Abstract
Background Several recent reports suggest an increasing incidence of oral squamous cell carcinoma (OSCC) among young persons in many regions of the world—a trend which is particularly concerning given the overall stabilization or even decline in incidence rates for head and neck cancer in general. The aim of this study is to determine whether there has been an increase in the number of cases of OSCC diagnosed in patients <40 years old by our biopsy service from 1971 to 2006. Methods A retrospective review of all OSCC cases diagnosed from 1971 to 2006 by the Emory University Hospital Oral Pathology biopsy service was performed. A comparison of demographic information, frequency, location and histologic grade was made between these cases as a whole and those occurring in a subset of patients <40 years old. Statistical procedures included chi-square analyses. Results From 1971–2006, 1,919 cases of OSCC were diagnosed, and 95 (5.0%) occurred in patients <40 years old. A total of 8 cases were diagnosed from 1971 to 1980, compared to 36 cases during the period 1981–1990, 31 during the period 1991–2000 and 21 cases from 2001 to 2006. The increase in OSCC incidence from the 1970s when compared to 1981–2000 was statistically significant (P < 0.002). A 1.7:1 male:female ratio was seen in all decades. The mobile (oral) tongue was the most common location in all decades (62.1%) in young patients. In contrast, tongue cancers accounted for 27.4% in patients ≥40. This difference between the two groups was statistically significant (P < 0.0001). Of great surprise, however, was the significant increase in tongue cancer during the study period in patients ≥40 which accounted for 37.1% of all OSCC diagnoses from 2001 to 2006, compared to 20.5% of OSCC cases from 1971 to 1980 (P < 0.0001). Conclusions We demonstrated a greater than fourfold increase in the incidence of oral squamous cell carcinoma (OSCC) in young patients <40 years old beginning in 1974 and peaking in the late 1980s, then remaining stable. The mobile tongue is the most common location for cancer in this age group accounting for 62.1% of cancers. However, the mobile tongue increasingly appears to be the most common site for oral cancer in all age groups.
Keywords: Oral cancer, Incidence, Tongue, Young
Introduction
Oral squamous cell carcinoma (OSCC) predominantly occurs in individuals during the fifth through eighth decades of life [1]. Among young persons, the occurrence of OSCC is rare, representing from 0.9 to 2.7% of all cases [2]. However, several recent reports suggest an increasing incidence of this disease among young persons in many regions of the world—a trend which is particularly concerning given the overall stabilization or even decline in incidence rates for head and neck cancer in general [3–5]. For instance, Chen et al. reported that between the 1960s and mid-1980s, there was a nearly fourfold increase in oral cancer incidence among male patients 30–39 years of age in the Connecticut Tumor Registry and University of Connecticut Oral Pathology Biopsy Service [6]. Moreover, Myers et al. found that the percentage of tongue SCC patients at University of Texas M.D. Anderson Cancer Center who were younger than 40 years at presentation increased from less than 10% in 1948 to between 15 and 25% in the mid-1990s [7]. Analyzing data from the Vaud Cancer Registry in Switzerland, Levi et al. calculated an increase in the age-standardized incidence rate of oral and pharyngeal cancer for young male adults from 39 per million during the years 1974–1979 to 65 per million during the years 1986–1992 [8]. Atula et al. found that among cases of tongue SCC registered in Finland, the percentage of cases occurring in young adults increased from 4.3% in the 1960s to 8.6% in the 1970s and 7.2% in the 1980s [9]. Oral cancer mortality rates for young patients also have been increasing over the last 30–50 years both in Europe [10, 11] and the United States [12]. Schantz et al. analyzing the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database noted a significant increase in tongue cancer incidence from 1973 to 1985 in patients younger than 40 years. After 1985, the incidence rates stopped rising but remained high [3]. Shiboski et al. also analysed the SEER database from 1973 to 2001 in adults aged 20–44 years [4]. A significant increase in both palatine tonsil/base of tongue and oral tongue SCC was noted in this age group compared to SCC in other oral and pharyngeal sites, which remained constant or even declined.
Prompted by these striking findings of increasing incidence and mortality, we wondered whether such trends were reflected in the large pool of OSCC cases seen at Emory University Hospital, located in a large metropolitan area of the Southeastern United States. Specifically, we aimed to establish whether there was an increase in the frequency of OSCC cases in patients <40 years of age recorded by the Emory University Hospital Oral Pathology biopsy service over a three-decade period spanning from 1971 to 2006.
Methods
A retrospective review was performed of all cases diagnosed as invasive SCC of the oral cavity by the Emory University Hospital Oral Pathology biopsy service from 1971 to 2006 after obtaining Institutional Review Board approval. Age at presentation, gender, primary site, and histologic grade were recorded. Cases occurring within the oral cavity (i.e., mobile tongue, floor of mouth, hard palate, gingiva/alveolar mucosa, buccal mucosa/vestibule/labial mucosa), lip vermilion, and intrabony mandible/maxilla were included in the study. We examined only biopsies from the oral or mobile tongue and did not include oropharyngeal sites, including tonsils and base of tongue.
Comparisons were made between OSCC patients less than 40 years of age and those greater than 40 years old to find out if there is difference between these two age cohorts. In addition, comparisons was made between data collected for the decades 1971–1980, 1981–1990, 1991–2000, and 2001–2006, for all subjects and separately for the two age cohorts, to detect possible variations and trends across decades. Pearson Chi-square statistics are used to analyze categorical variables while two sample t-tests are used to analyze continuous variables. All analyses are performed in SAS 9.1.
Results
A summary of data collected for all patients diagnosed with OSCC for the period 1971–2006 and for each period subdivision is given in Table 1. During the entire study period, out of a total of 159,407 total cases accessioned, 1,919 cases of OSCC were diagnosed in this population, representing 1.2% of total cases accessioned. With each decade, the percentage of OSCC diagnosed in the biopsy service remained relatively stable. Age data were available for all but 47 cases, with an average age at presentation of 63.7 years (range 18–95 years). The average age at presentation for female patients (66.4 years) was approximately 5 years greater than that for male patients (61.5 years) (P < 0.001). There was a slight male predilection with a male to female ratio of 1.2:1, which remained relatively unchanged during the 35–year study.
Table 1.
Oral squamous cell carcinoma (OSCC) incidence and demographic table for all subjects
| 1971–2006 | 1971–1980 | 1981–1990 | 1991–2000 | 2001–2006 | |
|---|---|---|---|---|---|
| OSCC incidence | |||||
| # OSCC patients | 1,919 | 446 | 474 | 669 | 463 |
| # total accessions | 159,407 | 29,157 | 41,473 | 49,241 | 39,536 |
| % of total accessions | 1.2 | 1.5 | 1.1 | 1.4 | 1.2 |
| Age (years) | |||||
| Average age | 63.7 | 63.1 | 63.0 | 63.9 | 64.6 |
| Average age of males | 61.5 | 61.8 | 60.4 | 61.2 | 62.8 |
| Average age of females | 66.4 | 64.7 | 66.1 | 67.2 | 66.8 |
| Gender | |||||
| % of female patients | 45.0 | 32.1 | 45.6 | 45.0 | 43.8 |
| (# females/total OSCC patients) | (863/1,919) | (143/446) | (216/474) | (301/669) | (203/463) |
A summary of data comparing and contrasting patients with OSCC <40 years of age and those ≥40 years for each study period 1971–2006 and for each decade is displayed in Table 2. Since no significant difference is detected between patients with age missing and patients with age recorded, we assume Missing Completely at Random here and omitted the missing data from this analysis. During a 35-year study period, a total of 95 patients <40 were diagnosed, representing 5.0% of all OSCC cases, and 0.1% of all cases accessioned. Eight patients were diagnosed in the 1970s. The first ever known diagnosis of OSCC in a patient <40 years of age in the Emory University Oral Pathology Service was in 1974. One case was reported each in 1977 and 1978, 2 cases in 1979, and 3 cases in 1980. Thirty-six patients presented in the 1980s, 31 patients presented in the 1990s, and from 2001 to 2006, 21 cases were reported. When we look at the percentage of OSCC patients <40 years of age among all OSCC patients, the overall chi-square test shows this number did not stay stable over the study period (P = 0.01). Specifically, there was a significant increase from the 1970s to the 1980s (2.56–7.59%, P = 0.003), a small decrease from 1980s to the 1990s (7.59–4.48%, P = 0.03), and then a plateau from 1990s to 2001–2006. A total of 1755 OSCC diagnoses were made during the 35 year study period in patients ≥40 years. The incidence of OSCC diagnosis in this cohort remained relatively stable over the 35-year study period, accounting for 1.1% of all accessions, and 95.0% of all OSCC diagnoses.
Table 2.
Demographic table by age group
| Patients younger than 40 | Patients, age 40 and older | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1971–2006 | 1971–1980 | 1981–1990 | 1991–2000 | 2001–2006 | 1971–2006 | 1971–1980 | 1981–1990 | 1991–2000 | 2001–2006 | |
| OSCC Incidence | ||||||||||
| % of total accessions | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 1.1 | 1.0 | 1.1 | 1.3 | 1.1 |
| (# OSCC/# total accessions) | (95/159,407) | (8/29,157) | (36/41,473) | (30/49,241) | (21/39,536) | (1,824/159,407) | (305/29,157) | (438/41,473) | (639/49,241) | (442/39,536) |
| % of all OSCC patients | 5.0 | 2.6 | 7.6 | 4.5 | 4.5 | 95.0 | 97.4 | 92.4 | 50.0 | 95.5 |
| (# OSCC/# total OSCC patients) | (95/1,919) | (8/313) | (36/474) | (30/669) | (21/463) | (1,824/1,919) | (305/313) | (438/474) | (639/669) | (442/463) |
| Age (years) | ||||||||||
| Average age | 32.5 | 30.9 | 31.8 | 32.6 | 34.2 | 65.3 | 64.0 | 65.6 | 65.4 | 66.0 |
| Average age of males | 32.8 | 31.2 | 33.0 | 31.9 | 35.1 | 63.1 | 62.7 | 63.0 | 62.7 | 63.7 |
| Average age of females | 32.1 | 30.3 | 29.7 | 33.7 | 33.6 | 68.1 | 65.4 | 68.5 | 68.6 | 68.9 |
| Gender | ||||||||||
| % of female patients | 42.1 | 12.5 | 36.1 | 40.0 | 57.1 | 45.2 | 68.6 | 46.3 | 45.4 | 39.2 |
| (# females/total OSCC patients) | (40/95) | (3/8) | (13/36) | (12/30) | (12/21) | (823/1,822) | (140/204) | (203/438) | (289/637) | (252/643) |
OSCC: oral squamous cell carcinoma
In the younger cohort, the average age at presentation of 32.5 years (range 18–39) was similar for both females and males. This is in contrast to the older cohort where females were on average 5 years older than males (68.1 vs. 63.1) (P < 0.001). Of the 95 OSCC in patient <40 years, 55 were males and 40 females. The male to female ratio was 1.4:1 which was slightly larger than the 1.2:1 ratio in the older cohort, but this difference was not statistically different (P = 0.558).
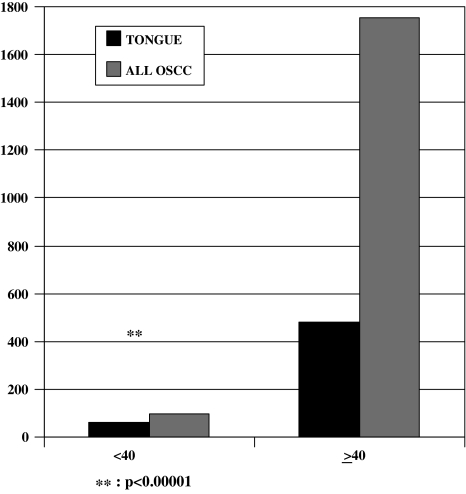
The anatomic location and histologic tumor grade for each age group from 1971 to 2006 are presented in Table 3. From 1971–2006, the most common sites for cancer in patients <40 years were overwhelmingly in the mobile tongue (62.1%), followed by lip vermilion (12.6%), and buccal mucosa/vestibule/labial mucosa (10.5%). This high incidence of cancer in the mobile tongue was statistically significant (P < 0.0001) when compared to the other sites of occurrence. In fact when we examined the last 5 years, 2001–2006, 85.7% (18/21) of OSCC cancers diagnosed in this age group were mobile tongue cancers. The percentage of mobile tongue cancer increased significantly from 1991–2000 to 2001–2006 (P = 0.02), while no significant change was detected for incidence in other locations and between other study periods. Also, the incidence in mobile tongue cancer in young patients is significantly higher than the incidence in patients ≥40 years old (62.1% vs. 27.4%, P < 0.0001) (Fig. 1).
Table 3.
Observed incidence rates of oral cancer by anatomic site for each age group and period, 1971–2006
| Patients younger than 40 | Patients, age 40 and older | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1971–2006 | 1971–1980 | 1981–1990 | 1991–2000 | 2001–2006 | 1971–2006 | 1971–1980 | 1981–1990 | 1991–2000 | 2001–2006 | |
| Anatomic location | ||||||||||
| N | 95 | 8 | 36 | 30 | 21 | 1755 | 302 | 412 | 610 | 431 |
| Mobile tongue | 62.1% | 62.5% | 55.6% | 53.3% | 85.7% | 27.4% | 20.5% | 20.6% | 28.5% | 37.1% |
| Floor of mouth | 4.2% | 12.5% | 0.0% | 10.0% | 0.0% | 22.1% | 25.8% | 25.0% | 21.3% | 17.6% |
| Gingiva/alveolar mucosa | 7.4% | 0.0% | 13.9% | 3.3% | 4.8% | 17.3% | 18.2% | 24.3% | 16.9% | 10.7% |
| Buccal mucosa/vestibule/labial mucosa | 10.5% | 0.0% | 13.9% | 13.3% | 4.8% | 15.2% | 10.3% | 16.5% | 16.7% | 15.1% |
| Lip vermilion | 12.6% | 25.0% | 11.1% | 16.7% | 4.8% | 7.1% | 7.3% | 6.3% | 4.8% | 11.1% |
| Hard palate | 2.1% | 0.0% | 2.8% | 3.3% | 0.0% | 4.2% | 3.3% | 3.2% | 4.9% | 4.9% |
| Retromolar trigone | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 4.0% | 5.3% | 3.4% | 4.4% | 3.2% |
| Mandible/maxilla | 1.1% | 0.0% | 2.8% | 0.0% | 0.0% | 2.7% | 9.3% | 0.7% | 2.5% | 0.2% |
| Histologic grade | ||||||||||
| N | 94 | 8 | 36 | 29 | 21 | 1819 | 305 | 438 | 636 | 440 |
| Well differentiated | 59.6% | 62.5% | 58.3% | 58.6% | 61.9% | 58.4% | 55.4% | 59.4% | 60.2% | 56.8% |
| Moderately differentiated | 24.5% | 12.5% | 16.7% | 31.0% | 33.3% | 30.8% | 23.6% | 29.5% | 33.5% | 33.2% |
| Poorly differentiated | 7.4% | 0.0% | 11.1% | 6.9% | 4.8% | 5.7% | 8.2% | 2.7% | 4.9% | 8.0% |
| Grade not available | 8.5% | 25.0% | 13.9% | 3.4% | 0.0% | 5.2% | 12.8% | 8.4% | 1.4% | 2.0% |
For histologic grade, number greater than total number of patients due to multiple sites recorded
Fig. 1.
From the years 1971–2006, cancer of the mobile tongue accounted for 62.1% of all oral squamous cell cancer (OSCC) cases in patients <40 years of age. This compares to cancer of the mobile tongue occurring in 27.4% of patients ≥40 years of age during the same time period. The incidence in mobile tongue cancer in young patients when compared to the incidence in patients ≥40 years old was highly statistically significant (P < 0.00001)
In patients ≥40 years of age, the most common sites of OSCC in order of decreasing frequency were mobile tongue (27.4%), floor of mouth (22.1%), and gingiva/alveolar mucosa (17.3%). Although not as dominant as in younger patients, the incidence of tongue cancer has been increasing (monotonically) among older patients. This increase is significant from the 1980s to the 1990s (20.6–28.5%, P = 0.005) and from the 1990s to 2001–2006 (28.5–37.1%, P = 0.0004). In contrast a decreasing trend (monotonically) was noted in the incidence of floor of mouth, while incidence rates of other locations exhibit some fluctuation.
The percentage of cases by histologic grade category are provided in Table 3. These observed histologic grades were similar throughout the three decades and were not significantly different between the younger and older cohorts.
Discussion
We demonstrated a sharp increase in the incidence of OSCC in young patients, beginning in 1974, peaking in the late 1980s, and remaining steady through 2006. Only 8 cases (2.3%) of OSCC were diagnosed in young patients in the 1970s from our biopsy service compared to 36 cases (6.8%) in the 1980s which represents a greater than fourfold increase (P < 0.002). When comparing the 1980s to the 1990s, and 1990s to 2001–2006, there were no statistically significant changes in the incidence of OSCC in this population. These findings reflect the published SEER data for the 1970s–1990s. The overall 35-year OSCC incidence in the biopsy service for the younger cohort was 5.2%. In the 1980s, 7.8% of all OSCC diagnosed in the biopsy service were in persons <40 years. These numbers are higher than the previously reported range of 0.4–4.8% [13].
In our study, there was a significantly greater proportion of oral cancer cases involving the mobile tongue in the young cohort compared to patients ≥40 years old (62.1% vs. 27.4%, P < 0.00001). Indeed several other authors examining OSCC in a young population also have found the mobile tongue to be the most common site of occurrence [2, 3, 13–15]. Martin-Granizo et al. reported 29% of cases affecting the tongue [2]. Slightly higher proportions of cases affecting the mobile tongue were found by other authors (e.g., 38% by Hart et al., 41% by Son and Kapp, 45% by Mackenzie et al.) [13, 15, 16]. In Shantz et al.’s review of the SEER database, tongue cancer in patients <40 years old increased 62% from 1973–1984 to 1985–1997 and accounted for 35% of OSCC [3]. Why our population had a higher percentage of mobile tongue cancers than other studies is difficult to determine.
An unexpected finding was the significant increase in mobile tongue cancers in patients ≥40 years of age. The SEER data shows a small, but steady decline of oral cancer in all anatomic sites during 1976–2004 including the oral tongue [1]. In our population, however, we observed a significant increase in oral tongue OSCC from the 80s to the 1990s (P < 0.005) and from the 1990s to 2001–2001 period (P < 0.004). This was the only anatomic site that consistently increased in incidence over the 35-year study period and accounted for a remarkable 37.1% of OSCC diagnoses in 2001–2006.
Significant decreases in OSCC diagnoses from the gingival/alveolar mucosa were noted during the same time period in the older cohort, and the other anatomic sites with the exception of lip were unchanged in incidence. We saw a significant increase in lip cancer diagnoses from the 1990s to 2001–2006 (P < 0.002), but not in the other time periods examined.
An interesting finding was the greater incidence of SCC of the lip vermilion in the young patient subset (12.6% of OSCC cases) than in the ≥40 patient population (7.1% of OSCC cases) (P < 0.05%). This increased incidence is in contradistinction to the SEER database, which showed a much higher incidence of lip cancer in patients ≥40 years old. Although lip cancer is generally included in oral cancer statistics, its etiology in part is thought to be distinct from that of intraoral cancer. SCC of the lip vermilion is typically caused by acute sun damage early in life or by chronic sun exposure, whereas SCC of the labial mucosa and other intraoral sites most often is associated with tobacco use with or without alcohol consumption.
There are five previous studies reported in the literature examining OSCC in patients 40 years of age and younger [2, 3, 13–15]. In many ways our findings are similar to those of prior studies. In our study the average age at presentation of the young patient subset was 32.5 years, similar to the average ages of 31.7 years reported by Son and Kapp [15] and 34.8 years reported by Martin-Granizo et al. [2]. The two youngest patients in our study were 19 years of age at presentation. Of the remaining patients in the young patient subset, 24 (25.2%) presented in the third decade of life, and 69 (72.6%) presented in the fourth decade of life. Similarly, Martin-Granizo et al. found a majority (79.2%) of young OSCC patients presenting in the fourth decade of life [2]. Thus, although OSCC is generally rare among patients 40 years of age and younger, when it does occur in this age group, it seems to have a peak incidence among patients in the fourth decade, in keeping with the generally accepted increased risk of OSCC with increasing age. The male: female ratio for cases in our young patient subset was 1.7:1, which was slightly greater than the ratio of 1.2:1 for the ≥40 OSCC population studied. A similar slight male predilection among OSCC patients 40 years and younger was reported by Martin-Granizo et al. and Mackenzie et al., who found male-to-female ratios of 1.6:1 and 1.9:1, respectively [2, 13]. In contrast, Cusumano and Persky [14] found a male-to-female ratio of 6.7:1, although this included both oral and oropharyngeal carcinoma. These authors did not comment on why there was such a dramatic male predilection among the cases they studied. Cusumano and Persky did note, however, that patients with oral cavity lesions were more often female whereas patients with oropharyngeal lesions were more often male.
OSCC seems to be extremely rare in the first decade. To the best of our knowledge, the youngest OSCC patient reported in the literature was a 7-year-old boy with a lesion of the maxillary alveolar ridge who was otherwise healthy [16]. Defining a general category of “young” patients as less than 40 years of age may be somewhat arbitrary, for it is not known whether the etiology and pathogenesis of OSCC in infancy and childhood is fundamentally different from that of OSCC in young adults. However, we believe that using this traditional definition was justified in order to allow for comparisons with previous studies and the SEER database. More than 95% of OSCC occur in people ≥40 years old with a mean age of onset in the seventh decade.
Our study was restricted to analysis of cases diagnosed by our biopsy service, and thus we are not presuming to extrapolate our findings in order to make conclusions concerning trends in the world population. Our patient population primarily hails from the Southeastern United States, although there are appreciable numbers of patients from other regions of the country as well. Cases seen by our biopsy service primarily include patients seen by health care providers (i.e., general dentists, oral surgeons, periodontists, and otolaryngologists) in private practice and managed care settings but also include a smaller number of patients receiving care at Emory University Hospital, which is a tertiary health care facility. In this respect, the patient population we studied may be more representative of the general population than previous studies solely limited to the experience of a tertiary health care facility and thus influenced by an inherent selection bias. Since the cases we analyzed were obtained from our biopsy service, in many cases information regarding risk factors (e.g., tobacco and alcohol usage), staging, treatment, and patient outcome were unavailable since most of the patients were not treated at Emory University Hospital. Although we attempted to gather some of this information by contacting individual providers, in many cases the information was not available due to lack of documentation, lack of patient follow-up, or purging of old patient records. Information regarding risk factors would have been interesting to examine, since the etiology of OSCC in young patients is not entirely clear. The primary risk factors for OSCC are tobacco and alcohol use. While some studies report that young patients parallel the OSCC population in general by displaying a high proportion of cases with a history of significant tobacco and alcohol use, others suggest that young patients usually lack such a history [3, 13, 17–19]. In addition, exposure to these risk factors must be of long enough duration to have a detrimental effect. Llewellyn et al. analyzed risk factors for oral cancer in patients 45 and younger and showed a significantly elevated odds ratios for oral cancer in patients who smoked ≥21 years [20]. This points to the potential influence of additional etiologic factors–such as genetic susceptibility, immunodeficiency, or viral infection–may be at work [2, 3, 12, 21, 22]. Lingen et al. studied the overexpression of p53 in individuals <40 years old with tongue cancer and no known risk factors [17]. They noted that 81% of these patients overexpressed p53 protein but did not exhibit mutations in exons 5–9 of the p53 gene, which are known to exist in at least 50% of OSCC in older patients. Thus, the molecular mechanisms of OSCC development may differ in young patients compared to older patients.
One might expect that a proportion of patients in our study had a history of smokeless tobacco use because the majority of patients in the population we studied were from the Southeastern United States. During the 35-year study period, especially from 1971 to 1987, this region of the country traditionally had high smokeless tobacco use rates [23]. However, the OSCC increase also was seen in young women whose use of smokeless tobacco is very low (prevalence 0.3%) [3]. Similarly, smokeless tobacco should induce cancer where the tobacco is held such as the gingiva, buccal mucosa and vestibule. Thus, one might expect to find a greater proportion of cases affecting the vestibule, buccal mucosa, labial mucosa, gingiva and alveolar mucosa compared to studies conducted in other geographic areas. However, in comparing our results with other studies, we did not find any significant differences in the proportion of cases affecting these sites. For example, we found that 16% of the young patient subset had lesions of the buccal mucosa/vestibule/labial mucosa or gingiva/alveolar mucosa. Similarly, Martin-Granizo et al. found that 16.7% of OSCC cases among young patients treated at a hospital in Madrid, Spain occurred in the alveolar or buccal mucosa, and Son and Kapp found that 22% of OSCC cases among young patients seen at Yale-New Haven Medical Center and affiliated hospitals affected the alveolar or buccal mucosa [2, 15]. In the ≥40 OSCC population, when combining the anatomic site of gingiva/alveolar mucosa and buccal mucosa/vestibule/labial mucosa these account for a total of 32.5% of OSCC diagnoses. We found it difficult to compare this finding to that of other studies, however, because most other studies in accordance with ICD-9 criteria designated locations outside of the tongue, floor of mouth, and gingiva simply as “other mouth” without further specification.
Other proposed risk factors include the use of marijuana, although no studies have shown a statistically significant elevation in the risk of OSCC with cannabis use in the younger cohort [20, 24]. Poor nutrition, including low consumption of fruits and vegetables, also has been suggested as a risk factor. Llewellyn et al. demonstrated that long-term consumption of a diet of fresh fruits and vegetables reduced the risk of OSCC in patients ≤45 years [20].
Conclusions
We have shown a sharply increasing trend in the number of OSCC in patients <40 years old, with a greater than fourfold increase from the 1970s to the 1980s from the Emory University Oral Pathology biopsy service. Our findings are similar to the SEER data as well as data presented from other Western countries. The mobile tongue accounted for the majority of OSCC diagnosed in this cohort accounting for almost 62.1% of all diagnoses. Although the etiologic factors responsible for oral cancer in young patients, most likely share some similarities to OSCC in the general population, there may be some distinct, but as yet, unidentified causes. Fortunately, oral cancer in persons <40 is still a rare event, however the rising trend is worrisome.
Of note, there was also a significant increase in mobile tongue cancer diagnosis in persons ≥40, accounting for almost 37.1% of cases from 2001 to 2006. Why our experience with rising rates of oral tongue cancer diagnoses in the older population is not as of yet reflected in the SEER data cannot be determined. Shantz et al. noted that the rising trend in tongue cancers started with patients born after 1938, and lifestyle factors may play a role. These authors pondered whether the incidence of tongue cancers may increase as the population ages [3]. Perhaps oral tongue cancer is a distinct disease process associated with unidentified risk factors that impact all age groups and warrants continued investigation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Presented in part at the annual meeting of the United States and Canadian Academy of Pathology, San Antonio, TX, March 2005.
References
- 1.Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al., editors. SEER cancer statistics review, 1975–2004. Bethesda, MD: National Cancer Institute. http://www.seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site (2007).
- 2.Martin-Granizo R, Rodriguez-Campo F, Gonzalez FJD. Squamous cell carcinoma of the oral cavity in patients younger than 40 years. Otolaryngol Head Neck Surg. 1997;117:268–75. doi: 10.1016/S0194-5998(97)70185-2. [DOI] [PubMed] [Google Scholar]
- 3.Schantz SP, Yu G-P. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Archives of Otolaryngol Head Neck Surg. 2002;128:268–74. doi: 10.1001/archotol.128.3.268. [DOI] [PubMed] [Google Scholar]
- 4.Shiboski CH, Schmidt BL, Jordan RCK. Tongue and tonsil carcinoma. Increasing trends in the US population ages 20–44 years. Cancer. 2005;103:1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DP, Irish JC. Head and neck squamous cell carcinoma in the young patient. Curr Opin Otolaryngol Head Neck Surg. 2005;13:207–11. doi: 10.1097/01.moo.0000170529.04759.4c. [DOI] [PubMed] [Google Scholar]
- 6.Chen JK, Eisenberg E, Krutchkoff DJ, Katz RV. Changing trends in oral cancer in the United States, 1935 to 1985: a Connecticut study. J Oral Maxillofac Surg. 1991;49:1152–8. doi: 10.1016/0278-2391(91)90406-C. [DOI] [PubMed] [Google Scholar]
- 7.Myers JN, Elkins T, Roberts D, Byers RM. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg. 2000;122:44–51. doi: 10.1016/S0194-5998(00)70142-2. [DOI] [PubMed] [Google Scholar]
- 8.Levi F, La Vecchia C, Randimbison L, Te VC. Cancer incidence and mortality in young adults in Vaud, Switzerland, 1974–1992. Int J Cancer. 1995;61:606–10. doi: 10.1002/ijc.2910610504. [DOI] [PubMed] [Google Scholar]
- 9.Atula S, Grenman R, Laippala P, Syrjanen S. Cancer of the tongue in patients younger than 40 years: a distinct entity? Arch Otolaryngol Head and Neck Surg. 1996;122:1313–9. doi: 10.1001/archotol.1996.01890240021006. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi S, Levi F, Lucchini F. Trends in cancer mortality in young adults in Europe, 1995–1989. Euro J Cancer. 1994;30A:2096–118. doi: 10.1016/0959-8049(94)00429-9. [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane GJ, Boyle P, Scully C. Rising mortality from cancer of the tongue in young Scottish males. Lancet. 1987;2(8564):912. doi: 10.1016/S0140-6736(87)91394-8. [DOI] [PubMed] [Google Scholar]
- 12.Depue RH. Rising mortality from cancer of the tongue in young white males. NEJM. 1986;315:647. doi: 10.1056/NEJM198609043151013. [DOI] [PubMed] [Google Scholar]
- 13.Mackenzie J, Ah-See K, Thakker N, Sloan P, Maran AG, Birch J, et al. Increasing incidence of oral cancer amongst young persons: what is the aetiology? Oral Oncol. 2000;36:387–9. doi: 10.1016/S1368-8375(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 14.Cusumano RJ, Persky MS. Squamous cell carcinoma of the oral cavity and oropharynx in young adults. Head Neck Surg. 1988;10:229–34. doi: 10.1002/j.1930-2398.1988.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 15.Son YH, Kapp DS. Oral cavity and oropharyngeal cancer in a younger population. Review of literature and experience at Yale. Cancer. 1985;55:441–4. doi: 10.1002/1097-0142(19850115)55:2<441::AID-CNCR2820550225>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Hart AKE, Karakla DW, Pitman KT, Adams JF. Oral and oropharyngeal squamous cell carcinoma in young adults: a report on 23 cases and review of the literature. Otolaryngol Head Neck Surg. 1999;120:828–33. doi: 10.1016/S0194-5998(99)70322-0. [DOI] [PubMed] [Google Scholar]
- 17.Lingen MW, Chang KW, McMurray SJ, Dlot DB, Kies MS, Mittal B, et al. Overexpression of p53 in squamous cell carcinoma of the tongue in young patients with no known risk factors is not associated with mutations in exons 5–9. Head Neck. 2000;22:328–35. doi: 10.1002/1097-0347(200007)22:4<328::AID-HED3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Earle A, Park C, Vlastou C. Oral squamous cell carcinoma in children. Ann Plast Surg. 1988;20:148–52. doi: 10.1097/00000637-198802000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Lipkin Squamous cell carcinoma of the oral cavity, pharynx, and larynx in young adults. Laryngoscope. 1985;95:790–3. doi: 10.1288/00005537-198507000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya S. An analysis of risk factors for oral cancer in young people: a case-control study. Oral Oncol. 2004;40:304–13. doi: 10.1016/j.oraloncology.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Slotman GJ, Swaminathan AP, Rush BF. Head and neck cancer in a young age group; high incidence in black patients. Head Neck Surg. 1983;5:293–8. doi: 10.1002/hed.2890050404. [DOI] [PubMed] [Google Scholar]
- 22.Oliver RJ, Dearing J, Hindle I. Oral cancer in young adults: report of three cases and review of the literature. Brit Dent J. 2000;188:362–5. doi: 10.1038/sj.bdj.4800481a. [DOI] [PubMed] [Google Scholar]
- 23.Giovino GA, Schooley MW, Zhu BP, Chrismon JH, Tomar SL, Peddicord JP, et al. Surveillance for selected tobacco-use behaviors—United States, 1900–1994. MMWR CDC Surveill Summ. 1994;43:1–43. [PubMed] [Google Scholar]
- 24.Hashibe Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1829–34. doi: 10.1158/1055-9965.EPI-06-0330. [DOI] [PubMed] [Google Scholar]