities of hemolytic streptococci. Jpn J Microbiol. 1967 Dec;11(4):323–326. doi: 10.1111/j.1348-0421.1967.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Pratt B. C. Cell-wall deficiencies in L-forms of Staphylococcus aureus. J Gen Microbiol. 1966 Jan;42(1):115–122. doi: 10.1099/00221287-42-1-115. [DOI] [PubMed] [Google Scholar]
- RAZIN S. FACTORS INFLUENCING OSMOTIC FRAGILITY OF MYCOPLASMA. J Gen Microbiol. 1964 Sep;36:451–459. doi: 10.1099/00221287-36-3-451. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Wittler R. G. The L form of Neisseria meningitidis. J Gen Microbiol. 1966 Jul;44(1):139–148. doi: 10.1099/00221287-44-1-139. [DOI] [PubMed] [Google Scholar]
- Ruuth E., Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989 Dec;112:133–160. doi: 10.1111/j.1600-065x.1989.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Saxne T., Palladino M. A., Jr, Heinegård D., Talal N., Wollheim F. A. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988 Aug;31(8):1041–1045. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Sher T., Rottem S., Gallily R. Mycoplasma capricolum membranes induce tumor necrosis factor alpha by a mechanism different from that of lipopolysaccharide. Cancer Immunol Immunother. 1990;31(2):86–92. doi: 10.1007/BF01742371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama K., Kuwano K., Furukawa M., Himeno Y., Satoh T., Arai S. Mycoplasmas induce transcription and production of tumor necrosis factor in a monocytic cell line, THP-1, by a protein kinase C-independent pathway. Infect Immun. 1990 Nov;58(11):3564–3567. doi: 10.1128/iai.58.11.3564-3567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C. S., Hamilton T. A. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol. 1989 Feb 15;142(4):1274–1280. [PubMed] [Google Scholar]
- Toon P., Brown P. E., Baddiley J. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis N.C.I.B. 8191. Biochem J. 1972 Apr;127(2):399–409. doi: 10.1042/bj1270399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITTLER R. G., MALIZIA W. F., KRAMER P. E., TUCKETT J. D., PRITCHARD H. N., BAKER H. J. Isolation of a Corynebacterium and its transitional forms from a case of subacute bacterial endocarditis treated with antibiotics. J Gen Microbiol. 1960 Oct;23:315–333. doi: 10.1099/00221287-23-2-315. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Wallis R. S., Amir-Tahmasseb M., Ellner J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990 May;87(9):3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibull C., Bickel W. D., Haskins W. T., Milner K. C., Ribi E. Chemical, biological, and structural properties of stable Proteus L forms and their parent bacteria. J Bacteriol. 1967 Mar;93(3):1143–1159. doi: 10.1128/jb.93.3.1143-1159.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. B., Smith P. F., Kahane I. Bacterial lipopolysaccharides and mycoplasmal lipoglycans: a comparison between their abilities to induce macrophage-mediated tumor cell killing and Limulus amebocyte lysate clotting. Biochem Biophys Res Commun. 1980 Nov 28;97(2):493–499. doi: 10.1016/0006-291x(80)90290-9. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]