Abstract
Background Evidence of an etiological role for human papillomavirus (HPV) in Schneiderian inverted papillomas IP arose in the late 1980’s; yet almost three decades later, the association between HPV and IP has yet to be universally accepted. This is probably due to the disparate HPV detection rates in IP reported in the literature. We analyzed the weight of published data in order to address the following questions: why do the HPV detection rates in IP vary so greatly? What is the relationship between low-risk (LR) and high-risk (HR) HPV types and HPV detection rates in IP? Is there a relationship between the presence and type of HPV in IP and recurrence and malignant progression? Materials and methods A search using the Pubmed search engine was performed to identify studies published in English from 01/87 through 12/06 using the MeSH terms ‘‘HPV’’ and ‘‘Inverted”, “Exophytic”, “Oncocytic Schneiderian” or “Fungiform papilloma’’. Data was abstracted from publications including histology, HPV target, HPV type, method of detection, etc. HPV results were stratified by histology and other variables. Tests for heterogeneity (between-study variability) were conducted, and weighted prevalence (WP) estimates and 95% confidence intervals (CI) were calculated using a random-effects inverse-variance model stratified on study. The association between HPV IP recurrence was estimated by random-effects inverse-variance weighted odds ratio (OR). Results Weighted estimates revealed similar detection rates across detection methods, 26.8% (95%CI 16.4–37.2%) by ISH, 25.2% (95%CI 14.7–35.6%) by consensus PCR, and 23.6% (95%CI 12.2–35.0%) by type-specific PCR. A preponderance of HPV 6/11 is found in IP as compared to HPV 16/18; the overall unadjusted ratio of LR to high-risk HR HPV types is 2.8:1 The HPV detection rates significantly increase (Wald t-test P < 0.02) in IPs with high-grade dysplasia (WP 55.8%, 95%CI 30.5-81.0%) and carcinoma (WP 55.1%, 95%CI 37.0–73.2%) as compared to IPs with no dysplasia or mild dysplasia (WP 22.3%, 95%CI 15.9–28.6%). Furthermore, the preponderance of LR HPV in benign IP (ratio LR/HR = 4.8:1) shifts in dysplastic and malignant IP. The LR/HR ratio is 1.1:1 for IPs with high-grade dysplasias, this ratio is inverted to favor HR HPV (1:2.4) for malignant IP. Recurrences developed in 44 of 236 patients; HPV was detected in 27 of 44 IPs (WP 57.9%, 95%CI 31.6–84.2%) that developed recurrences and in 24 of 192 IPs (WP 9.7%, 95%CI 4.4–15.0%) that did not develop recurrence. The presence of HPV was significantly associated with the likelihood of developing recurrence (weighted OR of 10.2, 95%CI 3.2–32.8). Conclusions We hypothesize that LR HPV may induce IP formation, and then are lost as infected cells are shed, as a “hit and run” phenomenon. HPV detection rates increase in dysplastic IP and SCC-ex-IP with increasing ratio of HR to LR HPV types, compared to nondysplastic IP. We believe that one explanation for the variation in HPV detection rates between different studies may be the actual histologic composition of the cohort. That is, if one series contains a higher frequency of dysplastic and malignant IP, it may have a higher detection rate than another series which contains only nondysplastic IP. We hypothesize that the higher rates of HPV detection in dysplastic and malignant IP may be related to HPV integration. The implication of this is that HPV sub-type testing may identify patients at risk for recurrence, or progression to dysplasia and malignancy, and thus may impact surveillance protocols.
Keywords: Inverted papilloma, HPV detection, Dysplasia, High-risk, Recurrence
Introduction
Schneiderian inverted papillomas (IP) are rare sinonasal tumors comprising approximately 0.5% of all nasal neoplasia. Historically, the etiology of IP had been ascribed to allergy or chronic sinusitis. Evidence of an etiological role for human papillomavirus (HPV) in IP arose in the late 1980’s. Yet almost three decades later, and after major technological advances that have increased the detection sensitivity of HPV, the association between HPV and IP has yet to be universally accepted. This is probably due to the disparate HPV detection rates in IP reported in the literature.
Few would dispute the causal role of HPV in the development and progression of cervical cancer. Biological similarities between cervical and the upper aerodigestive tract epithelium, specifically the oropharynx, have led to studies of HPV in the head and neck. Early HPV studies were inspired by the presence of koilocytosis in IP and “viral-like” clinical features such as multicentricity, tendency towards recurrence, and potential for malignant transformation. IP bears some histological resemblance to exophytic papillomas, which commonly contains high copy numbers of HPV. The relationship of HPV to the etiology of IP is not merely of academic interest. The weight of published evidence supports that HPV typing is clinically significant with respect to surveillance and potential therapy for patients with cervical dysplasias and laryngotracheal papillomatosis. We assert that it is important to examine the relationship of HPV to IP, as significant associations between HPV, IP and outcome parameters (disease recurrence or malignant progression) would potentially impact clinical surveillance protocols for these patients also. Therefore, we analyzed the weight of published data [1–35] regarding HPV and IP. We sought to address the following questions: why do the HPV detection rates in IP vary so greatly? What is the relationship between low-risk and high-risk HPV types and HPV detection rates in IP? Is there a relationship between the presence and type of HPV in IP and recurrence and malignant progression? Is HPV also involved in sinonasal carcinogenesis unrelated to IP?
Methods
We conducted an exhaustive literature search using the NIH Pubmed search engine to identify citations published in English from January 1987 through December 2006 using the MeSH terms ‘‘HPV’’ and ‘‘Inverted”, “Exophytic”, “Oncocytic Schneiderian” or “Fungiform papilloma’’. Additional searches including terms “HPV” and “Nasal”, “Paranasal”, “Sinonasal”, or “Squamous Cell Carcinoma” were also used. Priority was given to studies with type-specific HPV results from biopsy or tissue resections, and that clearly described the HPV testing methods. No limit was set a priori on number of cases given the rare nature of the disease, and no restriction on upper aerodigestive anatomic site was imposed.
Data Abstraction
Data abstracted from publications included year of publication, study country, number of cases, method of specimen preservation (fresh frozen or paraffin embedded), method of case procurement (retrospective selection from archives or prospective collection from cohort), histology of specimens (grade of dysplasia, carcinoma, or recurrent papilloma), target for HPV analysis (DNA or RNA), method of HPV detection and typing (polymerase chain reaction [PCR], in-situ hybridization [ISH] or Southern blot [SB]), HPV primers used (for PCR-based assays), HPV types genotyped or probed for, and overall and type-specific detection of HPV. Cases was separated by histological description (no or low-grade dysplasia, moderate to severe dysplasia, carcinoma, and recurrent papilloma), and by nosological classification (fungiform/exophytic papilloma, inverted papilloma and oncocytic Schneiderian papilloma). The geographic region of each study was classified into one of three categories: Europe (UK, Denmark, Germany, Switzerland and Finland), North America (USA), East Asia and Middle East (Japan, Korea and Egypt).
Results were summarized for HPV types 6/11 and 16/18, although additional types were also included when data were provided. Overall HPV detection rate was defined as persons testing positive for any HPV type divided by the total population and summarized by method of detection (PCR vs. ISH) and whether consensus primers were used. Studies varied substantially with respect to method of HPV detection and genotyping. All but eight of the 32 studies assessed tested for the four main HPV types (6, 11, 16 and 18), of which two tested for HPV 6, 11 and 16 [1, 2], two tested for HPV6 and 11 [3, 4], one tested for HPV 16 and 18 [5], and three tested only for HPV 16 [6], HPV 11 [7], and HPV 57 [8], respectively. Type-specific HPV detection for 6/11 and 16/18 were assessed only among those specimens tested for the specific HPV types in question; therefore, the sample size varies between the type-specific analyses. Multiple infections were separated into constituent types; thus, type-specific detection represents types found alone or with other HPV types. Consensus PCR primers MY09/11, PGMY09/11, GP5+/6+, and SPF10 were considered to amplify the 18 HPV types most commonly associated with cervical and head and neck cancers (6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 70, 73, 82, and 83) as well as additional high- and low-risk types. It is important to note that an HPV type was only considered tested for if it was, a) amplified by the primers and subsequently genotyped in analyses using PCR-based methods, or b) probed for using sequences specific to those types in the DNA-based (SB) and RNA-based (ISH) analyses.
Statistical Analysis
HPV results were stratified by histology and other variables believed to potentially impact HPV prevalence, including method of detection, geographic region, and specimen histology. Tests for heterogeneity (between-study variability) were conducted, and weighted prevalence estimates and 95% confidence intervals (CI) were calculated using a random-effects inverse-variance model stratified on study [36, 37]. Analysis of individual-level data (e.g., by gender, age, and smoking status) was not possible, as these were not available by HPV status in the studies evaluated. The association between HPV and occurrence of recurrent IP was estimated by random-effects inverse-variance weighted odds ratio (OR).
Results
There were 32 genotype-based investigations published in English peer-reviewed full manuscripts between 1987 and 2006, studying HPV in IP; these publications represent the basis of our literature review [1–9, 12–35]. These studies are summarized in the Appendix. Two studies using only Southern blot [7, 15] and one study probing only for HPV 57 [8] were omitted. Table 1 presents pooled detection rates from our review studies by methodology. Results were tabulated specifically to each technique; Brandsma studied cases by both SB and ISH, [13], and six studies used both ISH and PCR techniques [12, 16–18, 21, 31]. The inclusion of IP with dysplasia or carcinoma was slightly more common among studies using PCR (12/18) vs. ISH (10/18), although several (n = 4) studies used both methods of detection (see Appendix). A wide range of detection rates are seen for studies utilizing ISH techniques (0–79%), and PCR, either with type specific probes (0–77%) or consensus probes (2–67%). Interestingly, as detection thresholds have decreased, and the number of published reports using PCR-based techniques has increased, we see no increase in the overall range of HPV detection rates. Weighted estimates revealed similar detection rates across methods, 26.8% (95%CI 16.4–37.2%) by ISH, 25.2% (95%CI 14.7–35.6%) by consensus PCR, and 23.6% (95%CI 12.2–35.0%) by type-specific PCR. Analysis of heterogeneity by PCR primers used was not possible, as many of the PCR-based studies used several combinations of different primers.
Table 1.
HPV detection rates in IP––by method
| In situ hybridization [2, 4, 9, 12–14, 16–18, 20, 21, 24, 29, 31, 34] | Type specific PCR [1, 3,5, 6, 16–19, 23, 28] | PCR consensus [12, 21, 25–27, 30–33, 35, 36] | |
|---|---|---|---|
| Number of studies | 15 | 10 | 11 |
| Total case examined | 328 | 210 | 349 |
| Detection range | 0–79% | 0–77% | 2–67% |
| Weighted prevalence* | 26.8% (95%CI 16.4–37.2) | 23.6% (95%CI 12.2–35.0) | 25.2% (95%CI 14.7–35.6) |
| Pooled (crude) prevalence | 26% (84/328) | 22% (46/210) | 23% (80/349) |
* Weighted prevalence estimated by random-effects inverse-variance with continuity correction
In order to assess the sources for differences in HPV detection rates between individual studies, we further stratified the results from these studies [1–9, 12–35] by geographic location, histology (benign, recurrent, dysplastic, malignant) and viral subtype. There was insufficient reported data to determine whether age and gender influence HPV prevalence in IP, or by combined geographic and histologic categories. All studies demonstrated a male predominance with a median age in the sixth to seventh decade of life.
The location of each study was classified into one of three geographic locations: North America, Europe, and Asia. Given the large differences in diversity between regions across Asia (e.g., between East Asia and the Middle-East), countries from Asia were restricted to the Orient (East Asia) including Japan and Korea. After omitting a single study from the Middle East (n = 10 cases) [6], we see no significant differences in detection rates in studies conducted in North America (117 positive of 433 cases; weighted prevalence = 27.8%, 95%CI 18.2–37.3%) [4, 12–14, 16, 18–20, 22–27, 29, 30, 33] when compared with Europe (45 positive of 166 cases; weighted prevalence = 36.7%, 95CI 10.9–62.5%) [2, 3, 9, 21, 31, 32, 35] and East Asia (35 positive of 108 cases; weighted prevalence = 34.5%, 95%CI 6.3–62.7%) [1, 5, 28, 34].
HPV Types Distribution in Inverted Papillomas
A preponderance of HPV 6/11 has been detected in IP as compared to HPV 16/18; the overall unadjusted ratio of low-risk to high-risk HPV types is 2.8:1. Low-risk/high-risk coinfections are rare and found in three studies [2, 9, 29]. Several studies also specifically tested for HPV 31/33/35 [12, 16, 20–22, 24, 28, 31] as well as HPV 42–45, 51, 52, 56, 58 [1, 22, 24, 31] with negative results. Ogura [1] found HPV 57 in 1 of 3 cases and Wu [8] detected 57b in 9 of 15 IP studied; however this could not be confirmed by others [30].
HPV is More Commonly Detected in Exophytic Papillomas
HPV is detected more often in exophytic papillomas (EP) than IP. Table 2 summarizes the 15 studies comparing HPV in both IP and EP; some studies also included oncocytic Schneiderian papillomas (OSP). This included studies using only ISH [13, 20, 29, 34] as we believe there is no significant loss of detection sensitivity in this context (Table 1). Table 2 demonstrates a range of detection rates in IP from 0–72%, HPV was found in 61 of 375 cases with a weighted prevalence of 16.9% (95%CI 9.5–24.2%). The HPV detection in EP ranged from 0–100%, HPV was found in 63 of 104 cases with a weighted prevalence of 65.3% (95%CI 44.7–85.9%); and the overwhelming majority detected was HPV 6/11. Eight studies analyzed OSP and found HPV to be absent in all 25 cases tested.
Table 2.
HPV detection rates in different sinonasal papillomas
| Author | Year | Inverted papilloma* | Fungiform papilloma | Oncocytic Schneiderian Papilloma |
|---|---|---|---|---|
| Brandsma | 1987 | 2/9 (22.2%) | 3/4 (75.0%) | – |
| Judd | 1991 | 0/9 (0%) | 3/3 (100%) | 0/3 (0%) |
| McLachlin | 1992 | 4/17 (23.5%) | 3/5 (60.0%) | – |
| Kashima | 1992 | 7/29 (24.1%) | 4/26 (15.4%) | – |
| Sarkar | 1992 | 0/24 (0%) | 1/2 (50.0%) | 0/9 (0%) |
| Tang | 1994 | 0/26 (0%) | 6/7 (85.7%) | – |
| Buchwald | 1995 | 5/57 (8.8%) | 11/16 (68.8%) | 0/5 (0%) |
| Ogura | 1996 | 3/9 (33.3%) | 2/3 (66.7%) | – |
| Gaffey | 1996 | 2/20 (10.0%) | 5/5 (100%) | 0/3 (0%) |
| Mirza | 1998 | 5/28 (17.8%) | 0/1 (0%) | 0/1 (0%) |
| Weiner | 1999 | 6/83 (7.2%) | 17/17 (100%) | 0/2 (0%) |
| Kraft | 2001 | 1/29 (3.5%) | 3/5 (60.0%) | 0/3 (0%) |
| Fischer | 2005 | 4/6 (66.7%) | 2/3 (66.7%) | – |
| Katori | 2006 | 21/29 (72.4%) | 3/7 (42.9%) | – |
| Total | 60/375 (16.0 %) | 63/104 (60.6%) | 0/25 (0%) | |
| Weighted prevalence**(95%CI) | 16.9% (9.5–24.2) | 65.3% (44.7–85.9) | 0% | |
* Includes inverted papillomas with all histologies, including SCC-ex-inverted papilloma
** Weighted prevalence estimated by random-effects inverse-variance with continuity correction
HPV is More Often Detected in Dysplastic, Malignant and Recurrent IP
Table 3 summarizes the reported data on HPV positive IPs that included sufficient information regarding dysplasia and malignant progression with respect HPV viral type. We see that HPV detection rates significantly increase (Wald t-test p [ 0.02) in IPs with high-grade dysplasia (weighted prevalence of 55.8%, 95%CI 30.5–81.0%) and carcinoma (weighted prevalence of 55.1%, 95%CI 37.0–73.2%) as compared to IPs with no dysplasia or mild dysplasia (weighted prevalence of 22.3%, 95%CI 15.9–28.6%). Furthermore, the preponderance of low-risk HPV in benign IP (ratio low-risk/high-risk = 4.8:1) shifts in dysplastic and malignant IP. The low-risk/high-risk ratio is 1.1:1 for IPs with high-grade dysplasias, this ratio is inverted to favor high-risk HPV (1:2.4) for malignant IP.
Table 3.
Association of HPV in benign versus dysplastic versus malignant IP [2, 5, 12, 14, 21–25, 26–29, 31, 33]
| Diagnosis | Overall HPV | HPV 6/11 positive cases/Total | HPV 16/18 positive cases/Total | |||
|---|---|---|---|---|---|---|
| Pos/total | Weighted prevalence (95%CI)* | Pos/total | Pooled prevalence (range) | Pos/total | Pooled prevalence (range) | |
| IP, no dysplasia or low-grade dysplasia | 129/597 | 22.3% (15.9–28.6) | 102/597 | 17.1% (0–76.9%) | 21/597 | 3.5% (0–33.3%) |
| IP with moderate to severe dysplasia | 32/54 | 55.8% (30.5–81.0) | 17/54 | 31.5% (0–66.7%) | 15/54 | 27.8% (0–100%) |
| IP with SCC | 34/65 | 55.1% (37.0–73.2) | 9/65** | 13.8% (0–100%) | 22/65** | 33.8% (0–100%) |
* Weighted prevalence estimated by random-effects inverse-variance with continuity correction
** One additional carcinoma was co-infected with 6/11 and 16/18, and the remaining HPV in a carcinoma was untyped
We identified a total of 230 sinonasal squamous carcinomas, unrelated to IP, that were studied for HPV; these reports included sufficient information regarding the histological or clinical absence of IP [2, 3, 5, 9, 19, 25, 26, 35, 38–43]. HPV was detected in 46/230 of these carcinomas unrelated to IP, with a weighted prevalence of 21.8% (95%CI 11.6–31.9%). In most cases, high-risk HPV 16/18 are detected. This proportion is significantly lower than the rate of HPV detection in sinonasal squamous cell carcinomas that were associated with IP (weighted prevalence 55.1%, 95%CI 37.0–73.2).
We also investigated the association between HPV detection and disease recurrence in IP. Table 4 summarizes the data with respect to (benign) disease recurrence and HPV status from 9 studies that also included genotype data for recurrent IP [1, 2, 9, 12, 17, 25, 27, 28, 44]. Recurrences developed in 44 of 236 patients; HPV was detected in 27 of 44 IPs (weighted proportion of 57.9%, 95%CI 31.6–84.2%) that developed recurrences and in 24 of 192 IPs (weighted proportion of 9.7%, 95%CI 4.4–15.0%) that did not develop recurrence. The presence of HPV was significantly associated with the likelihood of developing recurrence (weighted OR of 10.2, 95%CI 3.2–32.8).
Table 4.
| HPV positive | HPV negative | Crude OR (95%CI) | Weighted OR* (95%CI) | |
|---|---|---|---|---|
| No recurrence (n = 192) | 24 | 168 | (Reference) | (Reference) |
| Recurrent IP (n = 44) | 27 | 17 | 11.1 (5.3–23.4) | 10.2 (3.2–32.8) |
* Weighted odds ratio (OR) and 95% confidence intervals (CI) estimated by random-effects inverse-variance method with continuity correction
Discussion
Methodology Does Not Impact HPV Detection Rates for IP
The widely disparate HPV detection rates reported for IP served as the impetus for this review. Our detailed literature analysis confirmed a wide range of detection rates for studies utilizing ISH, and PCR, either with type-specific probes or consensus probes. Interestingly, detection sensitivity has increased over the decades and the number of published reports has also increased, yet we see no increase in the overall range of HPV detection rates. Attempts to explain these divergent HPV infection rates generally focus on technical issues such as the nature of the specimens and detection method used. Technology has progressed from direct genome detection (dot blot, ISH) to target amplification (PCR) with the potential for tremendous increased sensitivity. For example, ISH is said to require 50 viral copies per nucleus for detection, as compared to PCR, which can detect a single viral copy per sample. ISH has the advantage of confirming target localization. PCR is generally touted as being superior to ISH due to its inherent increased detection sensitivity, yet this is not evident from our review. Five studies from Table 1 directly compared HPV detection in IP by ISH and PCR; two studies were uniformly negative [16, 18], two demonstrated increased HPV detection by ISH [17, 21] and one study demonstrated a non-significant increase in HPV detection by PCR as compared to ISH (23% versus 20% respectively) [12]. It should be noted that we cannot rule out the possibility that the differences in prevalence with respect to detection method may be due to geographic or histologic differences between study populations. Nonetheless, prevalence overall was slightly higher for ISH despite that studies involving IP with dysplasia or carcinoma were more likely to have used PCR than ISH.
Theoretically, PCR with consensus primers would cast a “wider detection net” for exploratory studies, as compared to type-specific oligonucleotide primer pairs. The use of HPV consensus primers, amplifies a highly conserved L1 region of HPV 1A, 2, 5, 6, 8, 11, 13, 16/18, 26, 27, 30, 31/33, 35, 39, 40, 41, 42, 44, 45, 47, 48, 51, 52, 53, 54, 55, 57, 58, 59, as well as uncharacterized types. If IPs were generally associated with HPV types other than HPV 6/11 or 16/18, then PCR with consensus primers would result in a significantly higher detection rate. Table 1 demonstrates that the use of consensus primers did not increase detection rates as compared to type-specific primers. However, a caveat is that HPV typing following PCR with consensus primers was usually performed only in cases positive by consensus primers. In total, there have been 11 PCR studies using consensus primers, all for the L1 domain. As the L1/L2 regions, as well as E1 through E5 regions may be lost upon viral integration, using only L1 primer (such as MY09/MY11) may not detect integrated HPV. The E6 region of HPV, however, is not usually lost. Therefore, a combination of early and late region primers has been recommended to increase detection sensitivity [45].
Specimen type and fixation, and length of tissue storage, are factors that affect DNA preservation and retrieval for PCR. DNA extraction from snap frozen tissue generally leads to better samples, and thus better greater sensitivity for molecular studies. Since IP are rare, collection of frozen tissue is not feasible and virtually all studies used formalin-fixed paraffin embedded archival tissue. The protein-DNA and DNA-DNA cross-links formed with formalin fixation hamper DNA extraction for the initial steps of PCR. Only two PCR based studies used prospectively collected frozen tissue samples [32, 35] with differing detection rates (4/6 or 67% and 3/26 or 12% respectively).
Primer pairs that target large DNA fragments (e.g. MY09/MY11 which form 450 bp amplicons) tend to perform poorly in archival samples compared with primers targeting shorter segments (e.g. GP5/GP6, which form 140 bp amplicons). Only one study compared these two primer sets for IP, and confirmed the increased detection sensitivity using the GP5/GP6 probes [30]. Increasing specimen age also impacts detection sensitivity; Kashima reported the HPV detection rate in sinonasal papillomas to decline from 20% in one-year old archival specimens to 2% in 6 year-old archival specimens [19].
It is unlikely that institutional factors impact the HPV detection rates in IPs. Head and neck SCC, for instance, have inherent biological variations due to tumor subsite. Referral patterns for different medical institutions and inherent population differences can impact stage at diagnosis and outcome. By contrast, IP tend to be an anatomically and histologically uniform group of neoplasia. They are uncommon and most studies are retrospective, studying archival tissue, which has inherent limitations, as discussed above.
Latent Sinonasal HPV Infection is Rare
Studies addressing HPV latency in the oral cavity demonstrate a bimodal age distribution, peaking among neonates, and then again in adolescents and young adults. Asymptomatic HPV infection in children younger than one year of age most likely represents vertical transmission [46]. A recent meta-analysis investigating neonatal oral and/or genital HPV recovery rates at birth found a pooled mother-to-child transmission rate of 18% for vaginal delivery and 8% for Cesarean section for HPV positive mothers [47]. The pooled relative risk for neonatal HPV exposure is 4.8 (95% confidence interval: 2.1–10.9) for HPV positive mothers. However the actual HPV infection rate following vertical transmission is unknown, but assumed to be very low. With respect to sinonasal infection, HPV has been recovered from neonatal nasopharyngeal secretions of 47% of vaginally delivered babies with HPV positive mothers [48]. As infants become older, the rate of latent oral HPV infection decreases, therefore, latent neonatal infection is probably cleared or the viral load is reduced to undetectable levels.
A number of studies have addressed the issue of latent sinonasal HPV infection beyond the neonatal period. HPV was found in only one of a total of 216 sinonasal mucosal samples, and in none of the 91 sinonasal polyps or sinusitis samples studied [2, 5, 8, 12, 13, 15, 17, 24, 30, 33, 35, 49–51]. The study by Bryan appears to be an outlier with an unusually high rate of HPV 6/11 detection in nasopharyngeal mucosa (9/15 or 60%) [3]. Only two studies from patients with IP specifically document the presence of HPV in Schneiderian mucosa in two samples procured adjacent to IP [13, 33]. Overall, we can conclude that latent HPV infection is highly uncommon to normal adult Schneiderian mucosa.
HPV is More Often Detected in Dysplastic and Malignant IP
HPV detection rates increase dramatically, when one stratifies HPV detection in IP for the presence of moderate-severe dysplasia and carcinomas arising in IP, (Ca-ex-IP). Caruana detected HPV in 29% (2/7) benign IP, 50% (4/8) dysplastic IP and in 50% (2/4) of Ca-ex-IP [26]. Likewise, Beck detected HPV in 54% (12/22) of IP, 71% (5/7) of dysplastic IP, and 70% (7/10) of Ca-ex-IP [22]. By contrast, the rate of HPV detection in sinonasal SCC that are unrelated to IP is lower (22.3%, 95%CI 15.9–28.6). Interestingly, El-Mofty noted that the HPV positive carcinomas tended to have a nonkeratinizing basaloid histomorphology, akin to the histology of HPV positive tonsillar carcinomas [42].
HPV Detection as a Predictor of Recurrence
It has been stated that HPV detection correlates with IP recurrence. All 4 recurrent cases of Siivonen were HPV positive (3 HPV 11, 1 HPV 16) [10]. Beck reported recurrence in 13 of 15 HPV positive IP patients and in none of 10 HPV negative patients [44]. Investigating the association between HPV detection and disease recurrence across studies in this review, we find that the presence of HPV in an IP is significantly associated with the likelihood of developing recurrence in IP.
Inverted Papilloma Represents a Benign Neoplastic Process
IP represent a benign proliferative neoplastic process. Califano demonstrated clonality with respect to X chromosome inactivation in all 4 informative IPs arising in women [52]. Loss of heterozygosity (LOH) was not detected in any IP, including those with dysplasia. Six patients in this study progressed to develop SCC; all of which demonstrated LOH of at least one microsatellite locus whereas the corresponding IPs did not contain any LOH.
In malignancies, the p53 gene status and HPV status can discern different pathways of carcinogenesis (wild type p53/high-risk HPV positive tumors versus mutated p53/HPV negative tumors). One might query as to whether genetic differences exist between benign IPs containing HPV and those which do not, as that might speak to different etiologies. However, there is no evidence for benign IP containing p53 mutations. Caruana studied 6 benign IP, and 2 of them were HPV positive, yet all had wild-type p53 sequenced; p53 mutations were found in dysplastic (3 of 8) and malignant (1 of 4) IPs [26].
HPV Integration in IP
The role of HPV integration in IP has been addressed in one study that found evidence supporting integration in one benign IP and two SCC-ex-IP [33]. Additional studies validating the issue of viral integration in IP would be interesting and necessary. Taken together, the above data support the hypothesis that IP represents a clonal tumor process, which requires additional promoting steps prior to malignant transformation. There is no data to explain why many benign IP remain HPV negative.
Does HPV produce a “Hit and Run” Phenomenon in the Pathogenesis of IP?
Viruses can transform or immortalize cell lines, but are not necessary for the maintenance of this phenotype, this has been referred to as the “Hit and Run” phenomenon [53]. Loss of HPV 18 from previously transfected, immortalized, transformed cell lines is consistent with this, vis-à-vis the maintenance of malignant phenotype [53]. The inability to detect either viral protein or genome does not necessarily negate a viral role in initiation.
We hypothesize that HPV is responsible for the initiation of IP but is not necessary for its maintenance. We offer the following explanation for the differences in HPV detection rates as detailed above. (Fig. 1) Low-risk HPV is detected in the majority of exophytic papillomas. The hyperkeratotic superficial epithelium of EP is permissive for HPV replication. We hypothesize that HPV reinfection of EP by assembled virions is a common event accounting for the high detection rate of low-risk HPV in EP. By contrast, for unknown reasons, IP tend to be nonkeratinizing; thus we would not expect much viral replication. As superficial IP epithelium is shed, HPV can be lost, accounting for the low HPV 6/11 detection rate in benign IP. Other steps are necessary for malignant IP progression; this would include either primary or secondary infection with high-risk (oncogenic) HPV infection and HPV integration. If viral integration is more common in dysplastic and malignant IP, this would explain the greater HPV 16/18 detection rates demonstrated in these lesions.
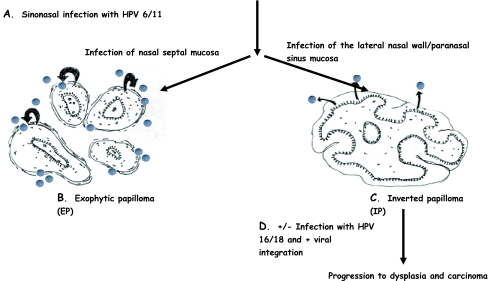
Fig. 1.
Hit and Run Hypothesis for HPV infection and Schneiderian papillomas. Figure hypothesized sequence of events in the pathogenesis of IP and EP. (A) Establishment of sinonasal HPV 6/11 infection is necessary for initiation. The biological and environmental factors which predispose an individual and particular sinonasal subsite towards infection are unknown. (B) In the nasal septum, the lamina propria is not loose and distensible, and the seromucinous ducts and glands are less concentrated as compared to the lateral nasal wall and paranasal sinus mucosa. HPV-induced epithelial hyperplasia of septal mucosa results in an exophytic growth pattern with fibrovascular cores, as seen in this diagram. EP tends to be keratinizing, the reasons for this are uncertain. The hyperkeratotic environment is permissive for viral replication (represented by grey spheres). Shedding superficial epithelial cells release assembled virions which reinfect the EP (black curved arrows); thus HPV 6/11 is commonly detected in EP. (C) The architecture of the lateral nasal wall and paranasal sinuses allows for HPV infection here to cause polypoid masses with squamous metaplasia and hyperplasia of the underlying seromucinous ducts, as seen in this diagram. For unknown reasons, IP tend to be nonkeratinizing; thus we would not predict viral replication and re-infection. As superficial epithelial cells are shed, HPV (grey spheres) can be lost from IP (black arrows), accounting for the low HPV 6/11 detection rate in IP. (D) Other steps are necessary for the progression to dysplasia and malignancy; they would include secondary infection with HPV 16/18 and/or integration of HPV 6/11 and/or HPV 16/18
Conclusion
The HPV detection rates in IPs vary widely. Increased assay sensitivity has not substantially changed the HPV detection rate in IP; we believe that this is not a function of limited detection sensitivity and it seems unlikely that future technologies will reveal new paradigms with respect to HPV detection rates in IP.
This review confirms that EP is associated with consistently higher HPV detection rates than IP. We believe that as EP are permissive for viral replication, resulting in epithelial re-infection and accounting for the high HPV DNA detection rates. As IP are generally non-keratinizing, they may not be able to maintain HPV infection. Therefore we hypothesize that low-risk HPV may induce IP formation, and then are lost as infected cells are shed. Interestingly, the HPV detection rates increase in dysplastic IP and SCC-ex-IP with increasing ratio of high-risk to low-risk HPV types, compared to nondysplastic IP. We believe that one explanation for the variation in HPV detection rates between different studies may be the actual histologic composition of the cohort. That is, if one series contains a higher frequency of dysplastic and malignant IP, it may have a higher detection rate than another series which contains only nondysplastic IP.
Dysplasia in IP results from either primary or secondary infection with high-risk HPV. Progression to malignancy requires additional steps such as loss of tumor suppressor genes and viral integration. The rate of integration may explain why HPV is detected in the majority of tonsillar SCC, despite the fact that these cancers are usually nonkeratinizing. Although HPV integration is not necessary for carcinogenesis, it is a common event in tonsillar SCC [54, 55]. Additional studies are needed to demonstrate the temporal association between viral integration and progression to dysplasia in IP. The implication of the present study is that HPV sub-type testing may identify patients at risk for recurrence, or progression to dysplasia and malignancy, and thus may impact surveillance protocols.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Appendix
Detection Methodology for HPV in Inverted Papillomas
| Author | Year | Target | Method* | HPV types | Positive cases/Subjects (%)* | Median Age | M:F | Comments |
|---|---|---|---|---|---|---|---|---|
| Respler | 1987 | DNA | SB | 11 | +1/2 (50%) | 12.5 | 1:1 | |
| Syrjanen | 1987 | DNA | ISH | 6/11/16/18 | 5/14 HPV 11 (36%) | 58 | 2:1 | 4 coinfections |
| 1/14 HPV 16 (7%) | 1 recurrence | |||||||
| 4/14 HPV 11 and 16 (29%) | ||||||||
| Brandsma | 1987 | DNA | SB, ISH | 2, 6/11, 16/18 | +2/9 HPV 11 (22% by SB) | NA | +1 HPV 6 (ISH) probably exophytic papilloma | |
| +1/6 HPV 6 ( 17% by ISH) | ||||||||
| Weber | 1988 | DNA | ISH | 6b, 11 | +16/21 (76%) | 45 | 2.5:1 | +1 case: with SCC, +3 cases with “atypia” |
| Brandwein | 1989 | RNA | ISH | 6/11, 16/18 | +3/7 (43%) HPV 6/11 | 60 | 6:1 | +3 cases with dysplasia +1 case:with SCC |
| +2/7 (29%) HPV 16/18 | ||||||||
| Siivonen | 1989 | DNA | ISH | 6/11, 16 | +8/21 (38%) HPV11 | 56 | 3.2:1 | +3 cases: with SCC |
| +8/21 (38%) HPV 16 | 3 cases with coinfection | |||||||
| Ishibashi | 1990 | DNA | SB | 6/11, 16/18 | +1/7 (14%) HPV 6 | 61 | 1.3:1 | |
| Bryan | 1990 | DNA | PCR | 6/11 | +10/13 (77%) HPV 6/11 | |||
| Judd | 1991 | DNA | ISH, PCR | 6/11, 16/18, 33 | 0/9 HPV+ | 67 | 1:1.3 | |
| Furuta | 1991 | DNA | ISH, PCR, DB | 6/11, 16/18 | +3/26 (12%) HPV 11 (DB & ISH) | NA | +1 case by DB & ISH with SCC. 1 additional IP with | |
| +1/26 (4%) HPV 16 (DB & ISH) | SCC + HPV 16 by PCR | |||||||
| Sarkar | 1992 | DNA | ISH, PCR | 6b/11, 16/18 | 0/24 HPV+ | |||
| McLachlin | 1992 | DNA | ISH, PCR | 6/11, 16/18, 31/33/35 | +3/17 (18%) HPV 6/11 | 57 | 14:1 | +1 case with dysplasia +1 HPV 16 case with SCC |
| +1/17 (6%) HPV 16 | ||||||||
| Kashima | 1992 | DNA | PCR | 6/11, 16/18 | +7/29 (24%) HPV 6/11 | NA | ||
| Wu | 1993 | RNA | ISH, PCR, SB | 57b | +12/15 (80%) HPV 57b | NA | +1 case with dysplasia +2 cases with SCC | |
| Tang | 1994 | DNA | ISH | 6/11, 16/18, 31/33/35 | 0/26 | NA | ||
| Buchwald | 1995 | DNA | ISH, PCR | 6/11, 16/18, 31/33/35, 45, 51, 52 consensus L 1 (MY09/MY11) | +4/57 HPV 6/11 (7%) | NA | +2 cases with SCC | |
| +1/57 HPV 18 (2%) | ||||||||
| Beck | 1995 | DNA | PCR | 6/11, 16/18, 31/33, 35, 57 consensus L1, MY09/MY11, E6 (WD72, WD76, WD66, WD67, WD154) | +18/39 (46%) HPV 6/11 | 59 mean | 2.6:1 | +5 with dysplasia +10 with SCC |
| +5/39 (13%) HPV 16/18 | ||||||||
| MacDonald | 1995 | DNA | PCR | 6/11, 16/18 | +8/20 (40%) HPV 6/11 | NA | +1 case with SCC | |
| +1/20 (5%) HPV 16 | ||||||||
| Gaffey | 1996 | DNA, RNA | ISH | 6/11, 16/18, 31/33, 35, 42–45, 51, 52, 56 | +1/20 (5%) HPV 16 | NA | +1 HPV 11 case with SCC | |
| +1/20 (5%) HPV 11 | ||||||||
| Shen | 1996 | DNA | PCR | 6/11, 16/18 consensus L1, MY09/MY11 | +17/46 (37%) HPV 6/11 | NA | Includes +1/6 with SCC (HPV 16) | |
| +1/46 (2%) HPV 16 | ||||||||
| Ogura | 1996 | DNA | PCR | 2a, 5b, 6b, 11, 16/18, 57, 58 | +2/9 (22%) HPV 16 | 51 | 1.4:1 | |
| +1/9 (11%) HPV 57 | ||||||||
| Caruana | 1997 | DNA | PCR | 6b, 11, 16/18, 31/33 consensus L1, MY09/MY11 | +2/19 (11%) HPV 6b/11 | 64 | 1.6:1 | +4/8 cases with dysplasia +2/4 cases with SCC |
| +7/19 (37%) HPV 16 | ||||||||
| Bernauer | 1997 | DNA | PCR | 6/11, 16/18 consensus L1, MY09/MY11 | +7/22 (33%) consensus primers | 53 | 2.5:1 | +2/2 cases with SCC, one of which was HPV 18 |
| +1/22 (5%) HPV 18 | ||||||||
| Hwang | 1998 | DNA | PCR | 6/11, 16/18, 33 | +3/42 (7%) HPV 6/11 + 2/42 (5%) HPV 16 | NA | +1 case with dysplasia +2 cases with SCC | |
| Mirza | 1998 | DNA | ISH | 6/11, 16/18 | +5/28 (18%) HPV 6/11 | NA | +1 case with dysplasia | |
| +2/28 (7%) HPV 16/18 | +1 case with SCC | |||||||
| +1 case with low-risk/high-risk coinfection | ||||||||
| Kassim | 1998 | DNA | PCR | 16 | 4/10 (40%) HPV 16 | 49 | All male | |
| Saegusa | 1999 | DNA | PCR | 16/18 | +6/28 (36%) HPV 16/18 | NA | ||
| Weiner | 1999 | DNA | PCR | 6b/11, 16/18 consensus L1, MY09/MY11, GP5+/GP6+ | +3/83 (4%) HPV 11 | NA | includes +1/7 with SCC. 6 SCC arose in IP, 1 arose in an oncocytic papilloma, derivation of one positive case not specified | |
| +2/83 (3%) HPV 16 | ||||||||
| +1/83 (1%) HPV 18 | ||||||||
| Kraft | 2001 | DNA | ISH, PCR | 6/11, 16/18, 31/33/51 consensus L1, MY09/MY11 | +1/29 (3%) HPV 11 | NA | ||
| Fischer | 2005 | DNA | PCR | consensus L1, CP66F, CP69F | +4/6 (67%) HPV+ | NA | Sequencing in +3 cases: homology with HPV 21/34/56/60/66/80 | |
| McKay | 2005 | DNA | PCR | 6/11, 16/18, 31/33, 35, 45, 52, consensus L1, MY09/MY11 | +1/14 (7%) HPV 11 + 2/14 (14%) HPV 18 | NA | HPV integration present in 4 cases with severe dysplasia or SCC | |
| Katori | 2006 | DNA | ISH | +12/29 (41%) HPV 6/11 | 52 mean | 1.9:1 | +5 cases with severe dysplasia and +4 cases with SCC. No mention of coinfection, we assume there was none | |
| +9/29 (31%) HPV 16/18 | ||||||||
| Hoffman | 2006 | DNA | PCR | 6/11, 16, consensus L1/L2, MY09/MY11 | +3/26 (12%) HPV 6/11 | 61 | 1.9:1 | |
| +2/26 (8%) other IP with consensus primers | ||||||||
* ISH––in situ hybridization; PCR––polymerase chain reaction; SB––southern blot; SCC––squamous cell carcinoma
References
- 1.Ogura H, Fujiwara T, Hamaya K, Saito R. Detection of HPV type 57 in a case of inverted nasal papillomatosis in Japan. Eur Arch Otorhinolaryngol. 1995;252:513–5. doi: 10.1007/BF02114763. [DOI] [PubMed] [Google Scholar]
- 2.Siivonen L, Virolainen E. Transitional papilloma of the nasal cavity and paranasal sinuses. ORL J Otorhinolaryngol Relat Spec. 1989;51:262–7. doi: 10.1159/000276071. [DOI] [PubMed] [Google Scholar]
- 3.Bryan RL, Bevan IS, Crocker J, Young LS. Detection of HPV 6 and 11 in tumours of the upper respiratory tract using the PCR. Clin Otolaryngol Allied Sci. 1990;15:177–80. doi: 10.1111/j.1365-2273.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 4.Weber RS, Shillitoe EJ, Robbins KT, Luna MA, Batsakis JG, Donovan DT, Adler-Storthz K. Prevalence of HPV in inverted papillomas. Arch Otolaryngol Head Neck Surg. 1988;114:23–6. doi: 10.1001/archotol.1988.01860130027009. [DOI] [PubMed] [Google Scholar]
- 5.Saegusa M, Nitta H, Hashimura M, Okayasu I. Down-regulation of p27Kip1 expression is correlated with increased cell proliferation but not expression of p21waf1 and p53, and HPV infection in benign and malignant tumours of sinonasal regions. Histopathology. 1999;35:55–64. doi: 10.1046/j.1365-2559.1999.00688.x. [DOI] [PubMed] [Google Scholar]
- 6.Kassim SK, Ibrahim SA, Eissa S, Zaki SS, El-Begermy MA, Abdou MH, Hassan MI, Khalifa A. Epstein Barr virus, HPV, and flow cytometric cell cycle kinetics in nasopharyngeal carcinoma and inverted papilloma among Egyption patients. Dis Markers. 1998;14:13–20. doi: 10.1155/1998/260392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Respler DS, Jahn A, Pater MM. Isolation and characterization of papillomavirus DNA from nasal inverting (Schneiderian) papillomas. Ann Otol Rhinol Laryngol. 1987;96:170–3. doi: 10.1177/000348948709600206. [DOI] [PubMed] [Google Scholar]
- 8.Wu TC, Trujillo JM, Kashima HK, Mounts P. Association of HPV with nasal neoplasia. Lancet. 1993;341:522–4. doi: 10.1016/0140-6736(93)90280-T. [DOI] [PubMed] [Google Scholar]
- 9.Syrjänen S, Happonen RP, Virolainen E, Siivonen L, Syrjänen K. Detection of HPV structural antigens and DNA types in inverted papillomas and squamous cell carcinomas of nasal cavities and paranasal sinuses. Acta Otolaryngol (stockh). 1987;104:334–41. doi: 10.3109/00016488709107337. [DOI] [PubMed] [Google Scholar]
- 10.Strauss M, Jenson AB. HPV in various lesions of the head and neck. Otolaryngol Head Neck Surg. 1985;93:342–6. doi: 10.1177/019459988509300310. [DOI] [PubMed] [Google Scholar]
- 11.Gaito RA, Gaylord WH, Hilding DA. Ultrastructure of a human nasal papilloma. Laryngoscope. 1965;75:144–52. doi: 10.1288/00005537-196501000-00016. [DOI] [PubMed] [Google Scholar]
- 12.McLachlin CM, Kandel RA, Colgan TJ, Swanson DB, Witterick IJ, Ngan BY. Prevalence of HPV in sinonasal papillomas: a study using PCR and in-situ hybridization. Mod Pathol. 1992;5:406–9. [PubMed] [Google Scholar]
- 13.Brandsma JL, Abramson AL, Scuibba J, Shah K, Barrezuela N. Papillomavirus infection of the nose. In: Steinberg BM, Brandsma JL, Taichman LP, editors. Cancer cells 5: papillomaviruses. Cold Spring Harbor, NY: Cold Spring harbor Press; 1987. p. 301. [Google Scholar]
- 14.Brandwein M, Steinberg B, Thung S, Biller H, Dilorenzo T, Galli R. HPV 6/11 and 16/18 in Schneiderian inverted papillomas. Cancer. 1989;63:1708–13. doi: 10.1002/1097-0142(19900501)63:9<1708::AID-CNCR2820630911>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi T, Tsunokawa Y, Matsushima S, Nomura Y, Sugimura T, Terada M. Presence of HPV type-6-related sequences in inverted nasal papillomas. Eur Arch Otorhinolaryngol. 1990;247:296–9. doi: 10.1007/BF00176541. [DOI] [PubMed] [Google Scholar]
- 16.Judd R, Zaki SR, Coffield LM, Evatt BL. Sinonasal papillomas and HPV: HPV 11 detected in fungiform Schneiderian papillomas by in-situ hybridization and PCR. Hum Pathol. 1991;22:550–6. doi: 10.1016/0046-8177(91)90231-D. [DOI] [PubMed] [Google Scholar]
- 17.Furuta Y, Shinohara T, Sano K, Nagashima K, Inoue K, Tanaka K, Inuyama Y. Molecular pathologic study of HPV infection in inverted papilloma and squamous cell carcinoma of the nasal cavities and paranasal sinuses. Laryngoscope. 1991;101:79–85. doi: 10.1288/00005537-199101000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar FH, Visscher DW, Kintanar EB, Zarbo RJ, Crissman JD. Sinonasal Schneiderian papillomas: HPV typing by PCR. Mod Pathol. 1992;5:329–32. [PubMed] [Google Scholar]
- 19.Kashima HK, Kessis T, Hruban RH, Wu TC, Zinreich SJ, Shah KV. HPV in sinonasal papillomas and squamous cell carcinoma. Laryngoscope. 1992;102:973–6. doi: 10.1288/00005537-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Tang AC, Grignon DJ, MacRae DL. The association of HPV with Schneiderian papillomas: a DNA in situ hybridization study. J Otolaryngol. 1994;23:292–7. [PubMed] [Google Scholar]
- 21.Buchwald C, Franzmann MB, Jacobsen GK, Lindeberg H. HPV in sinonasal papillomas: a study of 78 cases using in situ hybridization and PCR. Laryngoscope. 1995;105:66–71. doi: 10.1288/00005537-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Beck JC, McClatchey KD, Lesperance MM, Esclamado RM, Carey TE, Bradford CR. HPV types important in progression of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113:558–63. doi: 10.1016/S0194-5998(95)70046-3. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald MR, Le KT, Freeman J, Hui MF, Cheung RK, Dosch HM. A majority of inverted sinonasal papillomas carries Epstein-Barr virus genomes. Cancer. 1995;75:2307–12. doi: 10.1002/1097-0142(19950501)75:9<2307::AID-CNCR2820750920>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Gaffey MJ, Frierson HF, Weiss LM, Barber CM, Baber GB, Stoler MH. HPV and Epstein-Barr virus in sinonasal Schneiderian papillomas. An in situ hybridization and PCR study. Am J Clin Pathol. 1996;106:475–82. doi: 10.1093/ajcp/106.4.475. [DOI] [PubMed] [Google Scholar]
- 25.Shen J, Tate JE, Crum CP, Goodman ML. Prevalence of HPV in benign and malignant tumors of the upper respiratory tract. Mod Pathol. 1996;9:15–20. [PubMed] [Google Scholar]
- 26.Caruana SM, Zwiebel N, Cocker R, McCormick SA, Eberle RC, Lazarus P. p53 alteration and HPV infection in paranasal sinus cancer. Cancer. 1997;79:1320–8. doi: 10.1002/(SICI)1097-0142(19970401)79:7<1320::AID-CNCR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Bernauer HS, Welkoborsky HJ, Tilling A, Amedee RG, Mann WJ. Inverted papillomas of the paranasal sinuses and the nasal cavity: DNA indices and HPV infection. Am J Rhinol. 1997;11:155–60. doi: 10.2500/105065897782537160. [DOI] [PubMed] [Google Scholar]
- 28.Hwang CS, Yang HS, Hong MK. Detection of HPV in sinonasal inverted papillomas using PCR. Am J Rhinol. 1998;12:363–6. doi: 10.2500/105065898780182499. [DOI] [PubMed] [Google Scholar]
- 29.Mirza N, Montone K, Sato Y, Kroger H, Kennedy DW. Identification of p53 and HPV in Schneiderian papillomas. Laryngoscope. 1998;108:497–501. doi: 10.1097/00005537-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Weiner JS, Sherris D, Kasperbauer J, Lewis J, Li H, Persing D. Relationship of HPV to Schneiderian papillomas. Laryngoscope. 1999;109:21–6. doi: 10.1097/00005537-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Kraft M, Simmen D, Casas R, Pfaltz M. Significance of HPV in sinonasal papillomas. J Laryngol Otol. 2001;115:709–14. doi: 10.1258/0022215011908955. [DOI] [PubMed] [Google Scholar]
- 32.Fischer M. Investigation of a broad-spectrum PCR assay for HPV in screening benign lesions of the upper aerodigestive tract. ORL J Otorhinolaryngol Relat Spec. 2005;67:237–41. doi: 10.1159/000089347. [DOI] [PubMed] [Google Scholar]
- 33.McKay SP, Gregoire L, Lonardo F, Reidy P, Mathog RH, Lancaster WD. HPV transcripts in malignant inverted papilloma are from integrated HPV DNA. Laryngoscope. 2005;115:1428–31. doi: 10.1097/01.mlg.0000168091.50584.b4. [DOI] [PubMed] [Google Scholar]
- 34.Katori H, Nozawat A, Tsukuda M. Relationship between p21 and p53 expression, HPV infection and malignant transformation in sinonasal-inverted papilloma. Clin Oncol (R Coll Radiol). 2006;18:300–5. doi: 10.1016/j.clon.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman M, Klose N, Gottschlich S, Görögh T, Fazel A, Lohery C. Detection of HPV DNA in benign and malignant sinonasal neoplasms. Cancer Lett. 2006;239:64–70. doi: 10.1016/j.canlet.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Breslow NE, Day NE. Statistical methods in cancer research. Vol I: the analysis of case-control data. Lyon: IARC; 1980. [PubMed] [Google Scholar]
- 37.Rothman Greenland JR S. Modern epidemiology. 2. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 38.Furuta Y, Takasu Y, Asai T, Shinohara T, Sawa H, Nagashima K, Inuyama Y. Detection of HPV DNA in carcinomas of the nasal cavities and paranasal sinuses by PCR. Cancer. 1992;69:353–7. doi: 10.1002/1097-0142(19920115)69:2<353::AID-CNCR2820690213>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 39.Judd R, Zaki S, Coffield LM, Evatt BL. HPV type 6 detected by the PCR in invasive sinonasal papillary squamous cell carcinoma. Arch Pathol Lab Med. 1991;115:1150–3. [PubMed] [Google Scholar]
- 40.Tyan YS, Liu ST, Ong WR, Chen ML, Shu CH, Chang YS. Detection of EBV and HPV in head and neck tumors. J Clin Microbiol. 1993;31:53–6. doi: 10.1128/jcm.31.1.53-56.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchwald C, Lindeberg H, Pedersen BL, Franzmann MB. Human papilloma virus and p53 expression in carcinomas associated with sinonasal papillomas: a Danish epidemiological study 1980–1998. Laryngoscope. 2001;111:1104–10. doi: 10.1097/00005537-200106000-00032. [DOI] [PubMed] [Google Scholar]
- 42.El-Mofty S, Lu DW. Prevalence of high-risk HPV DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract. Am J Surg Pathol. 2005;29:1367–72. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 43.Mineta H, Ogino T, Amano HM, Ohkawa Y, Araki K, Takebayashi S, Miura K. Human papilloma virus (HPV) type 16 and 18 detected in head and neck squamous cell carcinoma. Anticancer Res. 1998;18:4765–8. [PubMed] [Google Scholar]
- 44.Beck JC, McClatchey KD, Lesperance MM, Esclamado RM, Carey TE, Bradford CR. Presence of HPV predicts recurrence of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113:49–55. doi: 10.1016/S0194-5998(95)70144-3. [DOI] [PubMed] [Google Scholar]
- 45.Tate JE, Yang YC, Shen J, McLachlin CM, Sheets EE, Crum CP. A comparison of early (E7) and late (L1) primer-mediated amplification of papillomaviral DNA in cervical neoplasia. Mol Cell Probes. 1996;10:347–51. doi: 10.1006/mcpr.1996.0047. [DOI] [PubMed] [Google Scholar]
- 46.Smith EM, Swarnavel S, Ritchie JM, Wang D, Haugen TH, Turek LP. Prevalence of human papillomavirus in the oral cavity/oropharynx in a large population of children and adolescents. Pediatr Infect Dis J. 2007;26:836–40. doi: 10.1097/INF.0b013e318124a4ae. [DOI] [PubMed] [Google Scholar]
- 47.Medeiros LR, Ethur AB, Hilgert JB, Zanini RR, Berwanger O, Bozzetti MC, Mylius LC. Vertical transmission of the HPV a systematic quantitative review. Cad Saude Publica. 2005;21:1006–15. doi: 10.1590/S0102-311X2005000400003. [DOI] [PubMed] [Google Scholar]
- 48.Sedlacek TV, Lindheim S, Eder C, Hasty L, Woodland M, Ludomirsky A, Rando RF. Mechanism for HPV transmission at birth. Am J Obstet Gynecol. 1989;161:55–9. doi: 10.1016/0002-9378(89)90232-9. [DOI] [PubMed] [Google Scholar]
- 49.Buchwald C, Franzmann MB, Jacobsen GK, Lindeberg H. HPV and normal nasal mucosa: detection of HPV DNA in normal nasal mucosa biopsies by PCR and in situ hybridization. Laryngoscope. 1994;104:755–7. doi: 10.1288/00005537-199406000-00018. [DOI] [PubMed] [Google Scholar]
- 50.Fukushima K, Ogura H, Watanabe S, Yabe Y, Masuda Y. HPV type 16 DNA detected by the PCR in non-cancer tissues of the head and neck. Eur Arch Otorhinolaryngol. 1994;251:109–12. doi: 10.1007/BF00179903. [DOI] [PubMed] [Google Scholar]
- 51.Eike A, Buchwald C, Rolighed J, Lindeberg H. HPV is rarely present in normal oral and nasal mucosa. Clin Otolaryngol Allied Sci. 1995;20:171–3. doi: 10.1111/j.1365-2273.1995.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 52.Califano J, Koch W, Sidransky D, Westra WH. Inverted sinonasal papilloma: a molecular genetic appraisal of its putative status as a precursor to squamous cell carcinoma. Am J Pathol. 2000;156:333–7. doi: 10.1016/S0002-9440(10)64734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwasaka T, Hayashi Y, Yokoyama M, Hara K, Matsuo N, Sugimori H. “Hit and run” oncogenesis by HPV 18 DNA. Acta Obstet Gynecol Scand. 1992;71:219–23. doi: 10.3109/00016349209009922. [DOI] [PubMed] [Google Scholar]
- 54.Koskinen WJ, Chen RW, Leivo I, Mäkitie A, Bäck L, Kontio R, et al. Prevalence and physical status of HPV in squamous cell carcinomas of the head and neck. Int J Canc. 2003;107:401–6. doi: 10.1002/ijc.11381. [DOI] [PubMed] [Google Scholar]
- 55.Kim SH, Koo BS, Kang S, Park K, Kim H, Lee KR, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418–25. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]