Abstract
Background Nasopharyngeal angiofibroma (also known as juvenile nasopharyngeal angiofibroma) is a rare fibroblastic tumor with a vascular component that occurs in the nasopharynx and posterolateral nasal wall of adolescent boys. The etiology of nasopharyngeal angiofibroma remains elusive. This investigation was undertaken to determine if human herpes simplex virus-8 and Epstein–Barr virus are possible etiologic viruses and to determine if they have any association with the age of the patient and/or the proliferative state of the lesion. Materials and methods Formalin fixed, routinely processed, and paraffin embedded surgical specimens of 15 angiofibromas were submitted to PCR for EBV and HHV-8, while in situ hybridization was also employed for EBV. Immunohistochemical analysis for ki-67 was performed using MIB immunostaining. Results None of the tumors were positive for HHV-8. The PCR technique produced a false positive reaction in five cases, with all cases non-reactive with EBV-ISH. The age of the patients did not show correlation with the Ki-67 labeling index. Conclusion Angiofibroma does not appear to be associated with either HHV-8 or EBV, thereby excluding these viruses as potential etiologic agents. The lack of a correlation between the proliferative index and the age of the patient suggests the proposed puberty induced, testosterone-dependent tumor growth may not play a significant role in tumor development.
Keywords: Nasopharyngeal juvenile angiofibroma, Immunohistochemistry, Vascular, Neoplasm, EBV, HHV-8, Ki-67, MIB, Males, Tumor growth, Adolescent, Angiofibroma, Molecular, In situ hybridization
Introduction
Nasopharyngeal angiofibroma (also known as juvenile nasopharyngeal angiofibroma) is a rare fibroblastic tumor with a vascular component that occurs in the nasopharynx of adolescent boys. This benign, locally aggressive neoplasm represents approximately 1% of all nasopharyngeal tumors [1].
Nasopharyngeal angiofibroma affects almost exclusively boys and adolescent to young men, with a peak in the 2nd decade of life. Patients usually present with nasal obstruction and/or recurrent, spontaneous epistaxis, nasal discharge, facial deformity (including proptosis) and/or diplopia. Tumors have the potential to spontaneously regress, but occasionally they are massively infiltrative, resulting in death due to intracranial complications. The unique gender predilection and potentially significant morbidity associated with a benign tumor provoke a scientific interest in their possible etiology.
As nasopharyngeal angiofibroma has a vascular component, angiogenesis is proposed to be an essential process required for tumor growth. In fact, vascular endothelial growth factor (VEGF), a key regulator of angiogenesis, was demonstrated to be expressed in neoplastic endothelial cells [2, 3]. Evaluation for the presence of estrogen, progesterone, and androgen receptors as possible etiologic agents in tumor development has resulted in conflicting data [4–7]. Testosterone-dependent, puberty-induced tumor growth has been proposed, with possible growth retardation when estrogen or progesterone receptors within the tumor are blocked [8].
Human herpes virus-type 8 (HHV-8) is associated with Kaposi sarcoma, a vascular neoplasm [9]. Epstein–Barr virus is commonly detected in lesions of the oropharynx and nasopharynx, and is specifically identified as an etiologic agent in nasopharyngeal carcinoma, Burkitt lymphoma and Hodgkin lymphoma, all tumors which show a predilection for the head and neck. The actions of EBV viral proteins mimic several growth factors, transcription factors, and antiapoptosis factors, thus leading to loss of control of various important cellular homeostatic pathways [10]. To the best of our knowledge in a review of Medline (1966–2008) the presence of these two viruses have not been studied in nasopharyngeal angiofibroma. This investigation was conducted to determine the presence of HHV-8 and EBV in 15 cases of nasopharyngeal angiofibroma. Further, ki-67 labeling index was determined and matched to the patient’s age to determine if there was a correlation between patient age and tumor proliferation as measured by MIB immunoreactivity.
Material and Methods
Tissue Samples
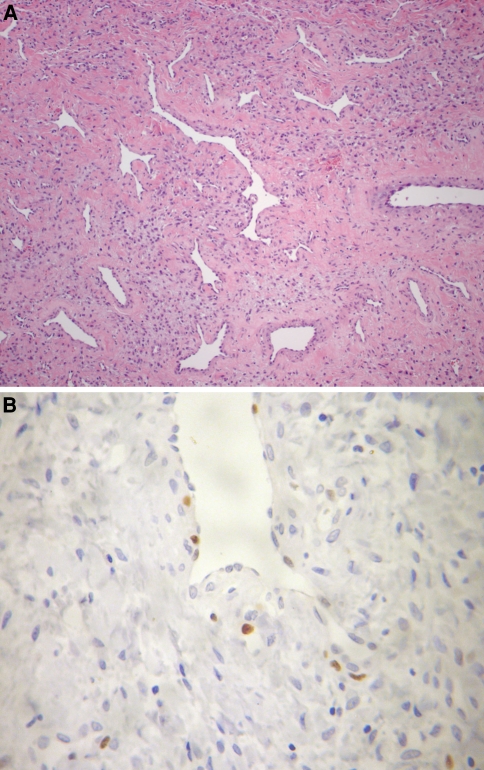
In all cases, tumor tissue consisted of formalin fixed, routinely processed, and paraffin embedded surgical specimens of nasopharyngeal angiofibroma retrieved from the Head and Neck Clinical Center, Guatemala. Hematoxylin and eosin-stained slides from all cases were reviewed to confirm the diagnosis of nasopharyngeal angiofibroma (Fig. 1a). Mitoses were counted by examining 10 high power fields (HPF) for each tumor in the region of highest cellularity and determining an average number per 10 HPF.
Fig. 1.
(a) The characteristic variable vascular proliferation set in a fibrous connective tissue with stellate fibroblastic cells seen in all of the nasopharyngeal angiofibromas. (b) Immunohistochemical reaction for Ki-67 showing many positive nuclei
Immunohistochemistry
Tissue sections were stained with Ki-67 antiserum (diluted 1:50, Clone MIB-1, DAKO, Carpinteria, CA, USA). Heat-induced epitope retrieval was performed with 10 mM citrate buffer pH 6.0 for 30 min in a steamer at 96°C. Primary antiserum incubation was performed diluted in BSA 0.5% for 18 h at 4°C, and followed by conventional streptavidin peroxidase method (LSAB, DAKO, Carpinteria, CA, USA). Peroxidase activity was developed with DAB (Sigma, St Louis, MI, USA) with timed monitoring using a positive control sample. The sections were counterstained with hematoxylin.
Multiple fields were examined and the percentage of positively stained cells was obtained for each case, regardless of staining intensity, after counting 1,000 endothelial and fibroblastic cells under high-power fields (magnification of 400×) (Fig. 1b).
DNA Extraction
DNA extraction for all specimens was performed using the silica-gel membrane microcolumns (DNEasy Tissue Kit, cat. #69506, Qiagen, Valencia, CA, USA). The paraffin embedded specimens were shaved into several 10-μm sheets. The specimens were treated with xylene to remove the paraffin. The specimens were resuspended in ATL buffer along with Proteinase K (both provided in the DNEasy Kit), and incubated at 55°C overnight. DNA purification was then performed using the microcolumns according to manufacturer protocol.
PCR Analysis
EBV and HHV-8 were identified by PCR according to previous validated procedures described in the literature [11, 12]. PCR amplification was performed in an Eppendorf-Master Cycler (Eppendorf, Hamburg, Germany). Positive and negative (PCR reagents without DNA) controls were routinely included.
For EBV-DNA amplification we performed PCR reaction with end volume of 25 μl containing Taq DNA polymerase (1 unit/reaction)(Phoneutria, Belo Horizonte, Brazil), PCR buffer (10 mM (NH4)2SO4, 10 mM KCl, 10 mM Tris–Cl pH 8.4, 3.0 mM MgCl2), 0.1 mM deoxynucleosidetriphosphates, 10 pmol/reaction primers and 200 ng genomic DNA. For reactions using the EBV primers, amplification was carried out using the following conditions: initial denaturation period of 1 min at 94°C followed by 40 cycles of 94°C (60 s), 56°C (50 s), 72°C (60 s) with a final extension period of 5 min at 72°C.
For HHV-8-DNA identification we used a 15 μl reaction containing: 1× Taq buffer, 1.5 mol/l MgCl2, 2.5 mol/l each of DNTP, 0.4 mol/l of each forward and reverse primers, 200 ng genomic DNA, and 0.5 units of Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA). For reactions using the HHV-8 primers, amplification was carried out using the following conditions: initial denaturation period of 3 min at 94°C followed by 2 cycles of 94°C (15 s), 66°C (20 s), 72°C (30 s); 2 cycles of 94°C (15 s), 64°C (20 s), 72°C (30 s); 2 cycles of 94°C (15 s), 62°C (20 s), 72°C (30 s); 30 cycles of 94°C (15 s), 60°C (20 s), 72°C (30 s) and with a final extension period of 3 min at 72°C.
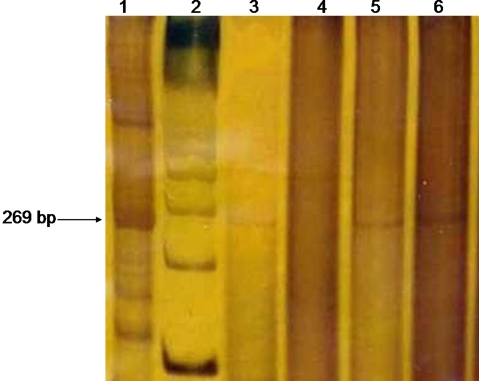
The EBV (269 bp) and HHV-8 (244 bp) amplified products were visualized by 6.5% polyacrilamide gel electrophoresis and silver stained.
EBER In Situ Hybridization (EBER ISH)
Deparaffinized sections were pretreated with Proteinase K for 20 min and incubated with fluorescein-conjugated (FITC) EBER-1 PNA probe (Dako, Carpinteria, CA) at 55°C for 3 h. The sections were rinsed in water and incubated with alkaline phosphatase-conjugated anti-fluorescein antibody for 30 min before adding NBT color substrate (bromochloroindole phosphate and nitroblue tetrazolium) to produce an alcohol-insoluble dark blue intranuclear stain in EBV-positive cells. Positive and negative controls were employed.
Statistical Analysis
The Mann–Whitney test or student test were used for the comparative analysis between the groups whenever appropriate. The association between age and Ki-67 labeling index was analyzed by the Spearman Correlation test. All the analyses were performed using the BioStat 4.0 software (Optical Digital Optical Technology, Belém, Brazil).
Results
The age, gender of the patients, together with PCR results for HHV-8 and EBV investigation and Ki-67 immunolabeling are summarized in Table 1. All the patients were boys or young adult men. The ages ranged from 8 to 23 years. All of the patients presented with masses with or without epistaxis. The tumors arose from the upper posterior nasal cavity or lateral nasopharyngeal wall. Although in some cases the lesions were quite expansive or destructive, none extended into the cranial cavity.
Table 1.
Summary of the demographics, polymerase chain reaction, in situ hybridization, and Ki-67 labeling index
| Case | Sex | Age | HHV-8 PCR | EBV PCR | EBV ISH | MIB (%) |
|---|---|---|---|---|---|---|
| 1 | M | 8 | − | + | − | 3.42 |
| 2 | M | 15 | − | − | − | 0.49 |
| 3 | M | 13 | − | − | − | 11.62 |
| 4 | M | 17 | − | − | − | 0.34 |
| 5 | M | 13 | − | + | − | 0.95 |
| 6 | M | 15 | − | − | − | 0.44 |
| 7 | M | 17 | − | + | − | 1.32 |
| 8 | M | 14 | − | − | − | 3.57 |
| 9 | M | 22 | − | − | − | 0.60 |
| 10 | M | 15 | − | − | − | 1.01 |
| 11 | M | 17 | − | − | − | 0.83 |
| 12 | M | 11 | − | + | − | 1.22 |
| 13 | M | 14 | − | − | − | 0.67 |
| 14 | M | 23 | − | + | − | 0.63 |
| 15 | M | 14 | − | − | − | 0.92 |
HHV-8: Human herpes simplex virus-8; EBV: Epstein–Barr virus; M: male; age in years
The tumors were submucosal, comprised of variably sized, disorganized vessels of variable thickness (Fig. 1a). The vessels were thin-walled, slit-like (“staghorn”) or dilated, ranging in caliber from capillary up to large, open, patulous vessels. Smooth muscle was noted around a number of vessels, but it was focal to circumferential depending on the caliber of the vessel. The endothelial cells were easily identified, but tended to be flattened or attenuated. The fibrous stroma consists of plump spindle, angular, to stellate shaped cells set in a fine to coarse collagen fiber background. In some areas, the stroma seemed to result in compression of the vessel lumens. The nuclei were hyper- to hypochromatic with coarse nuclear chromatin distribution. Nucleoli are indistinct. Mast cells were noted, but they were not the dominant finding.
The Ki-67 labeling index ranged from 0.34 up to 11.62%, with a mean labeling index of 1.87% of counted stromal and endothelial cell nuclei (Fig. 1b and Table 1). This result was correlated with the patient age, without a statistically significant finding (P = 0.14).
The stromal and the endothelial cells were non-reactive with HHV-8. By PCR reaction, five of the cases demonstrated a positive result (Fig. 2). However, the complete lack of EBV using the EBER in situ hybridization technique, confirmed an absence of EBV. The positive PCR result was interpreted to represent a false positive result related to endogenous lymphocytes in the background stroma.
Fig. 2.
Polymerase chain reaction (PCR) product analysis. The polyacrilamide gel illustrates the pattern obtained on different samples of nasopharyngeal angiofibroma (NA). Lane 1: positive control; Lane 2: molecular weight; Lanes 3, 5 and 6 illustrate “positive” amplification products for EBV; Lane 4: a “negative” case of angiofibroma for EBV
Discussion
Nasopharyngeal angiofibroma is a benign neoplasm that affects young males. Studies have suggested the participation of fibroblast growth factor, the transforming growth factor β1, vascular endothelial growth factor, and androgen receptors in nasopharyngeal angiofibroma tumorigenesis [2, 5, 13, 14]. Furthermore, specific genetic imbalances have been identified in angiofibromas by comparative genomic hybridization [15]. These authors found a high percentage of agreement in the observed gains and losses of DNA sequences in all of the three cases investigated, helping to confirm the neoplastic nature of the lesion. Aneuploidy is not identified in these tumors, a finding in accordance with the benign nature of the neoplastic proliferation histologically [16]. These same authors demonstrated chromosomal gains in a few cases of nasopharyngeal angiofibroma, suggesting that activation of oncogenes is more likely than the inactivation of tumor suppressor genes. In this study, we were unable to identify a statistically significant correlation between proliferative activity as reflected in the ki-67 immunohistochemical labeling and the age of the patient. It has been suggested that there may be a puberty-associated testosterone dependent tumor growth. [4–8] However, as the 8 and 11 year old patient in this study were not in puberty (lack of Tanner II or higher development characteristics), the puberty associated growth is not the only factor associated with tumor growth. In fact, the youngest patient in this series had a Ki-67 proliferation index nearly two fold higher than the mean for all of the patients.
The complete lack of HHV-8 by PCR evaluation supports the hypothesis that HHV-8 is an unlikely etiologic candidate. EBV is linked to the development of various lymphoid and epithelial neoplastic lesions, such as Burkitt lymphoma and nasopharyngeal carcinoma. EBV has a high level of prevalence in developing countries, although only about 50% of 5–9 year-old children in developed countries [17, 18]. There has been no specific etiologic association with nasopharyngeal angiofibroma. The presence of PCR product but lack of EBER in situ hybridization, strongly supports the bystander lymphoid cells in nasopharyngeal angiofibroma are the source of the EBV which is amplified by PCR, rather than an genuine “oncogenic” effect of the virus. It is our contention that no neoplastic fibroblastic or endothelial cells were infected and therefore EBV is not a potential candidate for tumorigenesis of angiofibroma. Specifically, the ability of in situ hybridization to allow for a direct visualization of any potential positive findings suggests it is a superior method for detecting EBV than PCR when lymphocytes may be present in the tumor, as is the case with angiofibroma.
Conclusion
Based on the results presented here, nasopharyngeal angiofibroma develops exclusive in boys and young men. We were unable to show any etiologic association between EBV and HHV-8 by molecular and immunohistochemical results. There does not seem to be an age associated proliferation index as reflected by Ki-67 immunohistochemistry labeling. This suggests that events other than puberty may be at play in the growth of these benign neoplasms. Further evaluation is required to more fully elucidate the complex etiology of this uncommon nasopharyngeal tumor.
Acknowledgements
Grant sponsor: Milênio CNPq/MCT and FAPEMIG, Brazil. Dr. RS Gomez is research fellow of CNPq, Brazil. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the affiliated Institutions. There is no financial conflict of interest.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Thompson LDR, Fanburg-Smith JC. Nasopharyngeal angiofibroma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Kleihues P, Sobin LH, series eds. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2005. pp. 102–103. [Google Scholar]
- 2.Brieger J, Wierzbicka M, Sokolov Y, Roth W, Szyfter W, Mann WJ. Vessel density, proliferation, and immunolocalization of vascular endothelial growth factor in juvenile nasopharyngeal angiofibromas. Arch Otolaryngol Head Neck Surg. 2004;130:727–31. doi: 10.1001/archotol.130.6.727. [DOI] [PubMed] [Google Scholar]
- 3.Saylam G, Yücel OT, Sungur A, Önerci M. Proliferation, angiogenesis and hormonal markers in juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol. 2006;70:227–34. doi: 10.1016/j.ijporl.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Lee DA, Rao RB, Meyer JS, Prioleau PG, Bauer WC. Hormonal receptor determination in juvenile nasopharyngeal angiofibromas. Cancer. 1980;46:547–51. doi: 10.1002/1097-0142(19800801)46:3<547::AID-CNCR2820460321>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Hwang HC, Mills SE, Patterson K, Gown AM. Expression of androgen receptors in nasopharyngeal angiofibroma: an immunohistochemical and clinicopathological study. Mod Pathol. 1998;11:1122–6. [PubMed] [Google Scholar]
- 6.Gatalica Z. Immunohistochemical analysis of steroid hormone receptors in nasopharyngeal angiofibromas. Cancer Lett. 1998;127:89–93. doi: 10.1016/S0304-3835(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 7.Liang J, Yi Z, Lianq P. The nature of juvenile nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg. 2000;123:475–81. doi: 10.1067/mhn.2000.105061. [DOI] [PubMed] [Google Scholar]
- 8.Farag MM, Ghanimah SE, Ragaie A, Saleem TH. Hormonal receptors in juvenile nasopharyngeal angiofibroma. Laryngoscope. 1987;97:208–11. doi: 10.1288/00005537-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 9.van der Wall I, Lamovec J, Knuutila S. Kaposi sarcoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Kleihues P, Sobin LH, series eds. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2005. p. 193–194
- 10.Chan JKC, Bray F, McCarron P, Foo W, Lee AWM, Yip T, et al. Tumours of the nasopharynx: nasopharyngeal carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, et al., editors. Pathology and genetics of head and neck tumours. Kleihues P, Sobin LH, series eds. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2005. pp. 85–97. [Google Scholar]
- 11.Telenti A, Marshall WF, Smith TF. Detection of Epstein–Barr virus by polymerase chain reaction. J Clin Microbiol. 1990;28:2187–90. doi: 10.1128/jcm.28.10.2187-2190.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen TL, Murr AH, Rabin J, Mhatre AN, Lalwani AK. Role of cytomegalovirus, Epstein–Barr virus, and human herpes virus-8 in benign lymphoepithelial cysts of the parotid gland. Laryngoscope. 2004;114:1500–5. doi: 10.1097/00005537-200408000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Schiff M, Gonzales AM, Ong M, Baird A. Juvenile nasopharyngeal angiofibroma contain an angiogenic growth factor: basic FGF. Laryngoscope. 1992;102:940–5. doi: 10.1288/00005537-199208000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Dillard DG, Cohen C, Muller S, Gaudio J, Reichman O, Parrich B, et al. Immunolocalization of activated transforming growth factor beta 1 in juvenile nasopharyngeal angiofibroma. Arch Otolaryngol Head Neck Surg. 2000;126:723–5. doi: 10.1001/archotol.126.6.723. [DOI] [PubMed] [Google Scholar]
- 15.Schick B, Brunner C, Praetorius M, Plinkert PK, Urbschat S. First evidence of genetic imbalances in angiofibromas. Laryngoscope. 2002;112:397–401. doi: 10.1097/00005537-200202000-00035. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich U, Brieger J, Gosepath J, Wierzbicka M, Sokolov M, Roth Y, et al. Frequent chromosomal gains in recurrent juvenile nasopharyngeal angiofibroma. Cancer Genet Cytogenet. 2007;175:138–43. doi: 10.1016/j.cancergencyto.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Slots J, Saygun I, Sabeti M, Kubar A. Epstein–Barr virus in oral diseases. J Periodontal Res. 2006;41:235–44. doi: 10.1111/j.1600-0765.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K, Tanaka-Taya K, Kazuyama Y, Ito YM, Hashimoto S, Fukayama M, et al. Prevalence of Epstein–Barr virus in Japan: trends and future prediction. Pathol Int. 2006;56:112–6. doi: 10.1111/j.1440-1827.2006.01936.x. [DOI] [PubMed] [Google Scholar]