Abstract
Clear cell changes may be observed in virtually any benign or malignant tumor of epithelial, mesenchymal, melanocytic and hematopoeitic derivation not be attributed to variable etiologies. In general, benign and malignant clear cell neoplasms of the head and neck are rare. They may involve various regions and may be of diverse derivations, with only 1–2% of tumors of the salivary glands, jaws and oral mucosa are primarily or almost exclusively composed of clear cells (Maiorano et al., Semin Diagn Pathol 14:203–212, 1997). This review will selectively discuss the clinicopathological features of salivary gland tumors with clear cell changes, which, at times, may pose a diagnostic challenge and dilemma.
Keywords: Clear cell tumors, Head and neck, Salivary gland tumors
Introduction
Clear cell tumors of the head and neck are rare. They may be observed in almost any benign or malignant tumor of epithelial, mesenchymal, melanocytic or hematopoeitic derivation. They are attributable to various factors including artifactual changes, improper cellular preservation and hydropic degeneration of organelles, or due to the accumulation of glycogen, mucopolysaccharides, lipid, mucin, or phagocytized foreign body material in the cytoplasm of tumor cells. Focal clear cell change in a tumor may become more extensive with tumor progression or it may appear secondarily, reflecting clonal evolution in that tumor [1–3]. These factors may collectively make the diagnosis of clear cell tumors difficult and challenging [1–5]. With few exceptions, most neoplasms with a clear cell component show sufficient original characteristic histomorphological features that would enable the pathologist to render a precise and accurate diagnosis. Nevertheless, these changes can potentially lead to difficulties and delays in establishing the diagnosis [1–3]. Salivary gland tumors account for less than 7% of neoplasms involving the head and neck region [4]. These tumors are also reported in various other sites including the skin, neck, thyroid gland, mastoid bone, middle ear, and jawbones [5–9].
Intraosseous salivary gland tumors may be derived from ectopic salivary tissue, may arise from the neoplastic transformation of the mucous cells found in the lining of dentigerous cysts, from embryonic remnants of submandibular glands found within the mandible, from bony entrapment of mucous cells of the retromolar pad during embryogenesis or theoretically, the may also arise from salivary tissue present in lingual cortical defect of the mandible [10–12]. The most common intraosseous salivary gland tumor reported is mucoepidermoid carcinoma, followed by adenoid cystic carcinoma [10]. While primary central clear cell carcinoma of salivary origin is extremely rare [10–12] it must be included in the differential diagnosis of central clear cell tumors and tumor- like conditions of bone. Clear cells can be observed in any type of benign and malignant salivary gland tumors, including benign mixed tumors, myoepithelioma/myoepithelial carcinoma, oncocytoma/oncocytic carcinoma, mucoepidermoid carcinoma, acinic cell carcinoma, polymorphous low-grade adenocarcinoma, and adenoid cystic carcinoma [13–18]. In most instances, clear cells constitute only a minor component of the cellular content of these neoplasms and the diagnosis is established based upon the identification of the classic histomorphological features and characteristic growth pattern of the designated tumor [1, 15]. Between 15–35% of all parotid gland tumors are malignant and 21–42% of these representing metastatic disease. The majority of metastatic parotid tumors are of cutaneous origin, primarily squamous cell carcinomas and melanomas (45 and 37% respectively). The remainder of metastatic tumors arise from the lung, breast, kidney, gastrointestinal tract, and prostate [19]. Distinguishing primary salivary tumors from metastatic tumors with clear cell features have important therapeutic, diagnostic and decision making considerations. The clinicopathological features of primary clear cell tumors of salivary glands and selective metastatic clear cell tumors to that region will be discussed.
Discussion
Modified myoepithelial cells constitute a significant component of numerous salivary gland neoplasms [20]. Both the benign myoepithelioma and its malignant counterpart; myoepithelial carcinoma are comprised exclusively of sheets and islands of myoepithelial cells that may be arranged in various patterns including plasmacytoid, epithelioid, and clear cell patterns. A marked cellular, mucoid, or hyalinized stroma may be seen in these tumors [16]. Many pathologists consider myoepithelioma to be in the spectrum of the benign mixed tumors (PA) but they characteristically lack ductal differentiation and myxoid–chondroid changes seen in benign mixed tumors (BMT) [21, 22].
Myoepitheliomas represents 1.5% of all salivary gland tumors and tend to involve both genders with equal frequency. Although it may be encountered anywhere within the maxillofacial region, the parotid gland is the most common site reported followed by the hard and soft palates [16]. Myoepithelial carcinomas tend to show a lobulated pattern and are composed to a similar range of cell types observed in the benign counterpart and although high mitotic rate, a cellular pleomorphic infiltrate and necrosis may be encountered [23–26]. The infiltrative and destructive growth pattern unequivocally remains to be the main diagnostic criteria in separating myoepithelial carcinoma from myoepitheliomas [16].
Clear cell myoepithelial carcinoma (CCMC) comprises about 16% of all myoepithelial carcinomas [23]. Its distinction from other salivary gland tumors with clear cell components is important since CCMC tends to behave more aggressively with a 50% recurrence rate and 40% metastatic rate [27]. This tumor characteristically reacts positively with anti S-100 protein, vitamin and high molecular weight cytokeratin, muscle specific actin (MSA), (HHF-35) and alpha smooth muscle actin (SMA) immunohistochemical stains. Calponin is thought to be superior to MSA and SMA in delineating the myoepithelial cells; yet it fails to stain half of the epithelioid cell type and 25% of the clear cell types [23, 27]. The documentation of strong positive staining with myoepithelial markers is important in differentiating the clear cell variant of myoepithelioma and myoepithelial carcinoma from other primary tumors with clear cell features in that region; including clear cell oncocytoma, mucoepidermoid carcinoma, acinic cell carcinoma and clear cell carcinoma.
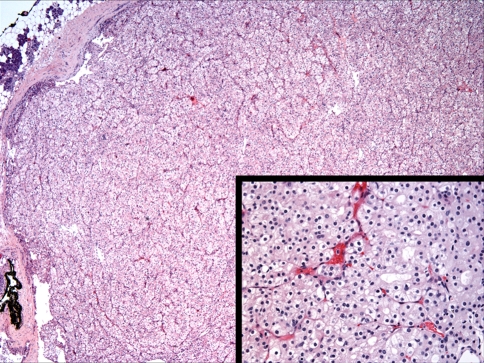
Oncocytes are modified epithelial cells that containing abundant mitochondria. They may be seen in many organs including the parotid gland, the minor salivary glands, larynx, nasal mucous membranes, thyroid and parathyroid glands [28] and although benign and malignant oncocytic neoplasms are well documented in the head and neck region, they are considered rare. Oncocytic tumors, or oncocytomas constitute approximately 1% of all salivary gland tumors with marked tendency for parotid gland involvement and a much lower occurrence in the minor glands [29, 30]. The mean age of patients is 85 years with no sex predilection reported. Oncocytomas, including the clear cell variant are well-demarcated lesions composed of large polyhedral eosinophilic epithelial cells with characteristic granular cytoplasm and small dark nuclei separated by thin vascular fibrous septae (Fig. 1). Clear cells may occasionally predominate in some lesions and may be attributed to intracytoplasmic glycogen deposition or to fixation artifact [15]. Nevertheless, oncocytomas, including the clear cell variant stain strongly positively for phosphotungstic acid hematoxylin (PTAH) and negatively for myoepithelial markers. It is noteworthy to mention that clear-cell oncocytoma of the salivary gland is a benign tumor with excellent prognosis [15–18]; when compared to the other salivary tumors with exclusively or predominantly clear cell features which are generally considered at least low-grade malignant tumors.
Fig. 1.
Histomorphological features of clear cell variant of oncocytoma of the parotid gland. The tumor is well demarcated and consists of monotonous clear cells, arranged in nests and organoid pattern and separated by thin fibrous spate. (Hematoxylin and eosin stain. Original magnification 20×.) (Inset: Hematoxylin and eosin stain. Original magnification 40× R. image)
Mucoepidermoid carcinoma is the most common malignant salivary gland tumor with more than half of the cases involving the major salivary glands and the balance involving the minor salivary glands of the palate, buccal mucosa, retromolar trigone, and lips [14–16]. It also has a predilection for females. MEC is characterized by three distinct cell types; epidermoid, mucous and intermediate cells mixed in various proportions with variable patterns. Clear cells constitute approximately 10% of the tumor population [15] but may occasionally form a large population of the tumor cells [31]. Clear cells are much more frequently encountered in mucoepidermoid carcinoma when compared to acinic cell carcinoma [14] and the existence of pure clear cell variant of acinic cell carcinoma is doubted [16, 32]. The clear cells in MEC typically stain positively with PAS; often with the absence or diminish intensity of staining after digestion with diastase confirming its glycogen content [15, 33]. Special staining for mucicarmine or alcian blue can readily identifies the mucous cell population, which is considered diagnostic since mucous cells are only rarely encountered in other salivary tumors with marked clear cell content [16, 31].
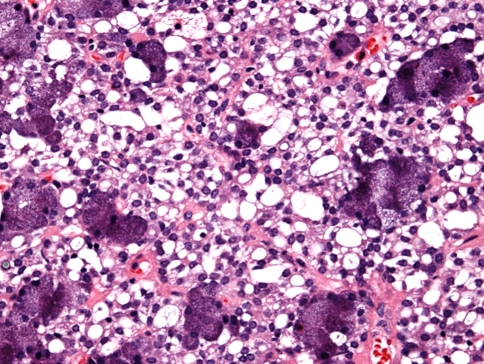
The parotid gland is the site of 83% of acinic cell carcinoma. Only 4% involve the submandibular gland [16] and the balance arise in the intraoral minor salivary glands. These tumors may be infiltrative or well circumscribed and may demonstrate variable growth patterns including the well defined serous acinar pattern, solid/tubular, papillary-cystic, microcystic, follicular non-specific glandular, vacuolated and clear cell patterns [1, 16, 34]. Clear cells are found in approximately 6% of acinic cell carcinomas (ACC) (Fig. 2) but in almost 1%, they constitute the major population of tumor cells [15]. The clear cells in ACC do not contain glycogen and the cytoplasmic clearing is most probably related to fixation artifacts, reduction in the numbers of organelles [14, 15, 18], or may reflect transformation of neoplastic acinar cells [14, 15, 18]. The identification of the PAS positive diastase resistant zymogen cytoplasmic granules can help in establishing the diagnosis especially when the typical histomorphological features are only focally identified. As in MEC, the percentage of clear cells in acinic cell carcinomas have no prognostic value or significance. Positive immunohistochemical staining to anti-S-100 protein, cytokeratin, Vimentin, tranferrin, alpha 1-antitrypsin, carcinoma embryonic antigen (CEA), glial fibrillary acidic protein (GFAP) and amylase have been reported [35, 36].
Fig. 2.
Clear cells in acinic cell carcinoma note the presence of serous tumor acini surrounding the clear cells. (Hematoxylin and eosin stain. Original magnification 60×)
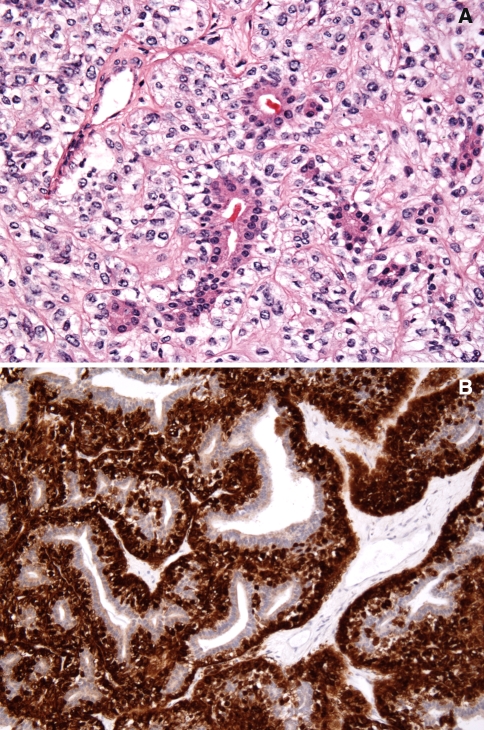
Epithelial–myoepithelial carcinoma (EMC) is an uncommon salivary gland tumor, characterized by variably sized duct-like structures of solid, cystic, spindle, tubular, organoid nodular, papillary, or cribriform tumor patterns. These consist of characteristic two cell types (biphasic pattern); ductal, cuboidal, intercalated duct like eosinophilic cells typically surrounding a small lumen and larger polygonal clear cells characteristically exhibiting myoepithelial differentiation which can be well delineated with anti S-100 protein immunohistochemistry staining. (Fig. 3a and b) [16, 17, 33]. Clear cell adenocarcinoma is an uncommon, yet important salivary gland tumor consisting of a monotonous and monomorphic pure clear cell population lacking the biphasic cellular pattern that characterizes epithelial myoepithelial carcinoma. The clear cells may or may not demonstrate glycogen content by PAS staining. Despite the presence or rare mucous cell differentiation in this tumor the absence of the epidermoid component should readily distinguish clear cell adenocarcinoma from mucoepidermoid carcinoma with prominent clear cell population. Despite the presence of occasional cystic spaces in clear cell carcinomas, ductal structures are generally absent and myoepithelial differentiation is never demonstrated [33, 37].
Fig. 3.
(a) Histomorphology of epimyoepithelial carcinoma of the salivary glands showing the class biphasic glandular-ductal pattern of the two cell types, inner basaloid cells and the outer clear cells, surrounded by a thickened and hyalinized connective tissue. (Hematoxylin and eosin stain. Original magnification 60×). (b) Immunohistochemistry staining of epimyoepithelial carcinoma of the salivary glands with anti-S-100 protein, showing distinct positive labeling of the outer myoepithelial cells, sparing the inner ductal cuboidal cells. (Immunohistochemistry stain. Original magnification 60×)
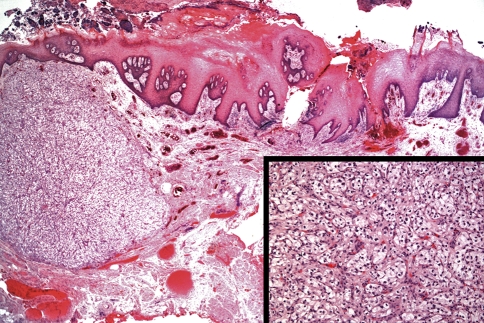
Renal cell carcinoma shows the greatest propensity to metastasize to various sites including lung, pleura, bones, liver, brain, and the thyroid gland [2, 14, 30]. Approximately 15–16% of these tumors may ultimately metastasize to the head and neck through hematogenous route [38] with 8% of these representing the initial presenting symptoms of the tumor [39]. Metastasis to the parotid gland is even more rarely encountered [40] and metastasis to the oral soft tissues is also rarely seen (Fig. 4).
Fig. 4.
Metastatic clear cell carcinoma to the oral soft tissues (including to the dorsal tongue area as seen in this case) is rarely seen. Note the rich sinusoidal type vascular network within the clear cell tumor. (Hematoxylin and eosin stain. Original magnification 10×/Inset = 20×)
It is essential to differentiated metastatic clear cell carcinoma of renal origin from primary salivary gland tumors with clear cell features (especially clear cell carcinoma) which may be challenging at times since the tumor shares chemical and ultrastructural qualities and features with other tumors [38–43]. Glycogen positivity, for example, can be demonstrated in both primary clear cell adenocarcinoma of salivary origin and metastatic renal clear cell carcinoma to that region. The demonstration of intracytoplasmic lipid would favor renal cell carcinoma and this would require the examination of frozen tissue. Additionally, the identification of a heterogeneous architecture and a rich dilated prominent sinusoidal vascular network favors metastatic renal cell carcinoma. Similarly, the identification of hemorrhage and hemosiderin coupled with pronounced pleomorphism and cytological atypia should alert the clinician to the possibility of metastatic disease. Needless to say, the identification of primary renal cell lesion on thorough workup should be also confirmatory; keeping in mind that renal cell carcinoma is known for its slow growth rate and its potential for late metastasis. Additionally, RCC reacts negatively with actins S-100, glial fibrillary acid protein and CEA as well as high-molecular-weight cytokeratin, cytokeratin cocktail, estrogen receptor, CA 125, inhibin, calretinin, CD34, and alpha-fetoprotein. While staining with anti CD10 antibodies is also diagnostic since it is seen in 90–94% of cases [38, 40].
Conclusion
Clear cell tumors of the salivary glands comprise a diverse group of benign and malignant tumors with variable clinicopathological characteristics. The distinction between different tumors of this group and this differentiation from metastatic disease is essential and can be facilitated by a combination of thorough clinical evaluation and chemical as well as histochemical staining characteristics.
Acknowledgement
The authors acknowledge the generous contribution of Dr. Omar Hameed form UAB, Department of Pathology for contributing the images in Fig. 3.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Maiorano E, Altini M, Favia G. Clear cell tumors of the salivary glands, jaws, and oral mucosa. Semin Diagn Pathol. 1997;14:203–12. [PubMed] [Google Scholar]
- 2.Nappi O, Mills SE, Swanson PE, Wick MR. Clear cell tumors of unknown nature and origin. A systematic approach to diagnosis. Semin Diagn Pathol. 1997;14:164–74. [PubMed] [Google Scholar]
- 3.Swanson PE. Clear cell tumors of bone. Semin Diagn Pathol. 1997;14:281–91. [PubMed] [Google Scholar]
- 4.Brookstone MS, Huvos AG. Central salivary gland tumors of the maxilla and mandible: a clinicopathologic study of 11 cases with an analysis of the literature. J Oral Maxillofac Surg. 1992;50:229–36. doi: 10.1016/0278-2391(92)90317-S. [DOI] [PubMed] [Google Scholar]
- 5.Miller AS, Winnick M. Salivary gland inclusion in the anterior mandible (report of a case with a review of the literature on aberrant salivary gland tissue and neoplasms) Oral Surg Oral Med Oral Pathol. 1971;31:790–7. doi: 10.1016/0030-4220(71)90135-6. [DOI] [PubMed] [Google Scholar]
- 6.Warnock GR, Jensen JL, Kratochvil FJ. Developmental disease. In: Ellis GL, Auclair PL, Gnepp DR, editors. Surgical pathology of the salivary glands. Philadelphia, PA: Saunders; 1991. p. 10–25.
- 7.Peters BR, Maddox HE, Batsakia JG. Pleomorphic adenoma of the middle ear and mastoid with posterior fossa extension. Arch Otolaryngol Head Neck Surg. 1988;114:676–8. doi: 10.1001/archotol.1988.01860180090038. [DOI] [PubMed] [Google Scholar]
- 8.Feigin GA, Robinson B, Marchevsky A. Mixed tumor of the mediastinum. Arch Pathol Lab Med. 1986;110:80–1. [PubMed] [Google Scholar]
- 9.Caironi C, Re S, Canali B. Mixed tumor of an ectopic salivary gland (presentation of a case) Minerva Chir. 1981;36:621–4. [PubMed] [Google Scholar]
- 10.Brookstone MS, Huvos AG, Spiro RH. Central adenoid cystic carcinoma of the mandible. J Oral Maxillofac Surg. 1990;48:1329–33. doi: 10.1016/0278-2391(90)90493-L. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Madrigal F, Pineda-Daboin K, Casiraghi O, Luna MA. Salivary gland tumors of the mandible. Ann Diagn Pathol. 2000;4:347–53. doi: 10.1053/adpa.2000.19395. [DOI] [PubMed] [Google Scholar]
- 12.Berho M, Huvos AG. Central hyalinizing clear cell carcinoma of the mandible and the maxilla a clinicopathologic study of two cases with an analysis of the literature. Hum Pathol. 1999;30:101–5. doi: 10.1016/S0046-8177(99)90308-8. [DOI] [PubMed] [Google Scholar]
- 13.Seifert G. Classification and differential diagnosis of clear and basal cell tumors of the salivary glands. Semin Diagn Pathol. 1996;13:95–103. [PubMed] [Google Scholar]
- 14.Eversole LR. On the differential diagnosis of clear cell tumors of the head and neck. Oral Oncol Eur J Cancer. 1993;29b(3):173–9. doi: 10.1016/0964-1955(93)90019-B. [DOI] [PubMed] [Google Scholar]
- 15.Ellis GL. Clear cell neoplasms in salivary glands: clearly a diagnostic challenge. Ann Diagn Pathol. 1998;2:61–78. doi: 10.1016/S1092-9134(98)80035-X. [DOI] [PubMed] [Google Scholar]
- 16.Ellis GL, Auclair PL. Tumors of the salivary glands. In: Ellis GL, Auclair PL, editors. Atlas of tumor pathology. 3rd series, fascicle 17. Washington, D.C.: Armed Forces Institute of Pathology; 1996. p. 318–24.
- 17.Ellis GL. “Clear cell” oncocytoma of salivary gland. Hum Pathol. 1988;19:862–7. doi: 10.1016/S0046-8177(88)80271-5. [DOI] [PubMed] [Google Scholar]
- 18.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177–84. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 19.Vara A, Madrigal B, Pérez del Río MJ, Díaz A, Mateos A, Sales C. Parotid metastasis from renal clear cell adenocarcinoma. Urol Int. 1998;61:196–8. doi: 10.1159/000030308. [DOI] [PubMed] [Google Scholar]
- 20.Dardick I, Nostrand AWP. Myoepithelial cells in salivary gland tumors-revisited. Head Neck Surg. 1985;7:395–408. doi: 10.1002/hed.2890070509. [DOI] [PubMed] [Google Scholar]
- 21.Simpson RHW, Kitara-Okot P. Clear cell salivary myoepithelioma. Pathol Res Pract. 1995;191:780. [Google Scholar]
- 22.Alos L, Cardesa A, Bombi JA, et al. Myoepithelial tumors of salivary glands: a clinicopathologic, immunohistochemical, ultrastructural and flow cytometric study. Semin Diagn Pathol. 1996;13:138–47. [PubMed] [Google Scholar]
- 23.Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761–74. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Nagao T, Shugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A, et al. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998;83:1292–9. doi: 10.1002/(SICI)1097-0142(19981001)83:7<1292::AID-CNCR4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Hungerman D, Roeser K, Buerger H, Jakel T, Loning T, Herbst H. Relative paucity of gross genetic alterations myoepitheliomas any myoepithelial carcinomas of salivary glands. J Pathol. 2002;198:487–94. doi: 10.1002/path.1234. [DOI] [PubMed] [Google Scholar]
- 26.Palma S, Guzzo M. Malignant myoepithelioma of salivary glands. Clinicopathological features of ten cases. Virch Arch A Pathol Anat. 1993;423:389–96. doi: 10.1007/BF01607152. [DOI] [PubMed] [Google Scholar]
- 27.Zarbo RJ. Salivary gland neoplasia. A review for the practicing pathologist. Mod Pathol. 2002;15(3):298–323. doi: 10.1038/modpathol.3880525. [DOI] [PubMed] [Google Scholar]
- 28.Chu W, Strawitz JG. Oncocytoma of the parotid gland with malignant change. Arch Surg. 1978;113:318–9. doi: 10.1001/archsurg.1978.01370150090022. [DOI] [PubMed] [Google Scholar]
- 29.Goode RK. Oncocytoma. In: Ellis GL, Auclair PL, Gnepp DR, editors. Surgical pathology of the salivary glands. Saunders: Philadelphia, PA; 1991. pp. 225–37. [Google Scholar]
- 30.Damm DD, White DK, Geissler RH, et al. Benign solid oncocytoma of intraoral minor salivary glands. Oral Surg Oral Med Oral Pathol. 1989;67:84–6. doi: 10.1016/0030-4220(89)90308-3. [DOI] [PubMed] [Google Scholar]
- 31.Hamper K, Mausch HE, Caselitz J, et al. Multiple expression of tissue markers in mucoepidermoid carcinomas and acinic cell carcinomas of the salivary glands. Virch Arch A Pathol Anat. 1989;414:407–13. doi: 10.1007/BF00718624. [DOI] [PubMed] [Google Scholar]
- 32.Perzin KH, LiVolsi VA. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979;44:1434–57. doi: 10.1002/1097-0142(197910)44:4<1434::AID-CNCR2820440439>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 33.Kusama K, Saito M, Kozu M, et al. Epithelial-myoepithelial carcinoma of the palate. J Oral Pathol Med. 1996;25:463–6. doi: 10.1111/j.1600-0714.1996.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 34.Batsakis JG, Bautina E. Metastases to major salivary glands. Ann Otol Rhinol Laryngol. 1990;99:501–3. doi: 10.1177/000348949009900618. [DOI] [PubMed] [Google Scholar]
- 35.Stanley RJ, Weiland LH, Olsen KD, Pearson BW. Dedifferentiated acinic cell (acinous) carcinoma of the parotid gland. Otolaryngol Head Neck Surg. 1988;98:155–61. doi: 10.1177/019459988809800210. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H, Fujita S, Okabe H, et al. Distribution of tissue markers in acinic cell carcinomas of salivary gland. Pathol Res Pract. 1992;188:692–700. doi: 10.1016/S0344-0338(11)80164-0. [DOI] [PubMed] [Google Scholar]
- 37.Batsakis JG, El-Naggar AK, Luna MA. Epithelial-myoepithelial carcinoma of salivary glands. Ann Otol Rhinol Laryngol. 1992;101:540–2. doi: 10.1177/000348949210100617. [DOI] [PubMed] [Google Scholar]
- 38.Som PM, Norton KI, Shugar JM, et al. Metastatic hypernephroma to the head and neck. AJNR Am J Neuroradiol. 1987;8:1103–6. [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman RL, Klein HZ. Glycogen-rich adenoma of the parotid gland. An uncommon benign clear-cell tumor resembling certain clear-cell carcinomas of salivary origin. Cancer. 1972;30:749–54. doi: 10.1002/1097-0142(197209)30:3<749::AID-CNCR2820300324>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Yang B, Ali SZ, Rosenthal DL. CD10 facilitates the diagnosis of metastatic renal cell carcinoma from primary adrenal cortical neoplasm in adrenal fine-needle aspiration. Diagn Cytopathol. 2002;27:149–52. doi: 10.1002/dc.10153. [DOI] [PubMed] [Google Scholar]
- 41.Ingelaere P, Simpson RH, Garth RJN. Metastatic renal cell carcinoma presenting as an aural polyp. J Laryngol Otol. 1997;111:1066–8. doi: 10.1017/S0022215100139350. [DOI] [PubMed] [Google Scholar]
- 42.Boles R, Cemy J. Head and neck metastases from renal carcinomas. Mich Med. 1971;70:616–8. [PubMed] [Google Scholar]
- 43.Madison JF, Frierson HF. Pathologic quiz case 2. Clear cell carcinoma, consistent with metastatic renal cell carcinoma. Arch Otolaryngol Head Neck Surg. 1988;114:570–1. [PubMed] [Google Scholar]