Abstract
Salivary gland cystadenomas are cystic neoplasms with diverse architecture and cytology. Cystadenomas may have a considerable intracystic epithelial component, but an epithelial proliferation in small ducts and cysts resembling atypical ductal hyperplasia of breast has not been documented. The patient was a 68-year-old man with a slow growing right submandibular mass. He has no recurrence 13 months after resection. The tumor was polycystic and measured 3.0 × 2.5 × 2.5 cm. The epithelium of the larger cysts was composed of flat, cuboidal, columnar, and apocrine-like cells. Many of the larger cysts showed “Roman bridges”, epithelial tufting, and papillae. The smaller cysts and ducts had apocrine-like cells forming secondary glandular lumens. The ductal cells were surrounded by clear myoepithelial cells. Nuclear pleomorphism and hyperchromasia was seen in the apocrine-like cells. Adjacent to the larger cysts, there was an adenomatoid proliferation of small ducts surrounded by myoepithelial cells. No mitotic activity, necrosis, or stromal invasion was identified. The ductal cells were diffusely positive for keratin 7 and androgen receptors with focal expression of keratin 19 and high-molecular weight keratin. S-100, estrogen and progesterone receptors, and BRST-2 were negative in the ductal cells. Recognition of a prominent intraductal epithelial component in cystadenomas is important to avoid a misdiagnosis of cystadenocarcinoma or low-grade salivary duct carcinoma. Cystadenomas join the list of salivary gland lesions with microscopic similarities to primary lesions of the breast.
Keywords: Salivary glands, Cystadenoma, Cystadenocarcinoma, Low-grade salivary duct carcinoma
Introduction
Salivary gland cystadenomas are well circumscribed or encapsulated, unicystic or polycystic neoplasms that often show papillary architecture [1–3]. The epithelial lining of cystadenomas is variable and ranges from flat, cuboidal, to tall columnar cells. Tumors with a predominance of mucous or oncocytic cells are classified as mucinous or oncocytic cystadenoma respectively [2, 4]. Apocrine epithelium, squamous metaplasia, psammoma bodies, and tyrosine crystals are occasional microscopic findings in these tumors [5–7].
Herein we describe an unusual cystadenoma of the submandibular gland. The tumor showed an intraductal epithelial proliferation with architectural features resembling atypical ductal hyperplasia of breast [8], a finding not previously documented in these lesions [2, 3, 9]. Recognition of these features in cystadenomas is important to avoid a misdiagnosis of cystadenocarcinoma or low-grade salivary duct carcinoma (LGSDC), a neoplasm resembling atypical ductal hyperplasia or low-grade intraductal carcinoma of breast [10]. LGSDC has also been designated “low-grade cribriform cystadenocarcinoma” in the 2005 WHO Classification of Head and Neck Tumours [11]. Along with sclerosing polycystic adenosis (SPA), LGSDC, and conventional salivary duct carcinoma; cystadenomas join the growing list of salivary gland lesions with microscopic similarities to primary lesions of the breast.
Clinical History
The patient was a 68-year-old man with no previous significant medical history. He presented with a slow growing, asymptomatic mass involving the right submandibular gland. The mass had been noted for 24 months. A fine needle aspiration biopsy was non-diagnostic. The patient remains well with no tumor recurrence 13 months after resection.
Materials and Methods
The surgical specimen was fixed in formalin and routinely processed with representative sections being embedded in paraffin and stained with hematoxylin and eosin. Mayer’s mucicarmine, PAS and PAS-D were performed in selected blocks. Immunohistochemical stains using standard avidin–biotin complex technique were performed in selected blocks using antibodies for epithelial membrane antigen (EMA, MC5, Ventana, prediluted), keratin 19 (CK19, Vector, 1:50), keratin 7 (CK7, Dako, 1:100), high-molecular-weight keratin (HMWK, Ventana 34BE12, prediluted), keratin 5/6 (CK 5/5, Dako 1:50), keratin 14 (CK14, Vector, 1:100), S-100 (Ventana, polyclonal, prediluted), p63 (Vector, 1:50), smooth-muscle actin (SMA, Ventana, prediluted), calponin (Dako, 1:100), androgen receptors (AR, Biogenex, 1:800), estrogen receptors (ER, Ventana, prediluted), and progesterone receptors (PR, Ventana, prediluted). All immunohistochemical stains were performed in an automated Ventana BenchMark® instrument (Tucson, AZ) with routine positive and negative controls.
Results
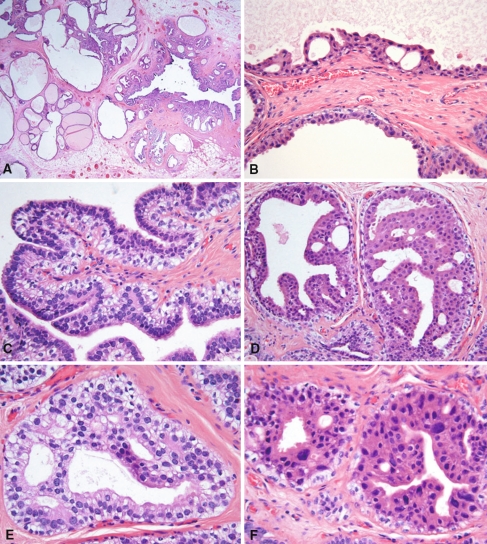
The tumor was well demarcated and measured 3.0 × 2.5 × 2.5 cm. It showed multiple cysts with serous contents. Microscopically, the tumor was uncapsulated, demonstrated a predominant polycystic architecture and was characterized by cytologic diversity. The cysts ranged from large unilocular spaces measuring up to 0.5 cm in diameter to small ducts (Fig. 1a). The inner lining of the larger cysts ranged from a luminal layer of flat atrophic ductal cells to cuboidal (Fig. 1b), columnar, and pseudostratified epithelium (Fig. 1c), as well as apocrine-like cells with eosinophilic cytoplasm and apical snouts (Fig. 1d and f).
Fig. 1.
The mass is composed of numerous cysts raging from large unilocular cysts to small dilated ducts (a). Large cyst lined by cuboidal ductal cells with eosinophilic cytoplasm with formation of “Roman arches” (b). Papilla with coarse core lined by pseudostratified epithelium and myoepithelial cells with clear cytoplasm (c). Small ducts with secondary glandular lumens and apocrine-like epithelium resembling atypical ductal hyperplasia of breast. Note the non-luminal layer with clear cytoplasm (d). Small duct with ductal cells with supra- and infranuclear vacuoles (e). Small ducts lined by ductal cells with apocrine-like appearance and nuclear pleomorphism. Note the outer layer of clear myoepithelial cells (f)
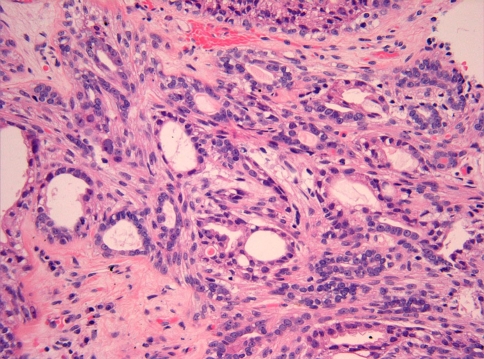
In many of the cysts there was an intraductal epithelial proliferation with formation of “Roman bridges” (Fig. 1b), epithelial tufting, and well formed papillae (Fig. 1c). The smaller cysts and ducts also had a variable degree of epithelial proliferation forming secondary glandular lumens of variable sizes similar to those of atypical ductal hyperplasia of breast (Fig. 1d and e). Most of the cells in these cysts and ducts were apocrine-like cells (Fig. 1d and f), although ductal cells with clear cytoplasm and infranuclear vacuoles were also present (Fig. 1e). The ductal cells were surrounded by a layer of myoepithelial cells of variable thickness (Fig. 1b–e). These myoepithelial cells had clear cytoplasm and contained glycogen. Nuclear pleomorphism and hyperchromasia was often seen in the apocrine-like cells but no mitotic figures were identified. In multiple areas adjacent to the largest cysts, there was an adenomatoid proliferation composed of small ducts surrounded by spindled myoepithelial cells (Fig. 2). No sclerotic nodules, inflammation, mitotic activity, necrosis, or stromal invasion was identified. The adjacent submandibular gland was unremarkable.
Fig. 2.
Adenomatous proliferation composed of small glands surrounded by spindled myoepithelial cells. The ductal cells show small to medium size nucleoli and occasional nuclear pleomorphism. This finding resembles sclerosing adenosis of breast
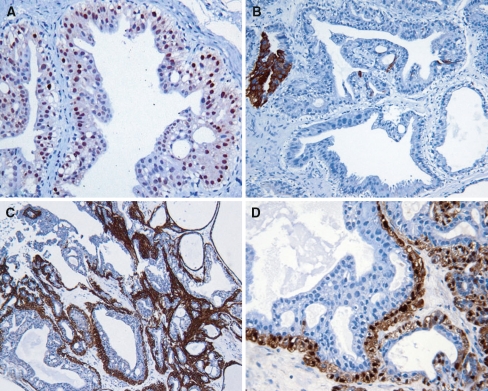
Immunohistochemistry showed that the ductal cells were diffusely positive for CK7 with strong nuclear expression of AR (Fig. 3a). Focal but strong staining for CK19 and HMWK was also present (Fig. 3b). EMA staining was seen in the apical surface of the ductal cells. S-100 was negative in the ductal cells. The myoepithelial cells demonstrated strong nuclear staining for p63 with diffuse expression of calponin (Fig. 3c), SMA, CK14, and S-100 (Fig. 3d). HMWK expression by myoepithelial cells was weak. Ductal and myoepithelial cells were negative for CK5/6. ER, PR, and BRST-2 were negative. The proliferating index measured by MIB-1 was less than 1%.
Fig. 3.
Ductal cells with strong nuclear expression of AR (a). CK19 is only focally positive in ductal cells (b). The myoepithelial cells are strongly positive for calponin (c) and S-100 (d)
Discussion
We have described a cystadenoma of the submandibular gland with a prominent intraductal epithelial proliferation in small ducts reminiscent of atypical ductal hyperplasia of breast, a hitherto unreported finding in these tumors. A direct comparison of this lesion with published reports of cystadenoma is difficult because most original publications describing the histopathology of these tumors are case reports [5, 7] or larger studies dealing with various types of salivary gland neoplasms [2]. Ellis et al. [1] had previously commented on the scanty information available regarding the microscopic features of salivary cystadenomas. Arguably, the most thorough description of the histopathologic spectrum of cystadenomas is found in Dardick’s Atlas of Salivary Gland Tumor Cytopathology, Oral & Surgical Pathology [3]. Dardick et al. [3] illustrated a considerable degree of intracystic epithelial proliferation in cystadenomas, but none of the Atlas’ illustrations resembles the lesion described herein. We also found nuclear enlargement and hyperchromasia in many of the apocrine-like cells of this lesion; however, this nuclear atypia was not accompanied by mitotic activity or increased MIB-1 index. This finding may be akin to the cellular atypia occasionally seen in apocrine metaplasia of the breast [12]. It has been reported that atypical ductal hyperplasia of the breast lacks expression of CK5/6 [13], interestingly the atypical intraductal epithelial proliferation in this lesion also lacked CK5/6 expression; however, experience with this antibody in salivary gland lesions is lacking therefore the biologic or diagnostic significance of this finding is unknown.
Like LGSDC, this tumor showed epithelial tufting, “Roman bridges”, and apocrine-like epithelium; however the mixture of broad and filiform papillae, columnar epithelium with infra- and supranuclear vacuoles, and prominent clear myoepithelial cells are not features of LGSDC [10, 14]. Furthermore, the immunophenotype of this lesion and LGSDC showed significant differences. Whereas CK19, HMWK, S-100, and BRST-2 expression by ductal cells is positive in LGSDC [9, 10], these markers were only focally positive (CK19, HMWK) or negative (S-100, BRST-2) in our case. Based on this combination of histopathologic and immunophenotypic findings, we think that this lesion is not related to LGSDC.
The differential diagnosis of this lesion also includes papillary cystadenocarcinoma and SPA. The spectrum of cystadenocarcinomas is broad and includes low and high grade tumors but in contrast with the tumor described herein; cystadenocarcinomas are invasive neoplasms [15–18]. The absence of invasion with desmoplastic reaction and an intact myoepithelial layer surrounding ducts and cysts in our case, argues against a diagnosis of cystadenocarcinoma. SPA is a rare tumor-like lesion recognized as a distinctive entity by Smith et al [19]. Features of SPA include lobular proliferation of cystically dilated ducts, abundant hypocellular collagenous nodules, ductal epithelial hyperplasia with cribriform architecture, frequent apocrine metaplasia, and variable inflammation [19, 20]. Our case had apocrine-like epithelium similar to that of SPA but lacked nodular or diffuse sclerosis and there was no significant inflammation.
Another unusual histopathologic finding in this neoplasm was the presence of an abluminal layer of clear myoepithelial cells thus resembling epithelial–myoepithelial carcinoma. Papillary and cystic areas have been described in 20–30% of epithelial–myoepithelial carcinoma [21, 22]; however, the multicystic appearance with only focal tubule formation, apocrine-like ductal epithelium, and absence of hyaline stroma in the tumor described herein, exclude a diagnosis of epithelial-myoepithelial carcinoma.
We have described a cystadenoma of the submandibular gland with an extensive intraductal epithelial proliferation of small ducts reminiscent of atypical ductal hyperplasia of breast. In addition, the tumor showed a prominent abluminal myoepithelial cells with clear cytoplasm. The frequency of these findings in cystadenomas is unknown, but it could be underreported features of these tumors. Along with SPA, LGSDC, and conventional salivary duct carcinoma; cystadenomas join the growing list of salivary gland lesions with microscopic similarities to primary lesions of the breast.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Auclair PL, Ellis GL, Gnepp DR. Other benign epithelial neoplasms. In: Ellis GL, Auclair PL, Gnepp DR, editors. Surgical pathology of the salivary glands. Philadelphia: W.B. Saunders Co.; 1991. pp. 252–68. [Google Scholar]
- 2.Waldron CA, el Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323–33. doi: 10.1016/0030-4220(88)90240-X. [DOI] [PubMed] [Google Scholar]
- 3.Dardick I, Kini S, Bradley G, Lee L, Leong I. Salivary gland tumor cytopathology, oral and surgical pathology. Ottawa: Pathology Images Inc; 2006. [Google Scholar]
- 4.Ellis GL, Auclair PL. Benign epithelial neoplasms. In: Ellis GL, Auclair PL, editors. Tumors of the salivary glands. Atlas of tumor pathology. Series III. Washington, DC: Armed Forces Institute of Pathology; 1996. pp. 39–153. [Google Scholar]
- 5.Michal M, Skalova A, Mukensnabl P. Micropapillary carcinoma of the parotid gland arising in mucinous cystadenoma. Virchows Arch. 2000;437:465–8. doi: 10.1007/s004280000274. [DOI] [PubMed] [Google Scholar]
- 6.Lim CS, Ngu I, Collins AP, McKellar GM. Papillary cystadenoma of a minor salivary gland: report of a case involving cytological analysis and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e28–33. doi: 10.1016/j.tripleo.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Gilcrease MZ, Nelson FS, Guzman-Paz M. Tyrosine-rich crystals associated with oncocytic salivary gland neoplasms. Arch Pathol Lab Med. 1998;122:644–9. [PubMed] [Google Scholar]
- 8.Page DL, Anderson TJ, Rogers LW. Epithelial hyperplasia. In: Page DL, Anderson TJ, editors. Diagnostic histopathology of the breast. Edinburgh: Churchill Livingstone; 1987. pp. 120–56. [Google Scholar]
- 9.Weinreb I, Tabanda-Lichauco R, Van der KT, Perez-Ordonezb B. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol. 2006;30:1014–21. doi: 10.1097/00000478-200608000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Delgado R, Klimstra DS, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78:958–67. doi: 10.1002/(SICI)1097-0142(19960901)78:5<958::AID-CNCR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Brandwein-Gensler M, Gnepp DR. Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. p. 233. [Google Scholar]
- 12.Izuo M, Okagaki T, Richart RM, Lattes R. DNA content in “apocrine metaplasia” of fibrocystic disease of the breast. Cancer. 1971;27:643–50. doi: 10.1002/1097-0142(197103)27:3<643::AID-CNCR2820270320>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Lacroix-Triki M, Mery E, Voigt JJ, Istier L, Rochaix P. Value of cytokeratin 5/6 immunostaining using D5/16 B4 antibody in the spectrum of proliferative intraepithelial lesions of the breast. A comparative study with 34betaE12 antibody. Virchows Arch. 2003;442:548–54. doi: 10.1007/s00428-003-0808-0. [DOI] [PubMed] [Google Scholar]
- 14.Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, Simpson JR, Simpson RH, Gnepp DR. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28:1040–4. doi: 10.1097/01.pas.0000128662.66321.be. [DOI] [PubMed] [Google Scholar]
- 15.Nagao T, Sugano I, Matsuzaki O, Hara H, Kondo Y, Nagao K. Intraductal papillary tumors of the major salivary glands: case reports of benign and malignant variants. Arch Pathol Lab Med. 2000;124:291–5. doi: 10.5858/2000-124-0291-IPTOTM. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi I, Kiyoshima T, Ozeki S, Shima K, Shigemura N, Matsuo K, Sakai H. Immunohistochemical and ultrastructural study of a papillary cystadenocarcinoma arising from the sublingual gland. J Oral Pathol Med. 1999;28:282–6. doi: 10.1111/j.1600-0714.1999.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 17.Pollett A, Perez-Ordonez B, Jordan RCK, Davidson MJ. High-grade papillary cystadenocarcinoma of the tongue. Histopathology. 1997;31:185–8. doi: 10.1046/j.1365-2559.1997.2270840.x. [DOI] [PubMed] [Google Scholar]
- 18.Foss RD, Ellis GL, Auclair PL. Salivary gland cystadenocarcinomas. A Clinicopathologic study of 57 cases. Am J Surg Pathol. 1996;20:1440–7. doi: 10.1097/00000478-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Smith BC, Ellis GL, Slater JL, Foss RD. Sclerosing polycystic adenosis of major salivary glands. A clinicopathologic analysis of nine cases. Am J Surg Pathol. 1996;20:161–70. doi: 10.1097/00000478-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Gnepp DR, Wang LJ, Brandwein-Gensler M, Slootweg P, Gill M, Hille J. Sclerosing polycystic adenosis of the salivary gland: a report of 16 cases. Am J Surg Pathol. 2006;30:154–64. doi: 10.1097/01.pas.0000186394.64840.1d. [DOI] [PubMed] [Google Scholar]
- 21.Seethala RR, Barnes EL, Hunt JL. Epithelial–myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007;31:44–57. doi: 10.1097/01.pas.0000213314.74423.d8. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca I, Soares J. Epithelial–myoepithelial carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World health organization classification of tumours. Head and neck tumours. IARC Press; 2005. p. 225–6.