Abstract
Intraoperative consultation (frozen section) plays an important part in the treatment of the head and neck cancer patient. The appropriate use of intraoperative consultations (frozen sections) usually results in a definitive diagnosis with immediate therapeutic impact while the patient is in the operating room. Among the determinations made by frozen section analysis include the evaluation of adequacy of surgical margins of resection; the differentiation between nonneoplastic, benign and malignant proliferations; the evaluation of lymph nodes for the presence of metastatic disease; the determination of specimen identification and specimen adequacy, including the verification of such organs as the parathyroid glands; and the determination of whether a given case requires special diagnostic testing best performed on frozen material, such as for lymphomas. This paper focuses on intraoperative consultation of mucosal lesions of the upper aerodigestive tract, including epithelial dysplasia and squamous cell carcinoma. Other issues that often not diagnosed or at issue at the time of surgery, including the diagnosis of microinvasive carcinoma and differentiating inflammatory lesions from neoplastic lesions are included for completion.
Keywords: Intraoperative consultation, Frozen section, Mucosal dysplasia upper aerodigestive tract, Squamous cell carcinoma, Margins of resection, Radiation change, Accuracy, Sentinel lymph nodes
Indications for Intraoperative Consultation of Mucosal Lesions
The indications for intraoperative consultation in mucosal (squamous cell) lesions of the upper aerodigestive tract include:
Render histologic assessment of the adequacy of resection (i.e., surgical resection margins) for the presence of dysplasia and/or carcinoma when definitive therapeutic intervention is planned immediately;
preliminary assessment of the nature of a planned procedure based on the extent and distribution of the neoplasm (e.g., subtotal versus total laryngectomy);
adequacy for diagnostic purposes;
determination for special handling (e.g., immunohistochemistry, flow cytometry, microbiologic cultures, other);
determination of neurotropism, lymph-vascular space invasion (LVI) or bone involvement that may necessitate resection of the involved bone (e.g., mandibulectomy);
if lymph nodes are excised then a frozen section may be requested to exclude the presence of metastatic disease and the need for a neck dissection.
Surgeon’s Expectations of the Intraoperative Assessment of Mucosal Lesions
establish the diagnosis of carcinoma/dysplasia and differentiate it from look-alike lesions;
confirm presence of absence of lesional tissue at the margins of resection;
when applicable, identify the presence of osseous involvement;
when applicable, identify the presence of nodal metastasis.
The accuracy of frozen sections has been analyzed for general surgical cases, as well as specifically for head and neck surgery. The studies on general surgical cases and otolaryngologic cases show similar findings with a 95 to 98% diagnostic accuracy rate, a 2 to 4% error rate (including less than 2% false negative and less than 1% false positive) and less than 4% of cases being deferred for permanent section evaluation [1–12]. Diagnostic errors may occur due to improper sampling, technical flaws, interpretive inaccuracies and in faulty communication.
Specimen Handling and Orientation
The evaluation of surgical margins of resection for the presence or absence of lesional tissue falls under the purview of the surgical pathologists. However, how the specimen is removed and the orientation of the specimen is the responsibility of the surgeon. This is particularly true for those cases in which the tumor is initially excised and the designated margins are separately removed, the tumor is removed in multiple parts or the specimen is a complex en bloc excision requiring proper orientation by the surgeon for those margins that are of critical concern. Once removed and properly oriented, the specimen becomes the responsibility of the surgical pathologist.
There is no standard method by which surgeons remove tissue and thereby request intraoperative consultation of the surgical resection margins. Some approaches include:
excision of the entire lesion, designation of specific margins and tissue selection by the pathologist for frozen section. There are some centers in which all of the circumferential resection margins are submitted for frozen section irrespective of the number of frozen sections that may be required to completely evaluate the circumferential margins. In other centers, a limited number of frozen sections (e.g., up to four––anterior, posterior, medial and lateral) are submitted as determined by the pathologist;
Note: The anatomy of the head and neck is complex and in any given resection the risk posed by removing the entire lesion and surrounding structures for intraoperative consultation includes:
loss of the 3-dimensional orientation of the specimen;
erroneous sampling of the areas of concern.
submission of biopsies from areas of clinical concern following the main resection and these biopsies are entirely submitted for frozen section.
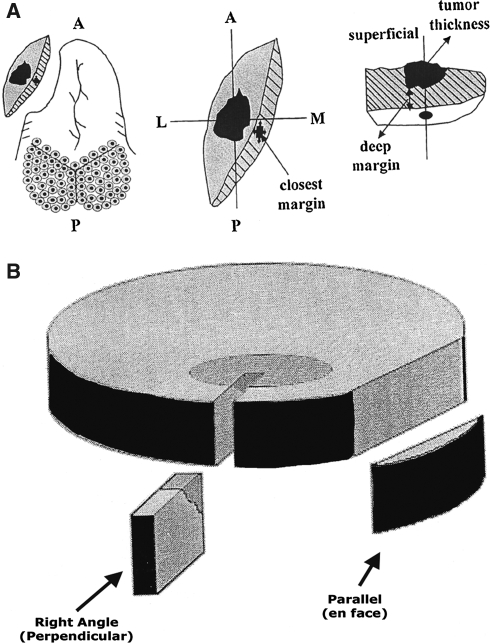
Right Angle (Perpendicular) versus Parallel (En-Face) Sections (Fig. 1)
Fig. 1.
Sectioning of surgical mucosal margins includes (a) right angle (perpendicular) versus (b) parallel (en face) each with advantages and disadvantage
The sectioning of surgical margins includes right angle (perpendicular) versus parallel (en face), each with advantage and diasadvantage [13]. The advantage of right angle section include that it is technically easier to obtain full thickness sections and the distance of the lesion to the resection margin can be viewed and measured. Right angle section disadvantages include the fact that it allows for evaluation of a relatively small area of the lesion/margin. The advantages of parallel section include it allows for evaluation of a larger area of the lesion/margin to include an entire margin if necessary. The disadvantages of parallel section include the fact that if the surgical margin is uninvolved the distance of the lesion to the resection margin cannot be viewed and/or measured. Further, due to retraction from the underlying connective tissues the superficial layers (i.e., mucosa and submucosa) may not be optimally seen. If parallel sections are made, the recommendation is to embed the tissue with the true surgical margin deepest in the block.
Surgical Resection Margins
Arguably, the most common request of the pathologist by the head and neck surgeon at the time of intraoperative consultation is the assessment of the surgical margins. Successful local control of a malignant tumor depends on complete surgical excision of all the disease. Undoubtedly the presence of gross residual cancer results in local persistence of disease and increased morbidity and mortality. There are many factors that impact on the assessment of the surgical margins, including the type of surgical specimen, proper orientation of the specimen, proper sectioning of the specimen and obviously the correct interpretation of the histopathologic changes. Unfortunately, even in the best situations an intraoperative report of negative margins may be followed a few days later by a permanent section diagnosis of positive margins.
Byers et al. [14] reported on the results of frozen section in 216 patients with neoplasms of the oral cavity, oropharynx and hypopharynx. Three groups were identified, including 68% of the patients in whom the tumors were initially adequately resected on the basis of negative surgical margins, 23% of the patients who had positive surgical margins that necessitated additional surgical resection to assure the presence of negative surgical margins, and the remaining 9% of patients in whom negative free margins by frozen section could not be obtained [14]. The follow-up of these three groups included local recurrence rates of 14.4%, 20% and 80%, respectively, with the third group having the worst survival rates. Byers and colleagues concluded that the probability of local recurrence in head and neck cancer is reduced when the resection margins are determined by intraoperative frozen section consultation [14]. The use of intraoperative frozen sections will also allow the surgeon to extend the surgical resection without loss of orientation of the operative field, a potential problem when additional surgery is required in a second operation [14]. Further, carcinoma remnants that have not been completely removed in the initial operation often are difficult to identify macroscopically, making their removal in a second operation more difficult [15]. Spiro et al. reported an intraoperative frozen section diagnostic accuracy of 89% for oral tongue cancer [16]. These authors found that the diagnostic accuracy was the same whether the sample was taken from the patient or from the surgical specimen.
Errors in interpretation and in sampling accounted for discrepancies. Cooley et al. found five discrepancies in 249 frozen sections of laryngeal squamous cell carcinoma in which the frozen diagnosis was negative but the permanent sections revealed dysplasia or carcinoma in situ [17]. These authors found that insufficient leveling of the frozen block resulted in these discrepancies. Their recommendation was that in examining margins for laryngeal squamous cell carcinoma the frozen section tissue should be completely sampled by examining several levels at the time of frozen section. Spiro et al. [16] reported that of the 131 samples taken intraoperatively whose margins were reported as negative, 13 were later reported to be positive following permanent sections. While the frozen section diagnosis of surgical margins are extremely accurate they are not entirely reliable in eliminating positive margins in the final diagnostic report.
Definition of a “Positive” Margin
Specimens in which no dysplasia or mild dysplasia is present at the surgical margins of resection are considered as completely excised (Fig. 2). “At the margin of resection” means that the neoplastic cells are seen in contact with or lie within millimeters of the pigment that was painted along the margin prior to sectioning (i.e., tumor across or up to the resection margin) (Fig. 3). In this situation, the specimen is considered incompletely excised, requiring a wider excision in order to be assured that all viable tumor cells have been adequately removed. Batsakis [18] in a comprehensive review from the pathologist’s perspective in the assessment of surgical excision margins in HNSCC made a number of cogent recommendations including:
the sole reliance of margins as assessed on the resected specimen should be discouraged and, when feasible, the intraoperative evaluation of tissue surrounding the specimen should be made and have that regarded as the “true margin”;
- the histologic definition of what constitutes a “positive” should be uniformly accepted and applied. There is discrepancy in the literature as to what constitutes a positive or negative resection margin. While some authors only include invasive carcinoma at the margin as positive excluding carcinoma in situ, dysplasia and gross residual disease, most pathologists would agree with the classification of positive margins, as defined by Loree and Strong:
- lesional tissue within 0.5 mm of the surgical margin (so-called close margins) with the exception of laryngeal lesions (see below);
- dysplastic epithelium at the margin;
- carcinoma in situ at the margin;
- invasive carcinoma at the margin.
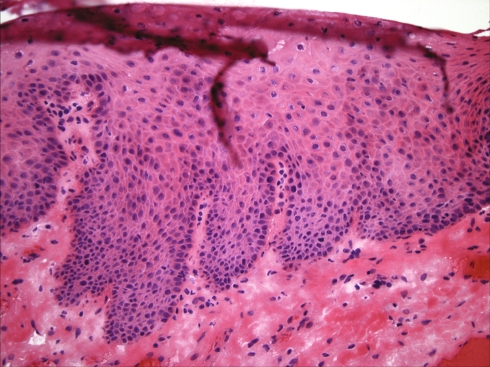
Fig. 2.
Surgical margin showing an absence of dysplasia
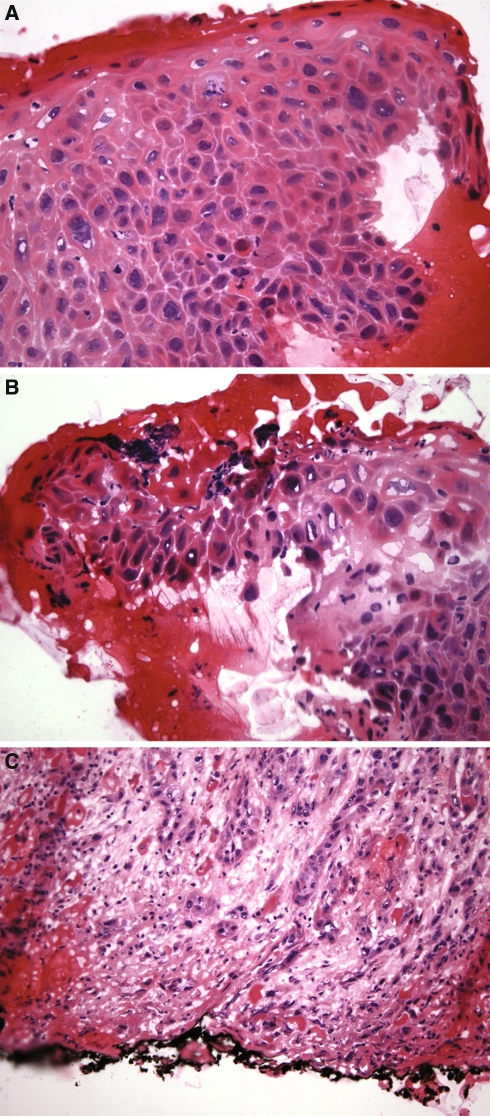
Fig. 3.
Illustrations depicting (a) moderate dysplasia, (b) severe dysplasia and (c) carcinoma at the margins of resection
Suffice to say that the evaluation of dysplasia under optimal circumstances can be subjective let alone at the time of frozen section where tissue distortion and artifact create additional diagnostic difficulties potentially resulting in misdiagnoses, including overdiagnosis and underdiagnosis. The diagnosis of severe dysplasia has equal biologic significance with carcinoma in situ and microinvasive carcinoma. A diagnosis of mild dysplasia also can be very problematic but is of questionable significance. In fact, the progression of low-grade dysplasia to higher grade epithelial lesions is so low as to make this diagnosis of little clinical significance, and often of minimal concern to the head and neck surgeon. However, the progression of moderate dysplasia to a higher grade lesion is nearly similar to that of severe dysplasia [19], so that a frozen section diagnosis of moderate dysplasia results in a therapeutic approach similar to that of severe dysplasia (Fig. 4).
Fig. 4.
Frozen section from a laryngeal carcinoma. The invasive carcinoma lies within 2 mm from the inked edge of the tissue specimen
Adequacy of Resection Margins
The presence of lesional tissue within 5 mm of the inked surgical margin irrespective of whether it is invasive carcinoma or carcinoma in situ/severe dysplasia places a patient at a nearly equal risk for local recurrence [18]. These margins are associated with approximately 80% incidence of recurrent disease, so that failure of local control is not inevitable with such positive margins [14]. The absence of positive margins does not guarantee local control of disease nor is it a reliable guide to the biologic behavior of a tumor. Spiro et al. [16] report that the presence of positive margins increased the likelihood of local recurrence but did not impact on survival since subsequent surgery and/or irradiation controlled tumor recurrence in some of their patients. The incidence of positive surgical margins is quite variable ranging from as low as 3% [20] in some studies to as high as 60% in other studies [21].
The question of how wide a tumor should be excised is the responsibility of the surgeon. However, for some specimens such as the laryngeal squamous cell carcinoma, free margins up to 5 mm may be sufficient while a similar tumor at another extralaryngeal site, such as the oral cavity and pharynx (oro-, hypopharynx) wider margins (1.0 cm) are optimal.
Factors Impacting of Margin Status and Prognosis
Certainly, this variability in “positive surgical margins” is dependent on multiple factors, not the least important of which may be the definition of what constitutes a positive margin. The factors that may impact on whether a margin is positive or negative, and in prognosis (control of disease) include:
clinical stage;
tumor size;
pattern of invasion;
adjuvant therapy;
histopathologic evaluation;
site dependency;
soft tissue margins.
More recently, Brandwein-Gensler et al. [22] suggests that resection margin status alone is not an independent predictor of local recurrence nor should resection margin status alone be used as the sole variable in deciding whether adjunctive radiation therapy is required; rather the need to give adjunctive radiation is suggested to be based on the following parameters:
positive margins;
perineural invasion;
osseous invasion;
- histologic risk assessment that includes score based on worst pattern of invasion:
- Patterns of invasion include:
- 1 = invasion in broad pushing front;
- 2 = invasion in “finger-like” broad pushing pattern or separate large tumor islands;
- 3 = invasive tumor islands of tumor at periphery greater than 15 cells/island;
- 4 = invasive tumor islands of tumor at periphery smaller than 15 cells/island or strands of tumor cells in single cell filing pattern regardless of island size;
- 5 = satellites of dispersed tumor infiltrates of any size with 1 mm or greater distance of intervening normal (non-fibrotic) tissue at the tumor-host interface.
- Perineural invasion;
- Lymphocytic response;
According to Brandwein-Gensler et al. [22] histologic assessement results in stratification of patients into low-risk, intermediate-risk and high-risk categories that define recommendations for adjuvant radiotherapy (Table 1).
Table 1.
Risk assessment for oral squamous cell carcinoma*
| Histologic variable | 0 | 1 | 3 |
|---|---|---|---|
| Perineural invasion | None | Small nerves | Large nerves |
| Lymphocytic response | Continuous band | Large patches | Little to none |
| Worst pattern of invasion at interface** | 1, 2 or 3 | 4 | 5 |
| Risk score*** | Risk for LR | Overall survival probability | Adjuvant treatment with RT |
|---|---|---|---|
| 0 | Low | Good | No benefit |
| 1 or 2 | Intermediate | Intermediate | No benefit |
| 3–9 | High | Poor | Beneficial regardless of 5 mm margins |
* Adapted from Brandwein-Gensler et al. ** Patterns of invasion: 1 = invasion in broad pushing front; 2 = invasion in “finger-like” broad pushing pattern or separate large tumor islands; 3 = invasive tumor islands of tumor at periphery greater than 15 cells/island; 4 = invasive tumor islands of tumor at periphery smaller than 15 cells/island or strands of tumor cells in single cell filing pattern regardless of island size; 5 = satellites of dispered tumor infiltrates of any size with 1 mm or greater distance of intervening normal (non-fibrotic) tissue at the tumor-host interface. *** = sum of all points; LR = Local recurrence; RT = Radiotherapy
Jacobs et al. [23] found that of the entry population of 696 patients in their study, 112 patients (16%) with stage III and IV operable tumors had positive surgical margins. These authors indicate that the 16% represented a national average rather than an institutional incidence. In comparison to patients with negative margins, patients with positive margins had a significantly higher rate of local failure (21% vs. 9%, P = 0.0003), and distant failure (20% vs. 12%, P = 0.42) but a similar incidence of regional (nodal) failure. Patients with positive surgical margins were most often seen in nonglottic primary cancers and with increasing incidence as the N stage increased. The positive-margin patients had a higher rate of distant metastasis and died more rapidly than positive-margin patients with lower nodal status. The survival of the positive-margin patients was approximately one half of the margin-negative patients. The addition of chemotherapy did not significantly alter the survival of positive-margin patients. These authors reported a median survival of 19 months in patients with positive margins essentially equating to the expected outcome for patients with inoperable cancer.
Tumor Size (“T” Staging)
Scholl et al. [24] reported that in 54 of 268 patients (20.1%) with lingual squamous cell carcinoma, the carcinoma was not initially completely removed (i.e., positive margins at frozen section). When additional surgery resulted in negative margins, the local recurrence rate was worse than if the initial margin was negative. Scholl et al. [24] also found that positive mucosal margins were more often present in T1 and T2 tumors and soft tissue margins were more common in T3 and T4 lesions. Looser et al. [20] reported that 71% of patients with positive margins had recurrent disease at the primary site in comparison with 32% of patients with the tumor-negative margins. Loree and Strong [25] found the incidence of positive margins was directly proportional to the increasing tumor size. Batsakis [26] notes that from 5% to 10% of carcinomas resist the surgical goal of clear margins regardless of T stage. He further notes that the survival of patients with free margins is related to the T stage of their carcinoma. All in all, the lower the clinical stage and/or pathologic class the better the ability to achieve local control and the overall better survival rates.
Pattern of Invasion
The pattern of invasive squamous cell carcinoma and the involvement of resection margins in oral squamous cell carcinoma were evaluated in a study by Spiro et al. [16]. The patterns of invasion were graded 1–4 as follows: Grade 1––invasion in a “pushing” manner with well-delineated border; Grade 2––invasion along the advancing edge in solid cords, bands or strands; Grade 3––invasion in small groups or cords of infiltrating cells; Grade 4––marked cellular dissociation in small groups and/or single cells. These authors found that those carcinomas invading with Grade 3 or 4 patterns were associated with an increase in nodal and distant metastasis, and a significant decrease in survival. The Grade 3 and 4 invasive patterns tended to be larger tumors (higher T stage) and occurred in younger patients (median of 56 years as compared to Grade 1 and 2 invasive patterns with a median age of 62 years).
Postoperative Radiotherapy
Other studies have shown that implications of a positive surgical margin for squamous cell carcinoma of the head and neck are associated with increased local failure and decreased survival rates. Loree and Strong [25] reported that the overall local recurrence rate in the group of tumor-positive margins was 36% as compared with 18% for the tumor-negative margin group. Further, these authors showed a statistically significant difference in the five-year survival rate between the tumor-positive margin patients (52% 5-year survival) as compared to the tumor-negative margin patients (**52% 5-year survival). Although postoperative radiotherapy was found to reduce the local recurrence rate in the positive-margin patients, the overall 5-year survival rates between margin-positive and margin-negative patients was not affected by the postoperative radiotherapy.
Scholl et al. [24] in their evaluation of oral tongue squamous cell carcinoma indicate in the presence of complete surgical removal of all disease to include macroscopic disease and histologically negative margins without neural invasion that adjunctive therapy does not improve local control as compared to surgery alone.
Beitler et al. [27] showed that the addition of adjuvant external radiation therapy and brachytherapy in patients with unexpectantly unsatisfactory surgical margins (i.e., microscopically positive or close margins) after surgical resection improves local control.
Histopathology
van Es et al. [28] reported that for T1 and T2 carcinomas of the mobile tongue and floor of mouth the single most important parameter in determining local recurrence is the histopathologic evaluation of margin status and that other histopathologic parameters were essentially irrelevant in predicting recurrence. The presence of positive margins does not always translate into failure of local control; however, the overwhelming majority of patients with recurrent disease at the primary site have positive margins. The ability to completely resect a squamous carcinoma of the head and neck weighs heavily in the surgeon’s decision to utilize surgery in the attempt to eradicate the cancer, and the presence of disease (gross or microscopic) at the surgical margins represents a key prognostic feature for patient survival.
Site Dependency
The identification of negative margins is not a guarantee for the absence of local recurrence. The larynx perhaps is an outlier in regard to positive-margins and local recurrence. As compared to extralaryngeal mucosal sites, patients with primary laryngeal squamous cell carcinoma with positive surgical margins have a significantly lower incidence of local recurrence. Bauer et al. [29] reported that of a total population of 111 patients with laryngeal squamous cell carcinoma 39 (35%) had positive surgical margins. Of these 39 patients, seven (18%) developed local recurrence while four of the remaining 72 patients (6%) with negative margins developed local recurrence. These findings suggest that margin status and local recurrence are site dependent and assists in explaining why surgeons are more apt to accept nearer margins for laryngeal carcinoma (free margins up to 2 mm) (Fig. 5) but require wider margins (5–10 mm) for carcinomas of extralaryngeal mucosal sites. The factors that may contribute to the lower incidence of local recurrences in laryngeal squamous cell carcinoma with positive margins supporting organ sparing (conservative) laryngectomy may include:
these patients have early stage carcinoma associated with a more favorable prognosis;
the submucosa of the glottic region has (quantitatively) less lymph-vascular spaces thereby decreasing the incidence of spread and lowering the incidence of locoregional failure.
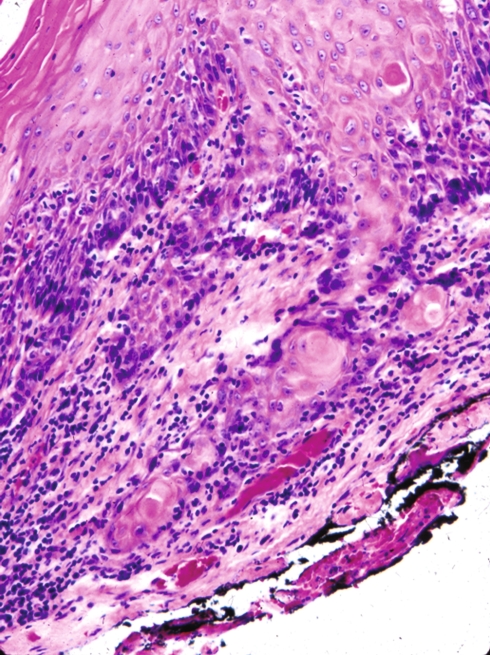
Fig. 5.
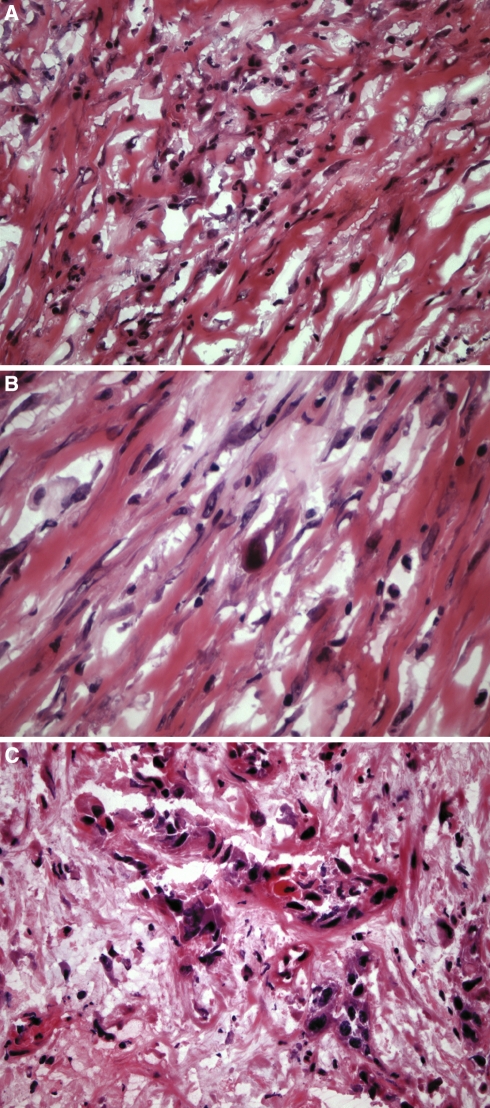
Postirradiation alterations include sialometaplasia that may histologically simulate squamous cell carcinoma. (a) At low magnification there is retention of the lobular architecture of the minor salivary glands, a key feature in trying to decide if there is or is not squamous cell carcinoma. (b) Only viewing the lesion at higher magnification may result in a diagnosis of carcinoma rather than sialometaplasia
Among the extralaryngeal sites with significant recurrence rates following negative surgical margin determination include the oral cavity and pharynx. A factor that contributes to the recurrence rates of pharyngeal and oral cavity carcinomas in the face of negative surgical margins is the tendency for submucosal spread by these carcinomas. Intraoral sites with negative margins but significant recurrence rates include the palate, tonsil, buccal mucosa, tongue, gingiva, floor of mouth and lip.
Soft Tissue Margins
Mucosal margins are not the only tissue margins. Surgical margins of resection may include all soft tissue components including adipose tissue, skeletal muscle, bone and neural structures. The latter are of particular concern in those tumors that have a propensity for neurotropism, such as adenoid cystic carcinoma of salivary glands. For oral cavity carcinomas, the presence of dentition significantly influences nerve-related spread. In evaluating spread of carcinoma within the mandible, McGregor and MacDonald [30, 31] found a fourfold increase in cancer spread related to the inferior alveolar nerve in edentulous, nonirradiated mandibles as opposed to partially dentate, nonirradiated mandibles. Nerve involvement was associated with extensive spread of carcinoma in the medullary parts of the bone.
Bone, in particular the osseous margins of resection of the mandibular region pose an especially significant issue in regard to carcinomas of the alveolar ridge, floor of mouth, lower buccal sulcus and lower retromolar region. Carcinomas of these sites are among those with the highest rate of recurrence. As such, the evaluation of the mandibular bone for the presence and extent of involvement is a key determinant in patient management [32]. The methods of evaluating for osseous involvement include: preoperative radiologic assessment; gross inspection; frozen section or imprint evaluation of bone from the resected osseous stump; frozen section of adherent soft tissues. The clinical determination of mandibular involvement is not reliable since one third of histologically proven carcinomatous invasion of the mandible showed no clinical indication of (preoperative) bone involvement [33]. Spread of oral carcinoma to the mandible typically occurs by direct invasion rather than by metastasis, lymph-vascular space spread or via nerves. Spread of carcinoma is nearly always through cancellous bone and its marrow spaces. The frozen section assessment is difficult given the inherent difficulties in performing frozen section on bone. Touch preparations or imprints of the cancellous bone of the stump or frozen sections of curetted material can be performed, and have shown with varying degree of success. Forest et al. [34] performing frozen sections on curetted material were able to accurately predict the adequacy of mandibular resection in 97% of the margins (31 of 32 margins). Alternatively, a portion of the soft tissue immediately adjacent to the periosteum may be submitted for frozen section; if negative this might indicate that there is sparing of the bone and if positive would indicate that there is bone involvement. However, the evaluation of the nonosseous soft tissue is not a reliable indicator vis-à-vis osseous involvement. Further, in irradiated mandibles it may not be possible to separate the periosteum. The size of the carcinoma does not appear to influence the incidence of bone involvement but proximity to bone does. In an evaluation of local control of oral and oropharyngeal carcinomas with clinically determined mandibular bone involvement, Dubner and Heller found a 19% recurrence rate following marginal mandibulectomy and 6% following segmental mandibulectomy [35]. In this study local recurrence was not dependent on tumor size, N stage, invasion of the mandible or radiotherapy. In those patients who recurred following segmental mandibulectomy, the recurrent disease was present within extraosseous soft tissues.
Tissue Shrinkage and Surgical Margins
The reliability of measuring the resection margins is impacted by postremoval changes especially related to tissue shrinkage. It is obvious that obtaining adequate tumor free surgical margins is critical for the successful management of the cancer patient. Any discussion on surgical resection margins would not be complete without a discussion on tissue shrinkage in the pathologic processing of resected tissues. Disparate surgical margin lengths of resected specimens between the in vivo measurements by the surgeon and the in vitro measurements by the pathologist have been reported for head and neck resection specimens [36, 37] and non-head and neck resection specimens [38–41]. Johnson et al. [37] found a mean tissue shrinkage of 31% (P < 0.0001) from the initial in-situ measurement by the surgeon to the final microscopic assessment of oral cavity and lingual surface mucosal margins by the pathologist. These authors reported that to obtain 5 mm of histopathologically clear margin an in-situ margin of resection of at least 8–10 mm needs to be taken. In their evaluation of oral squamous cell carcinomas Beaumont and Hains [36] found a reduction of 46% from the planned surgical margin before resection to the microscopically measured margin following pathologic preparation (minimum of 10 mm measured in situ surgical margin and average of 5.8 mm margin following fixation). These authors reported significant differences in the longitudinal diameter of the whole specimen from in situ to fresh states (P < 0.0004) and in the diameter of the tumor from the fresh state to fixed states (P < 0.0000) with the most significant shrinkage reported from the fresh state to the fixed state with a mean shrinkage of 4.82 mm. Similar issues with tissue shrinkage have been reported relative to colorectal resection specimens where the greatest proportion of shrinkage occurred immediately upon resection [39]. Johnson et al. [37] found that the greatest proportion of shrinkage occurred immediately upon resection. Although there are very few studies assessing the shrinkage of tissue margins it is apparent that a significant amount of tissue shrinkage occurs from the moment the tissue is excised to the time the pathologist reviews the histologic preparation of the excised tissues. Such tissue shrinkage should be taken into account and accommodated for by the surgeon at the time of the operation.
Time Factor
Another issue relative to the intraoperative histological evaluation of tumor resection margins is the time factor. The identification of negative surgical margins by frozen section may require a large number of samples. The result of numerous frozen sections in conjunction with the possibility of neck dissection may be the prolongation of surgery. In a prospective study of 24 patients with oral squamous cell carcinoma, Bähr and Stoll [15] advocate the removal of the regional lymphatic drainage while the frozen section examination of the surgical margins is being performed in order to reduce the time of surgery.
Microinvasive, Superficial or “Early” Invasive Squamous Cell Carcinoma (SCC)
Microinvasive SCC is a cancer that infiltrates into the superficial compartment of the lamina propria. For laryngeal lesions, some authors consider microinvasive cancer to include the presence of scattered malignant cells within the submucosa just below the basement membrane or within 1–2 mm of the basement membrane while other authors feel that microinvasive carcinoma is present when tongues or discrete foci of malignant epithelium invades through the basement membrane. Irrespective of its specific definition, a diagnosis of microinvasive carcinoma excludes those lesions that are restricted to the surface epithelium or carcinoma in situ (Tis) and those carcinomas that are deeply invasive into muscle and cartilage, and extralaryngeal structures (T2 or greater tumors). Of note, extension of the dysplastic process to involve the seromucinous glands is still considered as CIS and not invasive carcinoma. Clinical manifestations and appearance are similar to those of carcinoma in-situ. In the larynx, full cord mobility is present. Any dysfunction in vocal cord mobility (fixation) by definition means muscle invasion, which excludes a diagnosis of microinvasive cancer.
Histologically, microinvasive carcinoma can occur in two unrelated phases. The first is the development from and as a continuum of carcinoma in situ. The second is invasion from an epithelium demonstrating no evidence of CIS. In the upper aerodigestive tract, particularly in the larynx, severe dysplasia (i.e., carcinoma in situ) is not a prerequisite for the development of an invasive squamous cell carcinoma. Such invasive carcinomas “drop off” or “drop down” from the basal cell layer with the overlying mucosa showing no evidence of dysplasia. In all examples of invasive carcinoma the invasive nests must be cytologically malignant including dysplastic changes, dyskeratosis and mitotic figures, including atypical forms. The tumor nests have an irregular outline with infiltrative borders. The presence of invasive cancer generally results in a desmoplastic host response that includes edematous change immediately around the tumor nests with granulation tissue and fibrosis.
Microinvasive cancer is a biologically malignant lesion potentially capable of gaining access to lymphatic or vascular channels in the lamina propria that may result in metastatic disease. For microinvasive carcinoma of the laryngeal glottis, several studies have shown that the clinical significance is similar to CIS/severe dysplasia that and with appropriate therapy (excision and/or radiotherapy) progression of disease from a microinvasive to a more invasive carcinoma does not occur. This may be due to the earlier clinical manifestations produced by glottic cancers leading to an earlier diagnosis of cancer before it has invaded into deeper aspects of the larynx. Glottic microinvasive cancers are generally not associated with metastatic disease due to the fact that the glottic portion of the larynx has quantitatively less lymph-vascular spaces as compared to the supra- and subglottis. In contrast to the laryngeal glottis, supraglottic microinvasive carcinomas are associated with metastatic disease in approximately 20% of patients.
Pitfalls in the Intraoperative Assessment of Squamous Cell Carcinoma/Dysplasia (Differential Diagnosis)
Frozen section consultations on mucosal surface lesions can be useful especially in differentiating inflammatory and neoplastic lesions. Histologic grading of a mucosal malignancy (i.e., squamous cell carcinoma) may be problematic and is not advocated by frozen section. Artifactual distortion and sampling limitations may lead to erroneous conclusions relative to the histologic differentiation of the carcinoma.
Reactive Epithelial Changes
Reactive, infectious and neoplastic lesions (e.g., granular cell tumor) may be associated with an exuberant epithelial response (pseudoepitheliomatous hyperplasia (PEH)) that may be mistaken for squamous cell carcinoma. Despite this marked epithelial proliferation, there typically is an absence of significant cytomorphologic atypia and evidence of invasion with associated tissue response (i.e., desmoplasia) allowing for differentiating PEH from squamous carcinoma.
Postirradiation Changes
Postirradiation alterations may lead to false positive diagnosis due to the presence of bizarre cytologic alterations in the epithelium, minor salivary glands, fibroblasts, skeletal muscle and endothelial cells.
Distinguishing invasive squamous cell carcinoma from radiation change is challenging. Obviously, knowledge of prior radiation treatment would be imperative in the evaluation of a given specimen. If this information is known prior to surgery and intraoperative consultation then comparison of any previous material (e.g., biopsy) with the frozen section under review would be beneficial. Ultimately, the differentiation of radiation effect versus squamous cell carcinoma is based on histologic review. During the acute postirradiation phase (days to weeks) biopsies are seldom obtained [42]. Biopsies taken in the later postirradiation period (6–7 weeks following therapy to years later) show variable histologic changes, including a thinner than normal surface epithelium, surface ulceration, squamous epithelial atypia, atrophy of minor salivary gland acini, pseudoepitheliomatous proliferation of minor salivary glands, (atypical) squamous metaplasia of minor salivary glands (Fig. 5), submucosal fibrosis, vascular alterations characterized by telangiectatic capillaries often with prominent (plump) endothelial cells, myointimal proliferation, foamy histiocytes within the intima and thrombosis, as well as atypical (bizarre) fibroblasts (Fig. 6), and bizarre striated muscle degeneration [42]. The retention of the lobulated outline of the minor salivary glands, presence of “smudged” appearing nuclei of the atypical fibroblasts and absence of cohesive cellular grouping of the atypical fibroblasts scattered in scar formation or inflammatory tissue assists in distinguishing radiation change from carcinoma. Delayed radiation injury generally is not characterized by cellular inflammatory infiltrate. Familiarity with these findings should allow for their recognition and prevent misinterpretation. As previously indicated, the information that a patient received prior radiation to a given region being sent for intraoperative consultation should be a requirement.
Fig. 6.
(a, b) Atypical (bizarre) fibroblasts represent another postirradiation change that may create diagnostic difficulties with squamous cell carcinoma. Note the presence of “smudged” appearing nuclei of the atypical fibroblasts and absence of cohesive cellular grouping of the atypical fibroblasts which tissue assists in distinguishing radiation change from (c) squamous cell carcinoma
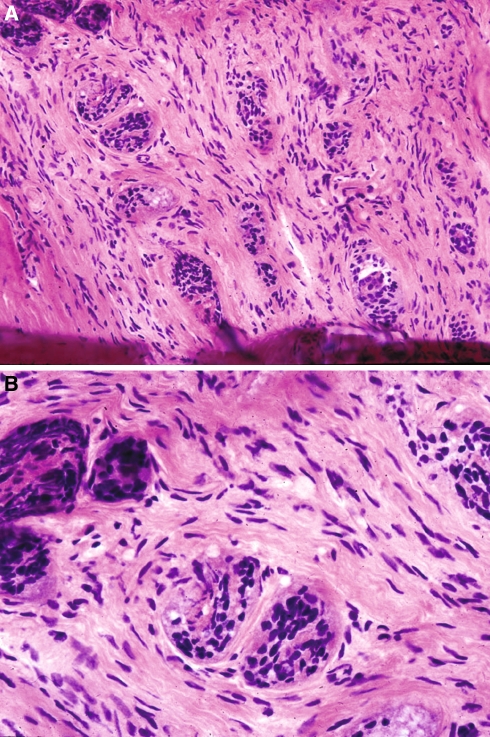
Juxtaoral Organ of Chievitz
Normal microscopic structure of unknown function found in the located bilaterally at the angle of the mandible (in the retromolar trigone of the oral cavity) near the buccotemporalis fascia and intimately associated with branches of the buccal nerve. Histologically, this structure is composed of nests or clusters of squamous cells with intercellular bridges surrounded by basaloid cells; the latter may show nuclear palisading (Fig. 7). Keratinization is not present although duct-like lumina may be seen. The cells are uniform with low nuclear-to-cytoplasmic ratio, minimal pleomorphism and absent mitotic figures; desmoplasia is absent. These structures may easily be misinterpreted as invasive carcinoma with perineural invasion. Familiarity with these structures, their characteristic location and overall relatively bland histomorphology should allow for distinguishing these normal benign structures from invasive carcinoma. Of course, detailed conversation with the surgeon would be imperative in identify these structures and not misdiagnosing them as invasive carcinoma.
Fig. 7.
Juxtaoral organ of Chievitz. (a, b) Histologically, this structure is composed of nests or clusters of squamous cells with intercellular bridges surrounded by basaloid cells. Given its appearance and intimate association with branches of the buccal nerve it may be misdiagnosed as infiltrative squamous cell carcinoma. In contrast to carcinoma, the cells are uniform with low nuclear-to-cytoplasmic ratio, minimal pleomorphism and absent mitotic figures
Contraindications
As with any surgical procedure, there are contraindications for the utilization of frozen sections. Frozen section consultation should not be used:
when the frozen section diagnosis will not have any impact on surgery or no immediate decision, such as satisfying the curiosity of the surgical team;
if the tissue specimen is small and additional sampling is not planned (in which situation, frozen sections may be equivocal and/or the material is artifactually distorted by the frozen section technique hampering histologic evaluation following permanent sections);
for heavily calcified or ossified tissue;
for certain lesions such as small cutaneous melanocytic lesions and lymphoproliferative lesions requiring special handling or extensive histologic evaluation for diagnosis.
Intraoperative “Rapid” Immunohistochemical Assessment
Intraoperative immunohistochemical assessment consisting of touch smear cytologic preparations and cytokeratin staining in 20 min from the time of tissue sampling. Limited studies to date [43] on gastric mucosal margins in patients undergoing gastrectomy for gastric (non-signet ring cell) carcinomas show accurate and rapid assessment of tumor margins. To date, such analyses have not been published relative to mucosal margins of the upper aerodigestive tract.
Molecular Biology in the Assessment of Surgical Margins (“Molecular Margins”)
Brennan et al. [44] utilized molecular biologic markers in assessing histopathologic negative surgical margins and negative lymph nodes for patients with squamous cell carcinoma of the head and neck. In 52% of the patients studied, p53 mutations were found in the tumor margins that were identified as free of tumor by conventional histologic examination. p53 mutations were found in 21% of the lymph nodes that were negative for tumor by conventional histologic examination. In 38% of the patients with p53 positive margins, the tumor recurred locally. The authors concluded that the presence of p53 mutations in surgical margins and in lymph nodes that were negative for tumor by light microscopic examination portended a substantially higher risk of local recurrent disease than those patients without p53 mutations in their surgical specimens. In their opinion, molecular biologic studies augmented conventional light microscopy in identifying cancer at surgical margins and in lymph nodes, and also may improve the prediction of local tumor recurrence.
Subsequent to the study by Brennan and colleagues, Ball et al. [45] evaluated p53 immunostaining of surgical margins in predicting local recurrence of oral and oropharyngeal SCC. These authors found that there was sample odds ratio test predicting a 5.333 times higher chance of local recurrence in patients with at least one p53 positive surgical margin. eIF4E is a translation initiation factor and powerful oncogene when overexpressed in model cell lines [46]. eIF4E has been reported to be elevated in HNSCC but not in benign lesions and overexpression of eIF4E may represent an early step in malignant transformation. Immunohistochemical analysis for eIF4E expression has shown it to be consistently elevated in patients with SCC. In a study of laryngeal and hypopharyngeal carcinoma [46], 92% (12 of 13 cases) of patients with negative eIF4E in histologically negative surgical margins were free of locoregional recurrences while 67% (12 of 18 patients) with overexpressed eIF4E in the surgical margins developed recurrences disease-free interval of 31.95 months). These authors reported significantly differences in the Kaplan-Meier survival curves for eIF4E-positive and eIF4E-negative margins (P = 0.0002). Molecular margins as presently constituted have not yet supplanted conventional methods of evaluating surgical resection margins.
Accuracy
Carcinoma
In general, the accuracy of frozen section diagnosis in head and neck surgery is high, and when deferred diagnoses are excluded, the reported accuracy ranges from 97 to 99% [11, 47, 48]. Several errors and other factors may account for any potential discrepancies in the frozen section-to-permanent section diagnosis. Errors can be divided into four categories including:
sampling errors;
interpretative errors;
technical errors;
communication errors.
In addition, there are other factors that may influence the result of a frozen section, including (but not limited to):
cooperation between the surgeon and pathologist;
quality assurance program allowing for continuous analysis and critical overview of the frozen section standards;
availability of other pathologists for consultation in challenging cases;
availability of technically competent staff;
equipment.
Lymph Nodes
The frozen section diagnosis of lymph nodes is considered to be extremely accurate. Gnepp in a review of the literature reported that an accuracy rate of 98.9%, excluding deferred diagnoses, with a 0.1% false-positive rate and a 1% false negative rate [49]. It should be noted that the lymph nodes represents the most frequently deferred specimen in frozen section diagnosis, especially in the diagnosis of a lymphoma. In general, the diagnosis of a carcinoma in a lymph node is not problematic at frozen section. Lymph node frozen section has also been utilized for accurate staging of the head and neck patient. Rassekh et al. [50] compared intraoperative node examination (palpation and inspection) to frozen section diagnosis in the identification of metastatic disease to cervical lymph nodes, thereby evaluating the surgeon’s ability to predict nodal stage. These authors prospectively studied 108 necks in 79 patients and reported that the overall reliability for intraoperative staging (palpation and inspection) was 59.3% (64 of 108) as compared to 92.3% (24 of 26) for frozen section biopsy, representing a highly significant difference (P < .005). Although frozen section biopsy was not performed on all cases, Rassekh et al. concluded that upstaging the neck without frozen section biopsy is much less reliable, and that frozen section biopsy is needed prior to converting a selective node dissection to a radical or modified radical neck dissection. Manni and van den Hoogan reached a similar conclusion [51].
Sentinel Lymph Nodes in Head and Neck Squamous Cell Carcinoma
There is increasing literature documenting the efficacy of sentinel lymph node evaluation in patients clinically staged as N0 necks (cN0) with diagnostic accuracy of over 90% and with few reported false negatives [52–57]. Elective treatment of cN0 necks by surgery and/or irradiation is controversial with some authorities advocating a conservative (watch and wait) approach and other authorities advocating treatment. The overall risk of occult metastases or neck recurrence in cN0 neck ranges from 10 to 30% prompting arguments for and against elective treatment. The majority of patients with cN0 necks likely do not harbor occult metastases, but patients with undetected and untreated metastases will experience high failure rates with increased morbidity and mortality; for this reason, sentinel lymph node procedure is gaining more support in the treatment of cN0 neck. To date, there have been limited studies of sentinel lymph node procedure in head and neck squamous cell carcinoma showing:
a sensitivity of greater than 90% in identifying at least one sentinel lymph node;
false negative rate of less than 10% (mean, 3.2%);
the clinical significance of identifying micrometastatic disease (i.e., isolated tumor cells and/or clusters of tumor cells measuring less than .2 mm as defined in breast cancer) relative to head and neck squamous cell carcinoma remains to be clarified but likely is significant in its prognostic import.
Conceptually, lymphatic drainage from a specific anatomic site is assumed to be predictable in the lymphatic drainage; therefore, metastatic tumor cells from a specific location will become deposited into the first (sentinel) node in a lymph node chain. Pathologic work-up, including handling of the gross resection specimen, number of histologic sections, use of special stains (i.e., immunohistochemistry) for the sentinel lymph node procedure for head and neck squamous cell carcinoma has not to date been standardized. Larger clinical trials are needed to determine the efficacy of the sentinel lymph node procedure for head and neck squamous cell carcinoma for staging, treatment and survival of the patient with cN0 neck. The application of sentinel lymph node procedure to head and neck squamous cell carcinoma has not as yet reached the level of standard of patient care.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Dehner LP, Rosai J. Frozen section examination in surgical pathology. Minn Med. 1977;60:83–94. [PubMed] [Google Scholar]
- 2.Ferreiro JA, Myers JL, Bostwick DG. Accuracy of frozen section diagnosis in surgical pathology: review of 1-year experience with 24,880 cases at Mayo Clinic Rochester. Mayo Clin Proc. 1995;70:1137–41. doi: 10.4065/70.12.1137. [DOI] [PubMed] [Google Scholar]
- 3.Holaday WJ, Assor D. Ten thousand consecutive frozen section. A retrospective study focusing on accuracy and quality control. Am J Clin Pathol. 1974;61:769–77. doi: 10.1093/ajcp/61.6.769. [DOI] [PubMed] [Google Scholar]
- 4.Howanitz PJ, Hoffman GG, Zarbo RJ. The accuracy of frozen-section diagnosis in 34 hospitals. Arch Pathol Lab Med. 1990;114:355–9. [PubMed] [Google Scholar]
- 5.Kaufman Z, Lew S, Giffel B, Dinbar A. Frozen-section diagnosis in surgical pathology. A prospective analysis of 526 frozen sections. Cancer. 1986;57:377–9. doi: 10.1002/1097-0142(19860115)57:2<377::AID-CNCR2820570231>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Rogers C, Klatt EC, Chandrasoma P. Accuracy of frozen-section diagnosis in a teaching hospital. Arch Pathol Lab Med. 1987;111:514–7. [PubMed] [Google Scholar]
- 7.Saltzstein SL, Nahum AM. Frozen section diagnosis: accuracy and errors; uses and abuses. Laryngoscope. 1973;83:1128–43. doi: 10.1288/00005537-197307000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Sawady J, Berner JJ, Siegler EE. Accuracy of and reason for frozen sections: a correlative, retrospective study. Hum Pathol. 1988;19:1019–23. doi: 10.1016/S0046-8177(88)80080-7. [DOI] [PubMed] [Google Scholar]
- 9.Zarbo RJ, Hoffman GG, Howanitz PJ. Interinstituional comparison of frozen-section consultation: a College of American Pathologists q-probe study of 79,647 consultations in 297 North American institutions. Arch Pathol Lab Med. 1991;115:1187–94. [PubMed] [Google Scholar]
- 10.Dinardo L, Lin J, Carageore L, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110:1773–6. doi: 10.1097/00005537-200010000-00039. [DOI] [PubMed] [Google Scholar]
- 11.Gandour-Edwards R, Donald PJ, Wiese D. The accuracy and clinical utility of frozen section in head and neck surgery. Evidence at a university medical center. Head Neck. 1993;15:33–8. doi: 10.1002/hed.2880150108. [DOI] [PubMed] [Google Scholar]
- 12.Gandour-Edwards RF, Donald PJ, Lie JT. Clinical utility of intraoperative frozen section diagnosis in head and neck surgery. A quality assurance perspective. Head Neck. 1993;15:373–6. doi: 10.1002/hed.2880150502. [DOI] [PubMed] [Google Scholar]
- 13.Ranchod M. Upper aserodigestive tract. In: Ranchod M (ed) Intraoperative consultations in surgical pathology: state of the art reviews. California Society of Pathologists; 1996, vol 3(2). p. 299–304.
- 14.Byers RM, Bland KL, Borlase B, Luna M. Prognostic and therapeutic value of frozen section determination in the surgical treatment of squamous carcinoma of the head and neck. Am J Surg. 1978;136:525–8. doi: 10.1016/0002-9610(78)90275-1. [DOI] [PubMed] [Google Scholar]
- 15.Bähr W, Stoll P. Intraoperative histological evaluation of tumor resection borders without prolonging surgery. Int J Oral Maxillofac Surg. 1992;21:90–1. doi: 10.1016/S0901-5027(05)80539-9. [DOI] [PubMed] [Google Scholar]
- 16.Spiro RH, Guillamondegui O, Paulino AF, Huvos AG. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck. 1999;21:408–13. doi: 10.1002/(SICI)1097-0347(199908)21:5<408::AID-HED5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 17.Cooley ML, Hoffman HT, Robinson RA. Discrepancies in frozen section mucosal margin tissue in laryngeal squamous cell carcinoma. Head Neck. 2002;24:262–7. doi: 10.1002/hed.10024. [DOI] [PubMed] [Google Scholar]
- 18.Batsakis JG. Surgical excision margins: a pathologist’s perspective. Adv Anat Pathol. 1999;6:140–8. doi: 10.1097/00125480-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Barnes L. Diseases of the larynx, hypopharynx, and esophagus. In: Barnes L, editor. Surgical pathology of the head and neck. Second edition, revised and expanded. New York: Marcel Dekker, Inc; 2001. pp. 127–237. [Google Scholar]
- 20.Looser KG, Shah JP, Strong EW. The significance of “positive” margins in surgically resected epidermoid carcinomas. Head Neck Surg. 1978;1:107–11. doi: 10.1002/hed.2890010203. [DOI] [PubMed] [Google Scholar]
- 21.Mantravadi RV, Haas RE, Leibner EJ, Skolnik EM, Applebaum EL. Postoprative radiotherapy for persistent tumor at the surgical margin in head and neck cancers. Laryngoscope. 1983;93:1337–40. doi: 10.1002/lary.1983.93.10.1337. [DOI] [PubMed] [Google Scholar]
- 22.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–78. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs JR, Ahmad K, Casiano R, et al. Implications of positive surgical margins. Laryngoscope. 1993;103:64–8. doi: 10.1288/00005537-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Scholl P, Byers RM, Batsakis JG, et al. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue. Prognostic and therapeutic implications. Am J Surg. 1986;152:354–60. doi: 10.1016/0002-9610(86)90304-1. [DOI] [PubMed] [Google Scholar]
- 25.Loree TR, Strong EW. Significance of “positive” margins in oral squamous cell carcinoma. Am J Surg. 1990;160:410–4. doi: 10.1016/S0002-9610(05)80555-0. [DOI] [PubMed] [Google Scholar]
- 26.Batsakis JG. Surgical margins in squamous cell carcinoma. Ann Otol Rhinol Laryngol. 1988;97:213–4. doi: 10.1177/000348948809700223. [DOI] [PubMed] [Google Scholar]
- 27.Beitler JJ, Smith RV, Silver CE, et al. Close or positive margins after surgical resection for head and neck cancer patient: the addition of brachytherapy improves local control. Int J Radiat Oncol Biol Phys. 1998;40:313–7. doi: 10.1016/s0360-3016(97)00717-7. [DOI] [PubMed] [Google Scholar]
- 28.Es RJJ, Amerongen N, Slootweg PJ, Egyedi P. Resection margin as a predictor of recurrence at the primary site for T1 and T2 oral cancers. Evaluation of histopathologic variables. Arch Otolaryngol Head Neck Surg. 1996;122:521–5. doi: 10.1001/archotol.1996.01890170055011. [DOI] [PubMed] [Google Scholar]
- 29.Bauer WC, Lesinski SG, Ogura JH. The significance of positive margins in hemilaryngectomy specimens. Laryngoscope. 1975;85:1–13. doi: 10.1288/00005537-197501000-00001. [DOI] [PubMed] [Google Scholar]
- 30.McGregor AD, MacDonald DG. Routes of entry of squamous cell carcinoma to the mandible. Head Neck Surg. 1988;10:294–301. doi: 10.1002/hed.2890100604. [DOI] [PubMed] [Google Scholar]
- 31.McGregor AD, MacDonald DG. Patterns of spread of squamous cell carcinoma within the mandible. Head Neck. 1989;11:457–61. doi: 10.1002/hed.2880110513. [DOI] [PubMed] [Google Scholar]
- 32.Cleary KR, Batsakis JG. Oral squamous cell carcinoma and the mandible. Ann Otol Rhinol Laryngol. 1995;104:977–9. doi: 10.1177/000348949510401212. [DOI] [PubMed] [Google Scholar]
- 33.Weisman RA, Kimmelman CP. Bone scanning in the assessment of mandibular invasion by oral cavity carcinoma. Laryngoscope. 1982;92:1–4. doi: 10.1288/00005537-198201000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Forest LA, Shuller DE, Lucas JG, Sullivan MI. Rapid analysis of mandibular margins. Laryngoscope. 1995;105:475–7. doi: 10.1288/00005537-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Dubner S, Heller KS. Local control of squamous cell carcinoma following marginal segmented mandibulectomy. Head Neck. 1993;15:29–32. doi: 10.1002/hed.2880150107. [DOI] [PubMed] [Google Scholar]
- 36.Beaumont DG, Hains JD. Changes in surgical margins in vivo following resection and after fixation. Aust J Otolaryngol. 1992;1:51–2. [Google Scholar]
- 37.Johnson RE, Sigman JD, Funk GF, Robinson RA, Hoffman HT. Quantification of surgical margin shrinkage in the oral cavity. Head Neck. 1997;19:281–6. doi: 10.1002/(SICI)1097-0347(199707)19:4<281::AID-HED6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 38.Sondenaa K, Kjellvold KH. A prospective study of the length of the distal margin after low anterior resection for rectal cancer. Int J Colorectal Dis. 1990;5:103–5. doi: 10.1007/BF00298480. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein NS, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. Am J Clin Pathol. 1999;111:349–51. doi: 10.1093/ajcp/111.3.349. [DOI] [PubMed] [Google Scholar]
- 40.Gardner ES, Sumner WT, Cook JL. Predictable tissue shrinkage during frozen section histopathologic processing for Mohs micrographic surgery. Dermatologic Surg. 2001;27:813–8. doi: 10.1046/j.1524-4725.2001.01017.x. [DOI] [PubMed] [Google Scholar]
- 41.Silverman MK. Verification of a formula for determination of preexcision surgical margins from fixed-tissue melanoma specimens. J Am Acad Dermatol. 2002;27:214–9. doi: 10.1016/0190-9622(92)70173-d. [DOI] [PubMed] [Google Scholar]
- 42.Fajardo LF. Radiation injury. In: Barnes L, editor. Surgical pathology of the head and neck. Second edition, revised and expanded. New York: Marcel Dekker, Inc; 2001. pp. 2171–90. [Google Scholar]
- 43.Matsusaka S, Nagareda T, Yamasaki H, et al. Immunohistochemical evaluation for intraoperative rapid pathological assessment of the gastric margin. World J Surg. 2003;27:715–8. doi: 10.1007/s00268-003-6792-3. [DOI] [PubMed] [Google Scholar]
- 44.Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–35. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 45.Ball VA, Righi PD, Tejada E, et al. P53 immunostaining of surgical margins as a predictor of local recurrence in squamous cell carcinoma of the oral cavity and oropharynx. Ear Nose Throat J. 1997;76:818–23. [PubMed] [Google Scholar]
- 46.Franklin S, Pho T, Abreo FW, et al. Detection of the protoncogene eIF4E in larynx and hypopharynx cancers. Arch Otolaryngol Head Neck Surg. 1999;125:177–82. doi: 10.1001/archotol.125.2.177. [DOI] [PubMed] [Google Scholar]
- 47.Ord RA, Aisner S. Accuracy of frozen sections in assessing margins in oral cancer resection. J Oral Maxillofac Surg. 1997;55:663–9. doi: 10.1016/S0278-2391(97)90570-X. [DOI] [PubMed] [Google Scholar]
- 48.Remsen KA, Lucente FE, Biller HF. Reliability of frozen section diagnosis in head and neck neoplasms. Laryngoscope. 1984;94:519–24. doi: 10.1288/00005537-198404000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Gnepp DR. Frozen sections. In: Gnepp DR, editor. Pathology of the head and neck. New York: Churchill Livingstone; 1988. pp. 1–24. [Google Scholar]
- 50.Rassekh CH, Johnson JT, Myers EN. Accuracy of intraoperative staging of the N0 neck in squamous cell carcinoma. Laryngoscope. 1995;105:1334–6. doi: 10.1288/00005537-199512000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Manni JJ, Hoogen FJA. Supraomohyoid neck dissection with frozen section biopsy as a staging procedure in the clinically node-negative neck in carcinoma of the oral cavity. Am J Surg. 1991;162:373–6. doi: 10.1016/0002-9610(91)90151-3. [DOI] [PubMed] [Google Scholar]
- 52.Shoaib T, Soutar DS, MacDonald DG, et al. The accuracy of head and neck carcinoma sentinel lymph node biopsy in the clinically N0 neck. Cancer. 2001;91:2077–83. doi: 10.1002/1097-0142(20010601)91:11<2077::AID-CNCR1235>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 53.Pitman KT, Johnson JT, Brown ML, Myers EN. Sentinel lymph node biopsy in head and neck squamous cell carcinoma. Laryngoscope. 2002;112:2101–13. doi: 10.1097/00005537-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Ross GL, Soutar DS, Shoaib T, et al. The ability of lymphoscintigraphy to direct sentinel node biopsy in the clinically N0 neck for patients with head and neck squamous cell carcinoma. Br J Radiol. 2002;75:950–8. doi: 10.1259/bjr.75.900.750950. [DOI] [PubMed] [Google Scholar]
- 55.Asthana S, Deo SV, Shukla NK, et al. Intraoperative neck staging using sentinel node biopsy and imprint cytology in oral cancer. Head Neck. 2003;25:368–72. doi: 10.1002/hed.10211. [DOI] [PubMed] [Google Scholar]
- 56.Civantos FJ, Gomez C, Duque C, et al. Sentinel node biopsy in oral cavity cancer correlation with PET scan and immunohistochemistry. Head Neck. 2003;25:1–9. doi: 10.1002/hed.10213. [DOI] [PubMed] [Google Scholar]
- 57.Loree TR. Sentinel lymph node biopsy for early stage clinical N0 squamous cell carcinoma of the oral cavity. Ann Surg Oncol. 2004;11:725–6. doi: 10.1245/ASO.2004.05.922. [DOI] [PubMed] [Google Scholar]