Abstract
Angiocentric lesions of the head and neck encompass a variety of benign and malignant lesions. Not unexpectedly the sequelae of an angiocentric process independent of its benign or malignant nature is one of tissue ischemia with a potential for either breakdown or reparative fibrosis. Therefore, the clinical presentations can be very similar despite a varied pathogenesis. Among the benign reactive infiltrates that will be considered are angiocentric eosinophilic fibrosis, Wegener’s granulomatosis, microscopic polyangiitis and cocaine associated mid line facial destruction. We will discuss other conditions which enter into the differential diagnosis either clinically or histologically including Erdheim Chester disease and mid line facial undermining unrelated to an angiocentric event specifically in the context of trigeminal trophic ulcer and relapsing polychondritis. The two main neoplastic conditions exhibiting angiocentricity are in the context of lymphomatoid granulomatosis and NK/T cell lymphoma; hence these two particular hematologic dyscrasias will be discussed in some detail in this review.
Keywords: Angiocentric lesions, Head, Neck, Eosinophilic angiocentric fibrosis (EAF), Wegener's granulomatosis (WG), Microscopic polyarteritis (PAN), Allergic granulomatosis of Churg Strauss (AGCS), Chronic cocaine abuse, Trigeminal trophic ulcer, Relapsing polychondritis, Lymphomatoid granulomatosis (LYG), Natural killer/T-cell lymphomas
Introduction
Head and neck pathologists are frequently confronted with nasal, nasopharyngeal and intraoral biopsies of necrotizing inflammatory lesions with evidence of vascular inflammation and or vascular damage. A broad spectrum of conditions may result in this constellation of findings although the various entities may be pathogenetically unrelated. In this review we will consider a very specific group of angiocentric conditions which can exhibit overlapping features clinically and histologically with each other. Most of the conditions are inflammatory non-neoplastic disorders however we will also consider in brief angioimmunoproliferative lesions representing distinct forms of B and T cell dyscrasia.
Benign Angiocentric Conditions
Eosinophilic Angiocentric Fibrosis
Eosinophilic angiocentric fibrosis (EAF) of the upper respiratory tract is a rare benign disorder of unknown etiology that is believed to represent a mucosal variant of granuloma faciale. Until 2003, only 14 cases of EAF had been described in the literature. The characteristic histological finding of a perivascular inflammatory infiltrate of neutrophils, lymphocytes and eosinophils with attendant mural and intraluminal fibrin deposition as well as onionskin whirling of stromal fibrotic tissue are useful for making the histologic diagnosis of EAF. Optimal treatment can be difficult as these cases respond poorly to oral medications. Surgical excision of the fibrotic tissue of EAF is the most common modality used to treat anatomic obstruction [1].
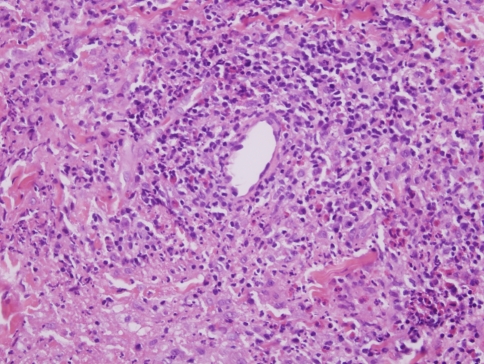
EAF represents a unique form of fibrosing small vessel vasculitis but is not associated with any systemic stigmata of vasculitis. The first documented case was by Homes and Panje who reported a case in 1983 of so called ‘intranasal granuloma faciale’, recognizing its similarity to the cutaneous entity of granuloma faciale from a histomorphologic and clinical perspective (i.e. no tendency toward multiorgan vasculitis) [2]. Further evidence of its pathogenetic relationship with granuloma faciale is the frequent occurrence of granuloma faciale in patients with EAF. In regards to granuloma faciale, this condition is a form of chronic cutaneous confined vasculitis with virtually all cases localized to the face and manifesting a middle aged male predilection. Granuloma faciale manifests as recurrent brown-red plaques on the forehead, cheeks, and ears with periods of relapses and partial remissions and in rare instances will co-exist with EAF [3]. Intranasal granuloma faciale was replaced by the term eosinophilic angiocentric fibrosis in 1985 [4, 5] (Fig. 1).
Fig. 1.
In early lesions of granuloma faciale a form of chronic leukocytoclastic vasculitis is observed. The hallmarks are nodular angiocentric mixed inflammatory cell infiltrates comprising lymphocytes, eosinophils, neutrophils and plasma cells. There is vascular ectasia, focal mural fibrin deposition and some leukocytoclastic debris. As the process evolves, the degree of inflammation lessens and there is progressive angiocentric fibroplasia, which can eventuate into an obliterative fibrous tissue reaction
There appears to be a slight female preponderance which is in contradistinction to the male gender bias observed in granuloma faciale. Patients tend to be in overall good health, but there appears to be a higher incidence of allergic and atopic disorders in patients with EAF than in the general population. The reported age at diagnosis ranges from 25 to 79 years with median and mean ages of 49 years and 47 years, respectively. Patients often have a history of chronic relatively mild symptoms. However, in some cases the symptoms may be significant including recurrent epistaxis and persistent nasal stuffiness. Non-specific mucosal thickening and submucosal fullness may be seen on physical examination while a soft-tissue, polypoid mass may be detected on radiographic imaging [5, 6]. In some instances when there is a significant active vasculitis a saddle nose deformity may eventuate. Conversely in cases where there is excessive fibroplasias, a convex hook nose deformity may eventuate.
As already alluded to, there are two defining morphologic features of EAF: vasculitis and fibrosis. In this regard, there are two separate histologic phases reflecting the temporal evolution of these lesions. Early lesions show an active necrotizing vasculitis affecting capillaries and venules with a prominence of eosinophils in an angiocentric array (Fig. 1). The more advanced lesions show a characteristic laminated scalloped pattern of fibrosis with accentuation around chronically injured vessels (Fig. 2a and b). The vessels typically show an onion bulb like pattern of laminated fibrosis, which can become very striking. Unlike classic cutaneous GF, in EAF the vasculitic changes may be very subtle while the fibrosis is extensive (3.4). In contrast in GF, the angiocentric inflammation may be prominent while the extent of fibrosis may be minimal.
Fig. 2.
(a and b) This photomicrograph depicts classic angiocentric eosinophilic fibrosis (a). While earlier phases resemble incipient inflammatory lesions of granuloma faciale, a more common pattern is characterized by angiocentric fibrosing vasculopathy from which the name of this conditions derives (i.e. angiocentric eosinophilic fibrosis). The vessels are thickened reflecting reduplications of the vascular basement membrane zones (b)
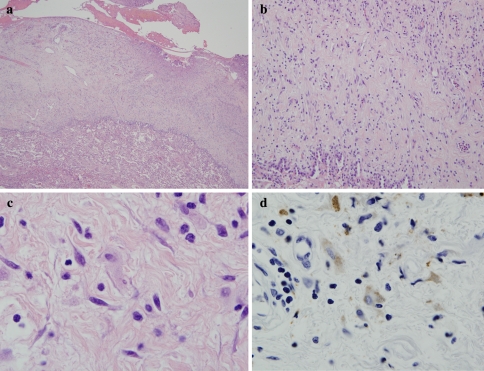
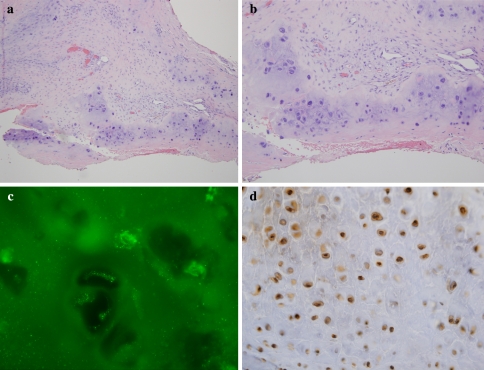
There are other causes of extensive upper airway and orbital fibrosis. These would include Erdheim Chester disease and idiopathic fibrosis. Erdheim-Chester disease is a rare non-Langerhans’ cell histiocytosis that invariably involves the long bones especially the knees and ankles. However about half of those affected have extraskeletal manifestations involving the hypothalamus-pituitary axis, lung, heart, retroperitoneum, skin, liver kidneys, spleen, and orbit [7]. The characteristic hallmark of Erdheim Chester disease is collagen deposition accompanied by a histiocytic infiltrate. The histiocytes do not exhibit CD1a or S100 positivity and are characteristically CD68 positive (Fig. 3a–c). Idiopathic fibrosis typically involves the retroperitoneum and mediastinum [8]. However, involvement of the orbit has been described [8, 9]. Cases showing dominant involvement of the nasopharynx have not been described.
Fig. 3.
In Erdheim Chester disease, the basic process is one of a non-Langerhans cell histiocytopathy associated with fibrosis. The biopsy is of lung showing marked thickening of the pleura largely attributable to collagen deposition (a and b). Higher power magnification reveals scattered histiocytes (c) which can be highlighted with a CD68 stain (d)
The etiology of EAF remains elusive. There appears to be a higher incidence of allergic and atopic disorders consisting of asthma, drug allergy, environmental allergy, urticaria and allergic rhinitis in patients with EAF than in the general population. One could therefore postulate that a potential trigger (viral or drug) may evoke a type III hypersensitivity reaction; the tissue eosinophilia and extensive collagen synthesis could be due to the Th2 dominant cytokine milieu associated with an atopic tendency [1].
Treatment of eosinophilic angiocentric fibrosis remains difficult. Oral corticosteroids, intralesional corticosteroids, as well as dapsone have been utilized for treatment with limited results. Local resection may result in progressive or persistent disease. There have been two reported cases where wide local excision in subglottic cases of EAF resulted in complete resolution of the disease [1, 6].
Wegener’s Granulomatosis
Wegener’s granulomatosis (WG) was first described by Dr. Wegener in 1936 and represents a necrotizing vasculitic syndrome that classically involves the upper and lower respiratory tract and the kidney [10]. The skin is involved in approximately 30% of cases. Immune complexes are not held to be important pathogenetically in the evolution of the vasculitis. Antibodies to cytoplasmic components of neutrophils are found in a high percentage of patients with active WG [11]. The antibodies are to a 29 KD serine protease designated proteinase 3. By indirect immunofluorescent analysis using neutrophils as substrate, a distinct pattern of granular cytoplasmic reactivity is observed, defining the so called cytoplasmic antineutrophilic cytoplasmic antibody (CANCA). The second family of antibodies produces a different pattern by indirect immunofluorescence, the so called perinuclear (P) ANCA and is rarely found in patients with WG [12–14]. Given the presence of granulomatous inflammation as an integral component of the inflammatory response in WG, cell mediated immunity may also play an integral role in its pathogenesis. In fact disease activity is associated with higher circulating numbers of Th1 cells, the main source of interferon gamma. It is possible that patients with WG exhibit cellular immune reactions to the same antigenic epitopes to which the antineutrophilic cytoplasmic antibody is directed (i.e. proteinase 3). In fact an initial limb of cellular and humoral immunity sensitization in WG patients may be staphylococcal aureus [11–13]. In particular most patients with WG are chronic nasal carriers of S. aureus which exhibits homology to proteinase 3.
Constitutional symptoms occur in approximately half of all patients. Pulmonary infiltrates, which may be clinically asymptomatic, have been identified in 71% of patients, head and neck manifestations in 95% with sinusitis being most common, skin lesions in 40–50% although the number is less with exclusion of uremic skin lesions (27%) with palpable purpura being most common, cough in 34%, ocular inflammation in 16%, epistaxis in 11% and renal involvement in 11% [13, 14]. Although the classic reports of WG describe concurrent upper respiratory tract, kidney and lung involvement, it is now apparent that a limited form of the disease exists whereby one organ is primarily involved such as isolated skin, lung, eye and or oral mucosa [15, 16]. The skin lesions fall under the designation of superficial cutaneous WG [17]. Among the head and neck manifestations are sinusitis, rhinitis, nasal crusting, saddle nose deformity, nasal and soft palate perforation, otitis media, tinnitus, tracheal and laryngeal stenosis and various ocular manifestations. The latter encompasses conjunctivitis, keratitis, episcleritis, uveitis, retinal vasculitis, optic neuropathy and orbital infiltration [16].
The histopathology of WG comprises (1) collagen degeneration, (2) extravascular inflammation with a conspicuous granulomatous component and (3) vasculitis. In WG limited to the skin, upper respiratory tract, mediastinum and/or retroperitoneum, extravascular inflammation without vasculitis may be seen. The nature of the extravascular inflammation includes neutrophils and admixed histiocytic giants cells. The giant cells exhibit a Langhans morphology but as well cells with a more irregular distribution of nuclei within the cytoplasm (i.e. foreign body like) are also characteristic (Fig. 4b). Cohesive epithelioid granulomata are not observed (Fig. 4a and b). The vasculitic lesions involve capillaries, venules, arterioles, small arteries and veins. There are, however, rare case reports of medium vessel involvement. There is necrosis of the vessel walls; the angiocentric infiltrate ranges from being frankly granulomatous to neutrophil-predominant (Fig. 5a and b) [17, 18]. At times vasculitis, necrobiosis, or extravascular inflammation can be seen in isolation. In regards to the typical findings in nasal biopsies, there is extravascular neutrophilic inflammation with scattered giant cells along with fibrin deposition. It may be difficult to isolate intact vessels showing a well defined vasculitic lesion. More commonly one observes features that are associated with vascular injury specifically in the context of fibrin deposition and ischemic undermining. In the zones of inflammation capillaries and venules may have a fibrinoid appearance although infiltration of the vessel walls with neutrophils and or histiocytes is not always seen (personal observations).
Fig. 4.
(a and b) In the setting of Wegener’s granulomatosis the pattern of granulomatous inflammation is varied. A well defined palisading morphology can be seen (a) however more commonly one will observe an intermingling neutrophils and scattered multinucleated giant cells (b). Vasculitis is not always observed. The giants include those with a langhans appearance but as well giant cells exhibiting an irregular disposition of multiple nuclei within the cell can also be observed (b)
Fig. 5.
(a and b) The vasculitis in WG is a small vessel one characterized by mural fibrin deposition with a concomitant infiltrate of inflammatory cells which can be frankly granulomatous in nature, a phenomenon depicted in (b)
Microscopic Polyangiitis
There are two forms of systemic PAN: macroscopic PAN (MaPAN) and microscopic polyangiitis . There is also a distinctive cutaneous vasculitis, benign cutaneous polyarteritis nodosa, which has a morphology resembling extracutaneous small artery involvement in macroscopic PAN-but is without any systemic organ involvement.
Microscopic polyangiitis is a necrotizing vasculitis involving capillaries, venules, and arterioles. The vasculitic sequelae in the kidney are in the context of a segmental necrotizing glomerulonephritis [19]. Lung involvement is characterized clinically by hemoptysis and histologically by a neutrophilic capillaritis [20]. Nasopharnygeal involvement is frequent with oral ulcers, sinusitis and epistaxis being among the more common manifestations [21]. Skin involvement is frequent.
The histomorphology is one of a severe pandermal LCV. Patients are typically P-ANCA positive [19] although concomitant C-ANCA positivity has also been described. Microscopic polyangiitis differs from WG by the absence of extravascular inflammation [22]. A patient with pANCA with myeloperoxidase specificity and a pulmonary-renal vasculitic syndrome almost invariably has microscopic polyangiitis. Cases of microscopic polyangiitis with CANCA positivity and a renal pulmonary vasculitic syndrome may be very difficult to distinguish from WG. The discriminating features include the absence of extravascular granulomatous inflammation and concomitant PANCA with myeloperoxidase specificity. In regards to the latter point it is extremely unusual to see a patient with polyangiitis who is only positive for CANCA positive. Typically there is concomitant PANCA with antibody titers in excess of the CANCA.
Allergic Granulomatosis of Churg Strauss
Allergic granulomatosis of Churg Strauss (AGCS) is a distinct syndromic complex associated with the development of profound tissue and peripheral blood eosinophilia with or without vasculitis in the setting of an atopic diathesis. Among the inciting triggers are inhaled allergens, desensitization shots and infection. In particular, allergic rhinitis and/or pulmonary involvement in the form of asthma along with marked peripheral blood eosinophilia occurs followed by evidence of vasculitis, in some cases up to 30 years after the onset of asthma [23]. Typically the asthma abates with the onset of vasculitis. Due to the frequency of nasal involvement there are some who consider that AGCS may be a defined by a distinctive tetrad namely asthma, tissue, peripheral blood eosinophilia, vasculitis, and nasal involvement, the latter occurring in 90% of patients with AGCS.
Among the established features of AGCS are (1) a history of asthma, (2) peripheral blood eosinophilia >10%, (3) mononeuropathy or polyneuropathy, (4) nonfixed pulmonary infiltrates, (5) paranasal sinus abnormalities (6) biopsy showing extravascular eosinophils. To render a diagnosis requires 4 of the 6 criteria listed above [22–24].
There are three essential phases in AGCS although not all patients will exhibit all three Common to all cases is a prodromal phase which last years and is characterized by asthma, allergic rhinitis and sinonasal polyposis. All develop peripheral blood eosinophilia and eosinophilic tissue infiltrates. The third phase is defined by systemic vasculitis and is usually only seen in patients who are ANCA positive, a point which will be alluded to presently.
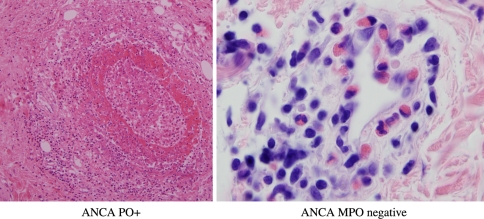
The pathology and hence clinical symptoms varies according to whether or not there is an associated ANCA. In MPO ANCA positive cases, vasculitis with dominant involvement of small arteries and venules is characteristic and essentially resembles the vasculitis encountered in microscopic polyangiitis, being typically neutrophil dominant (Fig. 6a). Among the common vasculitic manifestations are necrotizing crescentic glomerulonephritis, mononeuritis multiplex and palpable purpura. In contrast in cases which are ANCA negative, there may be an eosinophilic vasculopathy although not a true necrotizing vasculitis (Fig. 6b). Other features common to both the ANCA negative and positive cases is tissue eosinophilia and palisading granulomatous inflammation. The palisading granuloma differs from that seen in WG due to encrustation of connective tissue fibers with eosinophils. In nasal biopsies there is prominent tissue eosinophilia and depending on the ANCA status there may be supervening vasculitis (ANCA positive) and or infiltration of the vessel walls with eosinophils (ANCA negative) [25, 26]. In ANCA negative AGCS gastrointestinal disease, specifically eosinophilic gastroenteritis, and heart involvement (i.e. eosinophilic cardiomyopathy) are common while renal disease is uncommon; the dysfunction reflects damage directly attributable to eosinophil infiltration [24]). The most common cause of death in ANCA negative AGCS is a cardiomyopathy principally due to destruction of myocytes by eosinophils and less commonly a restrictive cardiomyopathy resembling Loffler’s eosinophilic endocarditis [24, 27, 28].
Fig. 6.
(a and b) In Churg Strauss disease, a necrotizing small vessel vasculitis morphologically indistinguishable from microscopic polyangiitis may be observed. This pattern is seen in patients who are ANCA positive (a). In contrast in ANCA negative Churg Strauss patients a necrotizing vasculitis is not seen although the vessels may be surrounded and permeated by an eosinophilic infiltrate
The Pathogenetic Basis of Vasculitis Triggered by ANCA
Antineutrophilic cytoplasmic antibodies are targeted against neutrophil and monocyte antigens. The evidence that these antibodies are pathogenic include the correlation of titers with disease activity, in vitro activation of neutrophils with ANCA, neonatal MPAN developing in infants from mothers with microscopic polyagiitis, remission with B cell depletion therapy and effective animal models with anti MPO ANCA [29].
Through indirect immunofluorescence using patient serum as the antibody source and alcohol fixed neutrophils as substrate, three main patterns are seen: C-ANCA, P-ANCA and atypical ANCA. The C-ANCA pattern is defined by a cytoplasmic pattern of immunofluorescence with central interlobular accentuation. This pattern typically reflects proteinase 3 specificity and is associated with active generalised WG. With P-ANCA the nuclear lobes are outlined. If the patient has antibodies to myeloperoxidase there is extension of staining into the nucleus. P-ANCA with myeloperoxidase specificity is seen in patients with microscopic polyangiitis and allergic granulomatosis of Churg and Strauss. PANCA without nuclear extension is seen in patients who do not have antibodies to myeloperoxidase and is found in the setting of inflammatory bowel disease, chronic active hepatitis, and primary sclerosing cholangitis [30, 31] (Fig. 7b). The third pattern is the so called atypical C-ANCA of which there are two main forms. The fluorescence is “flatter” and/or there is no interlobular fluorescence (Fig. 7a). The other form of the atypical ANCA is one showing both a CANCA and PANCA pattern. In these patients there are multiple antigenic specificities including PR3, MPO, bactericidal permeability increasing protein, cathepsin G, elastase, lactoferrin, and lysozyme. The atypical ANCA is observed in various autoimmune conditions, the commonest being those with rheumatoid arthritis and inflammatory bowel disease [11, 29].
Fig. 7.
This particular indirect immunofluorescent assay shows cytoplasmic granularity within a neutrophil without interlobular accentuation compatible with an atypical CANCA without reactivity to proteinase 3 (a). The PANCA is illustrated in (b) does not show nuclear extension and hence would not be associated with antibodies to myeloperoxidase. Both the atypical CANAC and PANCA without nuclear extension are seen in a variety of autoimmune inflammatory conditions
Antineutrophilic cytoplasmic antibodies are targeted against primary granules of neutrophils and lysosomes of monocytes. Although there are a variety of ANCAS, the most important in the pathogenesis of vasculitis are directed either against proteinase 3 or myeloperoxidase (Fig. 4).
The way ANCA induces vascular injury is unrelated to immune complexes and as well is not directed at vessel wall based antigenic epitopes. In this regard it is “pauci-immune”. What then defines the mechanisms of vascular injury triggered by ANCA? There are likely a series of integrated events that eventuate in vessel and perivascular injury. All of these will be addressed in seriatum. The first is one related to activation of neutrophils via ANCA. The binding of antineutrophilic cytoplasmic antibodies to neutrophil based granules results in the generation of oxygen free radicals with the subsequent release of various enzymes comprising elastase and certain metalloproteinases. These enzymes compromise the integrity of the vessel wall and adjacent tissues. Critical to the binding of ANCA to neutrophils is the upregulation of TNF alpha within neutrophils [32]. In particular TNF alpha mediated apoptosis leads to the surface displacement of the various neutrophil granules allowing access of ANCAs to its antigen epitope. Neutrophils containing bound ANCA adhere to endothelial cell monolayers. Once ANCA bound neutrophils adhere to endothelial cells, there is endothelial cell lysis. A critical event leading to enhanced TNF alpha production is localized infection.
Chronic Cocaine Abuse
Chronic cocaine abuse is a well established as a cause of destructive nasal, midfacial bones and soft tissue destruction. The etiology of this condition is related to the sympathetic mediated vasoconstrictive effects of the cocaine. Cocaine is derived from the leaf of erythroxylon coca plant and is available as a hydrochloride salt of IV/IN administration. The main effect of cocaine is one of release catecholamines from adrenal glands. The basis of the necrosis is one combined vasoconstriction and toxic effect on endothelial cells, the latter either reflecting a direct effect of the cocaine or the catecholamines. The hard palate, lateral nasal wall and nasal septum are the most commonly affected sites.
There is also a supervening irritant effect on the mucosal lining triggered by adulterating agents such as talc, mannitol, amphetamines and borax [33]. Destruction of the nasal and midfacial bones occurs after many years of chronic intranasal cocaine use with the amount of sniffed cocaine typically averaging in excess of 2 g/day. Among the clinical symptoms are epistaxis, chronic nasal discharge, saddle nose deformity, oronasal regurgitation of food and nasal speech [33].
Histomorphologically one sees necrosis of the nasal mucosa which is oftentimes pauci-cellular although a foreign body reaction to exogenous polarizable material, the latter reflecting various adulterants can be seen. Since the basis of the necrosis may be a direct toxic effect on endothelium, the microvasculature may exhibit endothelial cell necrosis and mural and luminal fibrin deposition imparting a pauci-cellular amorphous eosinophilic appearance to the vessel (Fig. 8a and b).
Fig. 8.
(a and b) In cocaine associated mid line facial destruction, the necrosis can be relatively pauci-cellular reflecting an ischemic sequelae. Higher power magnification of the mucosal vessels will reveal pauci-cellular fibrin deposition and endothelial cell injury, likely reflective of the direct toxic effect of the cocaine and or catecholamines on the mucosa
The differential diagnosis of cocaine associated necrosis of the nasal septum includes WG, tertiary syphilis and certain angiocentric lymphoproliferative conditions namely lymphomatoid granulomatosis and NK and NK like T cell lymphomas. The main differentiating features include a lack of lymphoid atypia, relatively pauci-cellular necrosis, vascular injury although without angiocentric mural inflammatory cell infiltrates, polarizable material in the tissue sample and an ANCA which in most instances is negative. However one caveat is that some patients with cocaine induced necrosis of the nasal septum will have a positive ANCA [34].
Differential Diagnosis of Other Conditions Associated with Mid Line Facial Destruction Not Specifically Associated with Vascular Compromise
Trigeminal Trophic Ulcer
This condition is a rare cause of facial ulceration [35]. Its exact pathogenetic basis is unknown; however, there does appear to be a temporal association between an insult to the trigeminal ganglia and other parts of the peripheral central nervous system in the trigeminal pathway. Among the temporal associations are alcohol ingestion, surgical ablation and severance of the sensory compartment of the trigeminal nerve. These procedures are done to control the pain of trigeminal neuralgia. Other associations include amyloid deposition within the trigeminal nerves. Overall the common factor is one of damage to the trigeminal nerve. The pathogenetic basis by which nerve injury results in compromise of tissue is unclear although an interference with normal sensory mechanisms may play a role. The classic manifestations are in the context of a crescent shaped ulcer involving the ala and nostrils extending onto the cheek and upper lip with sparing of the nasal tip. In most cases there is surgical removal of the Gasserian ganglion. A median time of 1 year passes between surgery and ulcer development. Self mutilation may be an additional factor [35, 36].
Differential Diagnosis of Mid Line Facial Destruction
Relapsing Polychondritis
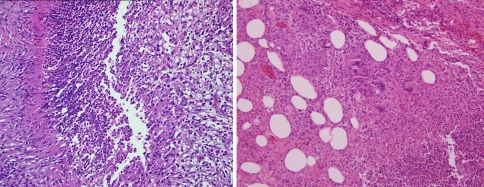
Relapsing polychondritis can cause considerable nasal destruction; antibodies directed against type II collagen is the primary mechanism causing damage to the nasal cartilage [37]. There has been an association with various underlying neoplastic disorders including tumors of the bladder, breast, bronchus, colon, lung pancreas, rectum and vocal cords [38]. Perhaps the most frequent association is in the context of underlying lymphoreticular and myeloid dyscrasia [39]. If there are concomitant mouth and genital ulcers, the syndromic complex falls under the designation of MAGIC (i.e. mouth and genital ulcers (MAG) with inflamed cartilage (IC) syndrome (40). Other sites of involvement include the ears, bronchus, trachea, heart valves and joints.
The characteristic findings pathologically include chondrocyte necrosis, foci of cartilage drop out with supervening fibrosis and granulation tissue response (Fig. 9a and b). Direct immunofluorescent studies conducted on cartilage show decoration of chondrocytes for IgG and components of complement activation including C3d, C4d, and C5b-9 (Fig. 9d). I have also conducted indirect immunofluorescent studies using human tracheal cartilage procured from either a surgical or autopsy specimen as substrate. In patients with known relapsing polychondritis a positive nuclear staining pattern is observed within the chondrocytes (Fig. 9c).
Fig. 9.
(a and b) In the setting of relapsing polychondritis there is chondrocyte necrosis with progressive cartilage replacement by granulation tissue (a and b). Indirect immunofluorescent testing using human trachea (autopsy specimen) shows positive granular staining of chondrocytes for IgG (c). C4d studies performed on paraffin embedded tissue will reveal nuclear decoration of chondrocytes (d)
Neoplastic Angiocentric Conditions of the Head and Neck
Lymphomatoid Granulomatosis (LYG)
Lymphomatoid granulomatosis (LYG) defines the concept of progressive lymphomagenesis generated by the combination of a lymphotropic viral trigger in concert with underlying immune dysregulation. Lymphomatoid granulomatosis is now established to represent an angiodestructive EBV associated B cell lymphoproliferative disease whereby lung, oral cavity, upper respiratory tract (20%), central nervous system and skin are the dominant sites of involvement. It was first reported in 1972 as a form of angiodestructive lesion with an indeterminate biological behavior Lipford and Jaffe coined the term angiocentric immunoproliferative lesions. In the inception of the disease most of the infiltrate is a dysregulated reactive CD4 T cell infiltrate responding to a few EBV infected B cells (Figs. 10a and b and 11a and b). Due to an inadequate antiviral response, the EBV infected B cells undergo continued growth immortalization and ultimately large B cell oncogenic transformation [41–44].
Fig. 10.
(a and b) In early lesions of lymphomatoid granulomatosis there are angiocentric infiltrates of small to intermediate sized lymphocytes that are predominantly T cells
Fig. 11.
(a and b) The T cells that define the earlier phases of the angioimmunoproliferative lesions of LYG are typically of the CD4 subset (a). Virtually no CD8 lymphocytes are seen (b). It is held that the T cells are responding to virally infected B cells
Because of its angiocentric nature and its tendency toward extranodal localization, it shares many features with NK and NK like T cell lymphoma and falls under the general rubric of angioimmunoproliferative lesions [45, 46]. The term LYG is a misnomer as the lesion is not granulomatous morphologically. The name stems from the nodular radiographic appearance resembling other forms of granulomatous disease including intrapulmonary fungal disease, mycobacterial infection or WG in concert with a low power microscopic appearance of multiple nodular zones of ischemic necrosis palisaded by rims of viable mononuclear cells. However, in contradistinction to reactive granulomatous disorders, the cells peripheral to the zones of necrosis are typically lymphocytes rather than histiocytes [45, 47].
Lymphomatoid granulomatosis characteristically involves extranodal sites with a predilection to affect the upper and lower respiratory tracts, central and peripheral nervous system, kidney and skin. Lymphomatoid granulomatosis has been described in children as well. Peripheral blood abnormalities mainly in the context of lymphopenia and leukopenia have been described. The prognosis is variable however many patients succumbing to their illness within 2 years of diagnosis. A variety of immune dysregulatory states have been associated with LYG including rheumatoid arthritis, Wiskott Aldrich syndrome, human immunodeficiency virus and following transplantation [44, 48].
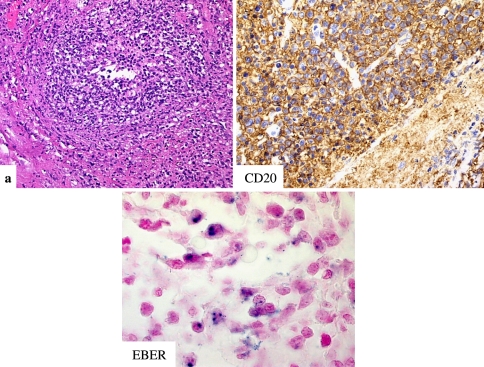
The histopathology is varied depending on the grade. In grade I lesions there is a dominance of reactive CD4 lymphocytes with only a few EBV infected B cells (<5 EBV affected B cells per high power field); reactive CD8 lymphocytes are very few in number. The T cells may show atypia and T cell clonality may occur. In grade II lesions the number of transformed EBV positive B cells increased but still insufficient for a diagnosis of lymphoma (5–20 EBV infected cells per high power field). In grade III lesions 20 or more infected EBV B cells per high power field are observed. In grade III lesions, a sheet like growth of EBV B cells signifies transition into diffuse large cell B cell lymphoma [43, 49–51] (Fig. 12a–c).
Fig. 12.
(a–c) In grade III lesions of lymphomatoid granulomatosis many angiocentric B cells are highlighted by the CD20 (b). EBER expression is observed amidst the neoplastic B cells (c)
In early lesions of LYG, the vasculopathy can assume two patterns. The first and most common is characterized by extensive infiltration of the vessel by CD4 positive lymphocytes unaccompanied by vessel wall injury. Due to the inherent immune dysregulation present in patients with LYG, the reactive T cell infiltrate may exhibit atypical cytomorphologic (in essence pseudolymphomatous) features defining a form of lymphomatoid vascular reaction [52–54]. The second pattern is one characterized by fibrinoid necrosis of the vessel wall with variable intraluminal thrombosis. The vascular damage may be mediated by the chemokines IP-10 and Mig, which are overexpressed in involved tissues. The basis for the upregulation of these chemokines likely reflects latent EBV infection within the neoplastic cells. EBV latent membrane protein can cause upregulation of both IP-1- and Mig, which have been shown to cause endothelial and vascular damage including fibrinoid necrosis [55].
EBV infection lies at the heart of LYG and is intrinsically linked to all aspects of its pathogenesis and pathophysiology [42, 43]. The initial lesions of LYG comprise reactive T cells with a variable admixture of plasma cells, histiocytes and rare transformed cells. The infiltrate is likely reflective of a dysregulated immunologic response to a few EBV infected B cells. If the normal T cell defense mechanisms to suppress this clone fail, (as might be expected in patients with intrinsic T cell dysfunction) neoplastic transformation is postulated to ensue amidst the infected B cells when these cells are exposed to additional oncogenic hits, eventuating in some cases to large cell B cell lymphoma. Of interest, the role of EBV in the propagation of the skin lesions is less consistent. In the one study by Angel and co-workers they evaluated 10 biopsies from 7 cases of primary cutaneous lymphomatoid granulomatosis using in situ hybridization, for the presence of EBV encoded RNAs (EBER-1 and EBER-2) [56]. Only one case showed positive staining with the EBER probes.
In NK and NK-like T cell lymphoma, EBV detected by molecular hybridization studies has been localized to T lymphocytes, in contrast to the dominant EBV B cell localization in the setting of LYG. The EBV associated genome has a uniform configuration consistent with a clonal process. Sixty percent of LYG cases exhibit B cell clonality, representing in most cases higher grade lesions (grade II/III). It is not uncommon to see B cell clonal divergence in patients with LYG; the clonal heterogeneity may be a function of site localization of the tumor deposits in a given patient. TCR-β and -γ rearrangements can also occur since over-expansion of an epitope specific T cell clone or a few T cell clones (restricted repertoire) responding to antigen (i.e. virally infected B cells) [50, 57].
Treatment modalities have included corticosteroids for early minimal disease, but for more advanced lesions, cyclophosphamide and aggressive combination chemotherapy are indicated. Interferon 2b because of its antiviral, antiproliferative and immunomodulatory effects has also been suggested. Dosing regimens have included cyclophosphamide at 100 mg and prednisone at 40 mg daily. Among the more recent therapeutic strategies are a reduction in immunosuppression and single agent therapy with rituximab; it is thought that early therapeutic intervention in patients of presenting with disease limited to the skin may on occasion lead to lesional resolution. When skin disease occurs in the setting of known pulmonary involvement the prognosis is not altered. In contrast, patients with neurologic involvement have a grave prognosis [58].
In summation, LYG is an immunodysregulatory disorder of B and T cells whereby the tissue destruction is attributable to the reactive T cells in the earlier phases of the disease while the sequelae of neoplastic B cell infiltration defines the basis of tissue compromise later in its evolutionary course.
Natural Killer/T-cell Lymphomas
The NK and NK-like T cell (i.e. collectively now designated as NK/T-cell) lymphomas are aggressive lymphomas which typically exhibit an extranodal presentation with characteristic involvement of the gastrointestinal tract, skin, nasal cavities and pharnyx. Bone marrow involvement at initial presentation is uncommon [59–62].
The NK/T-cell lymphomas were originally categorized as nasal versus non-nasal; now they are considered under the general rubric of extranodal NK/T cell lymphoma. Most of the NK cell lymphomas present with nasopharyngeal involvement with the majority of cases being reported from Asia. This lymphoma presents as a destructive mid line facial lesion. The 5-year survival is approximately 30%. It also falls under the designation of lethal mid line granuloma and sinonasal lymphoma and can present with palatal destruction, orbital swelling, and erythema. The initial clinical presentation may be one of disease localized to the aerodigestive tract, but this aggressive neoplasm tends to disseminate to various extranodal sites although bone marrow involvement is uncommon. Among the classic sites of tumor dissemination are skin, gastrointestinal tract, testis and cervical lymph nodes. The skin lesions are oftentimes ulcerated. Intestinal involvement presents with perforation. The clinical course may be complicated by hemophagocytic syndrome [59–62].
In the non-nasal category the main categories of lymphoma are nasal type, aggressive and blastoid. Nasal type is a confusing term as it suggests an initial presentation in the sinonasal region when in fact this tumor does not present initially in the sinonasal area, but instead in extranasal sites. Nevertheless the light microscopic, phenotypic and molecular profiles closely parallel those of the so called nasal NK/T-cell lymphoma, hence the designation. The blastoid form of NK lymphoma is unrelated to NK and NK like T cells and is now known to be a neoplasm derived from plasmacytoid monocytes [63–69].
In the classic nasal NK/T-cell lymphomas there is a male predominance whereby the median age at presentation varies from 43 to 64 years. In those lymphomas which are categorized as representing NK lymphomas as opposed to NK like T cell lymphomas, there is a frequent association with Epstein Barr virus infection, particularly in Asian patients and less often in Caucasians [60, 70]. The NK and NK like T cell lymphomas typically follow an aggressive clinical course with death occurring within 12 months after presentation. Although the nasal type of NK and NK like T cell lymphoma can initially present in the skin, cutaneous involvement in the context of known extracutaneous disease usually heralds an aggressive clinical course with refractoriness to chemotherapeutic and other intervention [60]. Rarely there may be a preceding phase of chronic peripheral blood NK cell lymphocytosis associated with neutropenia. Skin involvement is variable. When it occurs outside of the context of primary cutaneous NK cell lymphoma it usually portends a poor prognosis. Although these neoplasms do follow an aggressive clinical course, bone marrow involvement at the time of clinical presentation is uncommon.
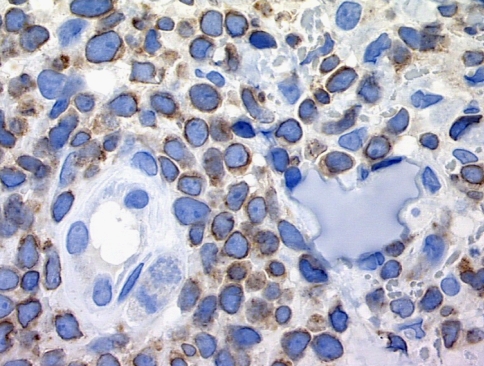
Light microscopically the NK and NK like T cell lymphomas appear similar being defined by an extensive nodular and diffuse infiltrate of intermediate size lymphocytes with nuclear contour irregularities and hyperchromasia. There is a striking tendency for the infiltrate to surround and permeate vessels with resultant luminal attenuation. Some vessels may exhibit mural fibrin deposition. The lymphomatoid vascular reaction leads to tissue undermining with variable epithelial and subepithelial necrosis (Fig. 13a and b).
Fig. 13.
(a–c) In both true NK and NK like T cell lymphomas the defining cell type is very atypical with marked nuclear hyperchromasia. Angiocentricity of the infiltrate occurs and leads to tissue ischemia due to attenuation of the vascular lumen (a). The cells are strikingly CD56 positive (b). In the NK lymphomas positivity for EBER is not uncommon amidst the neoplastic T cells while in the NK/T cell lymphomas EBER positivity would be unusual (c)
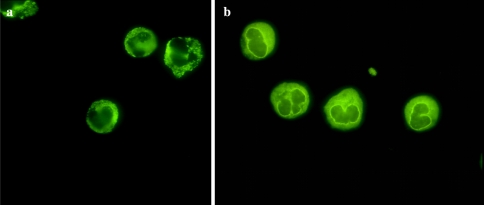
Natural killer cell lymphomas are so designated as the neoplasms derive from a cell whose normal phenotypic counterpart is the natural killer cell. Natural killer (NK) cells, also referred to as Tγ lymphocytes, differentiate from immature thymocytes to acquire characteristic phenotypic and genotypic properties and exhibit cytotoxic properties without prior sensitization [71, 72]. They are not major histocompatibility antigen (MHC) restricted in their function and hence belong to the innate immune system. These cells recognize and kill certain target cells, especially tumor and virally infected cells with altered expression of class I major histocompatibility complex antigens The NK cells express receptors for both the Fc portion of IgG and sheep erythrocytes. NK cells contain truncated cytoplasmic CD3, usually the ξ chain of CD3. However, they are without surface CD3 expression. The CD3 intracellular localization can only be reliably detected via paraffin embedded tissue using the technique of antigen retrieval with the CD3 ξ antibody. However using the routine polyclonal CD3 while a negative result may be seen, a distinct perinuclear staining pattern without surface extension can also be seen. In this regard CD3 staining does not exclude a diagnosis of NK lymphoma (Fig. 14). The cells also express CD2 and CD5 but neither CD4 nor CD8. They are CD56 positive [13]. CD56 is a neural cell adhesion molecule also designated NCAM. Other markers of NK cells such as CD11b, CD1c, CD16 and CD57 are variably positive and in some cases these additional markers are negative. The cells do not express TCR BF1 (αβ receptor) and TCR delta 1 and hence fail to demonstrates rearrangement of either the TCR β or γ chains [71, 72] (Figs. 9 and 10). Although clonality cannot be detected by TCR studies, the detection of single circularized episome form of EBV in the neoplasm and or molecular demonstration of X chromosome inactivation in female patients with NK cell lymphoma provides evidence for clonal restriction [73–75].
Fig. 14.
In this NK lymphoma cytoplasmic staining for CD3 is observed. The staining pattern is perinuclear, and there is a noticeable absence of surface CD3 staining
NK like T cells perform a similar function to the NK cells and share with true NK cells CD56 and cytoplasmic CD3 positivity however they demonstrate a rearrangement of the TCR and express CD3 on the surface. Collectively, both NK and NK like T cells account for 2–5% of peripheral blood lymphocytes. From a cytomorphologic perspective these cells demonstrate high cytoplasmic to nuclear ratios and have cytoplasms that contain prominent azurophilic granules in Giemsa stained smears Both NK cells and NK-like cytotoxic T lymphocytes are phenotypically mature lymphocytes whose cytoplasmic granules contain cytotoxic proteins that are capable of mediating cellular lysis in vitro. Among the cytolytic proteins are perforin (cytolysin), granzyme, Fas ligand (CD95L) and T cell intracellular antigen (TIA-1). TIA-1 is structurally related to the tumor necrosis factor receptor family that induces apoptosis when introduced into target cells [60, 63]. Lymphomas that manifest surface CD3 expression and TCRβ or gamma gene rearrangement are considered to represent NK like T cell lymphomas. The NK and NK like T cell lymphomas typically do not express either CD4 or CD8. Those lymphomas that fall under the designation of an NK like T cell lymphoma can on rare occasion be CD8 positive and/or CD4 positive, although the majority exhibits a null phenotype [60, 63].
Other hematologic malignancies can express cytotoxic proteins and hence would potentially enter into the differential diagnosis. Among cells with cytotoxic properties are NK cells, and cytotoxic αβ and γδ T lymphocytes. There is variation in the expression of cytotoxic proteins. The most ubiquitous is TIA-1; it is detected on virtually all cytotoxic cells. However, granzyme B and perforin expression are largely confined to activated cytotoxic cells. The various lymphoproliferative syndromes derived from cells with cytotoxic properties include NK/ T cell lymphoma, γδ T cell lymphomas which includes a certain subset of NK like T cell lymphomas, tumor stage mycosis fungoides, lymphomatoid papulosis (i.e. eosinophilic ulcer of the tongue), anaplastic large cell lymphoma and primary cutaneous CD8 positive epidermotropic cytotoxic T cell lymphoma.
Concluding Remarks
This review highlights the commonest angiocentric lesions of the head and neck. It is all too easy to misdiagnose these lesions due to the obscuration of the primary pathology by extensive necrosis, the infrequency with which we encounter these lesions and a relatively homogeneous initial clinical presentation despite a remarkably heterogeneous pathobiology. By being aware of the nuances that discriminate each of these conditions along with accurate clinical data a definitive and accurate diagnosis can be made in most cases.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Pereira EM, Millas I, Reis-Filho JS, Maeda SA, Franco M. Eosinophilic angiocentric fibrosis of the sinonasal tract: report on the clinicopathologic features of a case and review of the literature. Head Neck. 2002;24(3):307–11. doi: 10.1002/hed.10041. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DK, Panje WR. Intranasal granuloma faciale. Am J Otolaryngol. 1983;4(3):184–6. doi: 10.1016/S0196-0709(83)80041-6. [DOI] [PubMed] [Google Scholar]
- 3.LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24(5):440–3. doi: 10.1097/00000372-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Roberts PF, McCann BG. Eosinophilic angiocentric fibrosis of the upper respiratory tract: a mucosal variant of granuloma faciale? A report of three cases. Histopathology. 1985;9(11):1217–25. doi: 10.1111/j.1365-2559.1985.tb02801.x. [DOI] [PubMed] [Google Scholar]
- 5.Paun S, Lund VJ, Gallimore A. Nasal fibrosis: long-term follow up of four cases of eosinophilic angiocentric fibrosis. J Laryngol Otol. 2005;119(2):119–24. doi: 10.1258/0022215053419989. [DOI] [PubMed] [Google Scholar]
- 6.Burns BV, Roberts PF, Carpentier J, Zarod AP. Eosinophilic angiocentric fibrosis affecting the nasal cavity. A mucosal variant of the skin lesion granuloma faciale. J Laryngol Otol. 2001;115(3):223–6. doi: 10.1258/0022215011907037. [DOI] [PubMed] [Google Scholar]
- 7.Sheu SY, Wenzel RR, Kersting C, Merten R, Otterbach F, Schmid KW. Erdheim-Chester disease: case report with multisystemic manifestations including testes, thyroid, and lymph nodes, and a review of literature. J Clin Pathol. 2004;57(11):1225–8. doi: 10.1136/jcp.2004.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy JM, White VA, Harris G, Simons KB, Kennerdell J, Rootman J. Idiopathic sclerosing inflammation of the orbit: immunohistologic analysis and comparison with retroperitoneal fibrosis. Mod Pathol. 1993;6(5):581–7. [PubMed] [Google Scholar]
- 9.Holodny AI, Kirsch CF, Hameed M, Sclar G. Tumefactive fibroinflammatory lesion of the neck with progressive invasion of the meninges, skull base, orbit, and brain. AJNR Am J Neuroradiol. 2001;22(5):876–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Wegener F. Ueber generalisierte, septische Gefaesserkrankungen. Verh Dtsch Ges Pathol. 1936;36:202–10. [Google Scholar]
- 11.Kallenberg CG, Heeringa P, Stegeman CA. Mechanisms of disease: pathogenesis and treatment of ANCA-associated vasculitides. Nat Clin Pract Rheumatol. 2006;2(12):661–70. doi: 10.1038/ncprheum0355. [DOI] [PubMed] [Google Scholar]
- 12.Haubitz M. ANCA-associated vasculitis: diagnosis, clinical characteristics and treatment. Vasa. 2007;36(2):81–9. doi: 10.1024/0301-1526.36.2.81. [DOI] [PubMed] [Google Scholar]
- 13.Hewins P, Tervaert JWC, Savage COS, Kallenberg CGM. Is Wegener’s granulomatosis an autoimmune disease? Curr Opin Rheumatol. 2000;12(1):3–10. doi: 10.1097/00002281-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Stein SL, Miller LC, Konnikov M. Wegerner’s granulomatosis: case report and literature review. Pediatr Dermatol. 1998;15(5):352–6. doi: 10.1046/j.1525-1470.1998.1998015352.x. [DOI] [PubMed] [Google Scholar]
- 15.Patten SF, Tomecki KJ. Wegener’s granulomatosis: cutaneous and oral mucosal disease. J Am Acad Dermatol. 1993;28(5 Pt 1):710–8. doi: 10.1016/0190-9622(93)70098-E. [DOI] [PubMed] [Google Scholar]
- 16.Pakrou N, Selva D, Leibovitch I. Wegener’s granulomatosis: ophthalmic manifestations and management. Semin Arthritis Rheum. 2006;35(5):284–92. doi: 10.1016/j.semarthrit.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Comfere NI, Macaron NC, Gibson LE. Cutaneous manifestations of Wegener’s granulomatosis: a clinicopathologic study of 17 patients and correlation to antineutrophil cytoplasmic antibody status. J Cutan Pathol. 2007;34(10):739–47. doi: 10.1111/j.1600-0560.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 18.Barksdale SK, Hallahan CW, Kerr GS, Fauci AS, Stern JB, Travis WD. Cutaneous pathology in Wegener’s granulomatosis. A clinicopathologic study of 75 biopsies in 46 patients. Am J Surg Pathol. 1995;19(2):161–72. [PubMed] [Google Scholar]
- 19.Savage COS, Winearls CG, Evans DJ, et al. Microscopic polyarteritis: presentation, pathology, and prognosis. QJ Med. 1985;56:467–83. [PubMed] [Google Scholar]
- 20.Haworth SJ, Savage CO, Carr D, Hughes JM, Rees AJ. Pulmonary haemorrhage complicating Wegener’s granulomatosis and microscopic polyarteritis. Br Med J (Clin Res Ed) 1985;290(6484):1775–8. doi: 10.1136/bmj.290.6484.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metaxaris G, Prokopakis EP, Karatzanis AD, Sakelaris G, Heras P, Velegrakis GA, et al. Otolaryngologic manifestations of small vessel vasculitis. Auris Nasus Larynx. 2002;29(4):353–6. doi: 10.1016/S0385-8146(02)00062-7. [DOI] [PubMed] [Google Scholar]
- 22.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 23.Keogh KA, Specks U. Churg-Strauss syndrome: update on clinical, laboratory and therapeutic aspects. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23(1):3–12. [PubMed] [Google Scholar]
- 24.Garini G, Corradi D, Vaglio A, Buzio C. Churg-Strauss syndrome. Recenti Prog Med. 2003;94(12):573–81. [PubMed] [Google Scholar]
- 25.Sergent JS, Cockshin MD, Christian CL, et al. Vasculitis with hepatitis B antigenemia: long-term observation in nine patients. Medicine (Baltimore) 1976;55:1–18. doi: 10.1097/00005792-197601000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Garcia G. Allergy-related hypereosinophilia. Presse Med. 2006;35(1 Pt 2):135–43. doi: 10.1016/S0755-4982(06)74536-5. [DOI] [PubMed] [Google Scholar]
- 27.Pagnoux C, Guilpain P, Guillevin L. Churg-Strauss syndrome. Curr Opin Rheumatol. 2007;19(1):25–32. doi: 10.1097/BOR.0b013e3280119854. [DOI] [PubMed] [Google Scholar]
- 28.Crowson AN, Mihm MC, Jr, Magro CM. Cutaneous vasculitis: a review. J Cutan Pathol. 2003;30(3):161–73. doi: 10.1034/j.1600-0560.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 29.Schultz H, Weiss J, Carroll SF, Gross WL. Antineutrophil cytoplasmic antibodies: major autoantigens, pathophysiology, and disease associations. Semin Arthritis Rheum. 1995;25(3):143–59. doi: 10.1016/S0049-0172(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ. Serologic markers in inflammatory bowel disease: state of the art. Rev Gastroenterol Disord. 2004;4(4):167–74. [PubMed] [Google Scholar]
- 31.Terjung B, Spengler U. Role of auto-antibodies for the diagnosis of chronic cholestatic liver diseases. Clin Rev Allergy Immunol. 2005;28(2):115–33. doi: 10.1385/CRIAI:28:2:115. [DOI] [PubMed] [Google Scholar]
- 32.Savage CO. The evolving pathogenesis of systemic vasculitis. Clin Med. 2002;2(5):458–64. doi: 10.7861/clinmedicine.2-5-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Cosola M, Turco M, Acero J, Navarro-Vila C, Cortelazzi R. Cocaine-related syndrome and palatal reconstruction: report of a series of cases. Int J Oral Maxillofac Surg. 2007;36(8):721–7. doi: 10.1016/j.ijom.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Scheenstra RJ, Buren M, Koopman JP. A patient with both cocaine-induced nasal septum destruction and antibodies against anti-neutrophil cytoplasmic antibodies (ANCA); potential confusion with Wegener’s disease. Ned Tijdschr Geneeskd. 2007;151(43):2395–9. [PubMed] [Google Scholar]
- 35.Rashid RM, Khachemoune A. Trigeminal trophic syndrome. J Eur Acad Dermatol Venereol. 2007;21(6):725–31. doi: 10.1111/j.1468-3083.2007.02250.x. [DOI] [PubMed] [Google Scholar]
- 36.Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949–52. doi: 10.1016/j.jaad.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 37.Rapini RP, Warner NB. Relapsing polychondritis. Clin Dermatol. 2006;24(6):482–5. doi: 10.1016/j.clindermatol.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Cohen PR. Paraneoplastic relapsing polychondritis. Arch Dermatol. 2007;143(7):949–50. doi: 10.1001/archderm.143.7.949. [DOI] [PubMed] [Google Scholar]
- 39.Yanagi T, Matsumura T, Kamekura R, Sasaki N, Hashino S. Relapsing polychondritis and malignant lymphoma: is polychondritis paraneoplastic? Arch Dermatol. 2007;143(1):89–90. doi: 10.1001/archderm.143.1.89. [DOI] [PubMed] [Google Scholar]
- 40.Kötter I, Deuter C, Günaydin I, Zierhut M. MAGIC or not MAGIC—does the MAGIC (mouth and genital ulcers with inflamed cartilage) syndrome really exist? A case report and review of the literature. Clin Exp Rheumatol. 2006;24(5 Suppl 42):S108–12. [PubMed] [Google Scholar]
- 41.Liebow AA, Carrington CRB, Friedman PJ. Lymphomatoid granulomatosis. Human Pathol. 1972;3(4):457–558. doi: 10.1016/S0046-8177(72)80005-4. [DOI] [PubMed] [Google Scholar]
- 42.Veltri RW, Raich PC, McClung JE, Shah SH, Sprinkle PM. Lymphomatoid granulomatosis and Epstein Barr virus. Cancer. 1982;50:1513. doi: 10.1002/1097-0142(19821015)50:8<1513::AID-CNCR2820500811>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Medeiros LJ, Jaffe ES, Chen YY, Weiss LM. Localization of Epstein-Barr viral genomes in angiocentric immunoproliferative lesions. Am J Surg Pathol. 1992;16(5):439–47. doi: 10.1097/00000478-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Peiper S. Angiocentric lymphoproliferative disorders of the respiratory system: incrimination of Epstein Barr virus in pathogenesis. Blood. 1993;82(3):687–9. [PubMed] [Google Scholar]
- 45.Guinee D, Jr, Jaffe E, Kingma D, et al. Pulmonary lymphomatoid granulomatosis. Evidence for a proliferation of Epstein-Barr virus infected B-lymphocytes with a prominent T-cell component and vasculitis. Am J Surg Pathol. 1994;18:753–64. doi: 10.1097/00000478-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Lin BT. Lymphomatoid granulomatosis. Am J Surg Pathol. 1999;23(9):1162–3. doi: 10.1097/00000478-199909000-00029. [DOI] [PubMed] [Google Scholar]
- 47.McNiff JM, Cooper D, Howe G, et al. Lymphomatoid granulomatosis of the skin and lung. An angiocentric T-cell-rich B-cell lymphoproliferative disorder. Arch Dermatol. 1996;132(12):1464–70. doi: 10.1001/archderm.132.12.1464. [DOI] [PubMed] [Google Scholar]
- 48.Haque AK, Myers JL, Hudnall SD, et al. Pulmonary lymphomatoid granulomatosis in acquired immunodeficiency syndrome: lesions with Epstein-Barr virus infection. Mod Pathol. 1998;11(4):347–56. [PubMed] [Google Scholar]
- 49.Lipford EF, Jr, Margolick JB, Longo DL, Fauci AS, Jaffe ES. Angiocentric immunoproliferative lesions: A clinicopathologic spectrum of post thymic T cell proliferations. Blood. 1988;72:1674. [PubMed] [Google Scholar]
- 50.Jaffe ES, Wilson WH. Lymphomatoid granulomatosis: pathogenesis, pathology, and clinical implications. Cancer Surv. 1997;30:233–48. [PubMed] [Google Scholar]
- 51.Katzenstein ALA, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979;43:360–72. doi: 10.1002/1097-0142(197901)43:1<360::AID-CNCR2820430151>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 52.Tas S, Simonart T, Dargent J, Kentos A, Antoine M, Knoop C, Estenne M, Dobbeleer G. Primary and isolated cutaneous lymphomatoid granulomatosis following heart-lung transplantation. Ann Dermatol Venereol. 2000;127(5):488–91. [PubMed] [Google Scholar]
- 53.Sebire NJ, Haselden S, Malone M, Davies EG, Ramsay AD. Isolated EBV lymphoproliferative disease in a child with Wiskott-Aldrich syndrome manifesting as cutaneous lymphomatoid granulomatosis and responsive to anti-CD20 immunotherapy. J Clin Pathol. 2003;56(7):555–7. doi: 10.1136/jcp.56.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saxena A, Dyker KM, Angel S, Moshynska O, Dharampaul S, Cockroft DW. Posttransplant diffuse large B-cell lymphoma of “lymphomatoid granulomatosis” type. Virchows Arch. 2002;441(6):622–8. doi: 10.1007/s00428-002-0694-x. [DOI] [PubMed] [Google Scholar]
- 55.Teruya-Feldstein J, Jaffe ES, Burd PR, et al. The role of Mig, the monokine induced by interferon-gamma, and IP-10, the interferon-gamma-inducible protein-10, in tissue necrosis and vascular damage associated with Epstein-Barr virus-positive lymphoproliferative disease. Blood. 1997;90(10):4099–105. [PubMed] [Google Scholar]
- 56.Angel CA, Slater DN, Royds JA, et al. Epstein-Barr virus in cutaneous lymphomatoid granulomatosis. Histopathology. 1994;25(6):545–8. doi: 10.1111/j.1365-2559.1994.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 57.Jaffe ES, Chan JK, Su IJ, et al. Report of the workshop on nasal and related extranodal angiocentric T/natural killer cell lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996;20(1):103–11. doi: 10.1097/00000478-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Wilson WH, Kingma DW, Raffeld M, et al. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferon-alpha 2b. Blood. 1996;87(11):4531–7. [PubMed] [Google Scholar]
- 59.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: A proposal from the international lymphoma study group. Blood. 1994;84:1361. [PubMed] [Google Scholar]
- 60.Jaffe ES, Krenacs L, Raffeld M. Classification of T-cell and NK-cell neoplasms based on the REAL classification. Ann Oncol. 1997;8(Suppl 2):17–24. doi: 10.1023/A:1008249325615. [DOI] [PubMed] [Google Scholar]
- 61.Jaffe ES, Sander CA, Flaig MJ. Cutaneous lymphomas: a proposal for a unified approach to classification using the R.E.A.L./WHO classification. Ann Oncol. 2000;11(Suppl 1):17–21. doi: 10.1023/A:1008336732086. [DOI] [PubMed] [Google Scholar]
- 62.Greer JP, Kinney MC, Loughran TP Jr. T cell and NK cell lymphoproliferative disorders. Hematology (Am Soc Hematol Educ Program). 2001;259–81. [DOI] [PubMed]
- 63.Hirakawa S, Kuyama M, Takahashi S, et al. Nasal and nasal-type natural killer/T-cell lymphoma. J Am Acad Dermatol. 1999;40(2 Pt 1):268–72. doi: 10.1016/S0190-9622(99)70204-5. [DOI] [PubMed] [Google Scholar]
- 64.Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186–94. doi: 10.1038/sj.leu.2403955. [DOI] [PubMed] [Google Scholar]
- 65.Nava VE, Jaffe ES. The pathology of NK-cell lymphomas and leukemias. Adv Anat Pathol. 2005;12(1):27–34. doi: 10.1097/01.pap.0000151318.34752.80. [DOI] [PubMed] [Google Scholar]
- 66.Cheung MM, Chan JK, Wong KF. Natural killer cell neoplasms: a distinctive group of highly aggressive lymphomas/leukemias. Semin Hematol. 2003;40(3):221–32. doi: 10.1016/S0037-1963(03)00136-7. [DOI] [PubMed] [Google Scholar]
- 67.Santucci M, Pimpinelli N, Massi D, Kadin ME, Meijer CJ, Muller-Hermelink HK, et al. Cytotoxic/natural killer cell cutaneous lymphomas. Report of EORTC cutaneous lymphoma task force workshop. Cancer. 2003;97(3):610–27. doi: 10.1002/cncr.11107. [DOI] [PubMed] [Google Scholar]
- 68.Petrella T, Comeau MR, Maynadie M, et al. Agranular CD4+ CD56+ hematodermic neoplasm’ (blastic NK-cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol. 2002;26(7):852–62. doi: 10.1097/00000478-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Petrella T, Bagot M, Willemze R, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123(5):662–75. doi: 10.1309/GJWNPD8HU5MAJ837. [DOI] [PubMed] [Google Scholar]
- 70.Aviles A, Diaz NR, Neri N, et al. Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol. 2000;22(4):215–20. doi: 10.1046/j.1365-2257.2000.00307.x. [DOI] [PubMed] [Google Scholar]
- 71.Hallett WH, Murphy WJ. Natural killer cells: biology and clinical use in cancer therapy. Cell Mol Immunol. 2004;1(1):12–21. [PubMed] [Google Scholar]
- 72.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2005. [DOI] [PubMed]
- 73.Gaal K, Sun NC, Hernandez AM, Arber DA. Sinonasal NK/T-cell lymphomas in the United States. Am J Surg Pathol. 2000;24(11):1511–7. doi: 10.1097/00000478-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Gaal K, Weiss LM, Chen WG, et al. Epstein-Barr virus nuclear antigen (EBNA)-1 carboxy-terminal and EBNA-4 sequence polymorphisms in nasal natural killer/T-cell lymphoma in the United States. Lab Invest. 2002;82(7):957–62. doi: 10.1097/01.lab.0000020416.66825.a0. [DOI] [PubMed] [Google Scholar]
- 75.Siu LL, Chan V, Chan JK, Wong KF, Liang R, Kwong YL. Consistent patterns of allelic loss in natural killer cell lymphoma. Am J Pathol. 2000;157(6):1803–9. doi: 10.1016/S0002-9440(10)64818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]