Abstract
Benign fibro-osseous lesions of the craniofacial complex are represented by a variety of disease processes that are characterized by pathologic ossifications and calcifications in association with a hypercellular fibroblastic marrow element. The current classification includes neoplasms, developmental dysplastic lesions and inflammatory/reactive processes. The definitive diagnosis can rarely be rendered on the basis of histopathologic features alone; rather, procurement of a final diagnosis is usually dependent upon assessment of microscopic, clinical and imaging features together. Fibrous dysplasia and osteitis deformans constitute two dysplastic lesions in which mutations have been uncovered. Other dysplastic bone diseases of the craniofacial complex include florid osseous dysplasia, focal cemento-osseous dysplasia and periapical cemental dysplasia, all showing a predilection for African descent individuals; although no specific genetic alterations in DNA coding have yet to be uncovered and most studies have been derived from predominant high African descent populations. Ossifying fibromas are neoplastic lesions with four subtypes varying with regard to behavior and propensity for recurrence after surgical excision. The clinicopathologic and molecular features of this unique yet heterogeneous group of diseases are reviewed.
Keywords: Benign fibroosseous lesions, Ossifying fibroma, Cementoosseous dysplasia, Osteitis deformans, Cementoma, Fibrous dysplasia
Benign fibro-osseous lesions (BFOL) of the jaw, facial and skull bones are a variant group of intraosseous disease processes that share microscopic features [1–4]. Whereas some are diagnosable histologically, most require a combined assessment of clinical, microscopic and radiologic features. In turn, some BFOL of the craniofacial complex are unique to that location whereas others are encountered in bones from other regions [5, 6]. Reactive, neoplastic, developmental and dysplastic pathologic processes are subsumed under the rubric of BFOL and treatment varies from disease to disease. This review will discuss the clinical, microscopic and imaging aspects of BFOL of the craniofacial complex with updated information on underlying molecular pathogenetic mechanisms of disease.
Classification
Prior to reviewing the features of each entity subsumed under the heading of BFOL of the craniofacial complex, we will proceed with a nosology of these lesions and as the discussion progresses, we will attempt to support the basis for this classification (Table 1). Whereas some investigators include giant cell lesions of bone with BFOL, lesions of this nature will not be included here with the exception of the trabecular variant of Ossifying Fibroma which is essentially a BFOL yet may contain foci of multinucleated giant cells.
Table 1.
Classification of benign fibro-osseous lesions of the craniofacial complex
| I. Bone dysplasias |
| a. Fibrous dysplasia |
| i. Monostotic |
| ii. Polyostotic |
| iii. Polyostotic with endocrinopathy (McCune-Albright) |
| iv Osteofibrous dysplasiaa |
| b. Osteitis deformans |
| c. Pagetoid heritable bone dysplasias of childhood |
| d. Segmental odontomaxillary dysplasia |
| II. Cemento-osseous dysplasias |
| a. Focal cemento-osseous dysplasia |
| b. Florid cemento-osseous dysplasia |
| III. Inflammatory/reactive processes |
| a. Focal sclerosing osteomyelitis |
| b. Diffuse sclerosing osteomyelitis |
| c. Proliferative periostitis |
| IV. Metabolic Disease: hyperparathyroidism |
| V. Neoplastic lesions (Ossifying fibromas) |
| a. Ossifying fibroma NOS |
| b. Hyperparathyroidism jaw lesion syndrome |
| c. Juvenile ossifying fibroma |
| i. Trabecular type |
| ii. Psammomatoid type |
| c. Gigantiform cementomas |
aOsteofibrous dysplasia is found in the fibula and tibia only
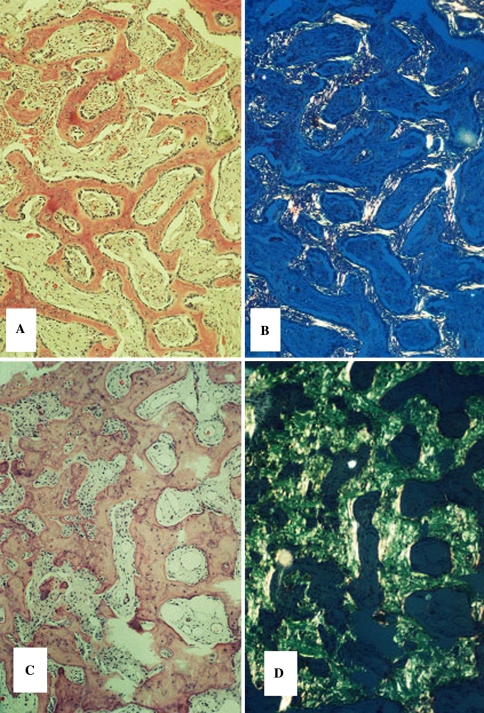
By definition, all BFOL possess an osseous and a fibrous tissue component. Table 2 lists the variations in histology among BFOL. These variant appearances may be unique to one disease yet in other instances, three or four entities may share the same histology even though they represent separate and distinct clinicopathologic entities [2, 3, 5–10]. Essentially, the stromal element of BFOL may be quite homogeneous yet hypercellular with monomorphic appearing fibroblasts, whereas in others, the stroma is more mature or collagenous and then in yet others a storiform fibroblastic pattern prevails. The ossifications in BFOL can be quite heterogeneous even within a specific disease entity. Newly formed bone shows a woven pattern of collagen fiber orientation when viewed under polarized light. Mature bone exhibits a lamellar pattern as does dental cementum although the latter is microlamellar (Fig. 1). Many BFOLs are found to have both irregular trabeculae as well as spheroidal cementicle calcifications, so-called “cemento-ossifying” lesions. The ossification patterns seen in BFOL often represent the “age” of the lesion; formative processes in the early stages are more cellular and osteoblastic rimming of trabeculae is more prominent than older lesions of longer duration in which the stroma is more mature. As each entity is reviewed, the predominant histopathologic patterns will be specified (Genetic lesions are listed in Table 3).
Table 2.
Microscopic similarities and dissimilarities among fibro-osseous lesions
| Fibrous element variations |
| Homogeneous plump monomorphic fibroblasts, hypercellularity, thin collagen fibers |
| Mature, hypocellular |
| Fasiculated, Storiform |
| Ossification (trabeculation) variations |
| Metaplastic woven bone |
| “Chinese/Hebrew” figure trabeculae |
| Lamellar bone trabeculae |
| Osteoblastic rimming |
| Mosaic resting/reversal lines |
| Trabecular paralleling |
| Cemental woven |
| Cemental microlamellar |
| Sharpey fiber fringe |
| Droplet (psammomatoid) |
| Curvilinear conglomerates (“Ginger root”) |
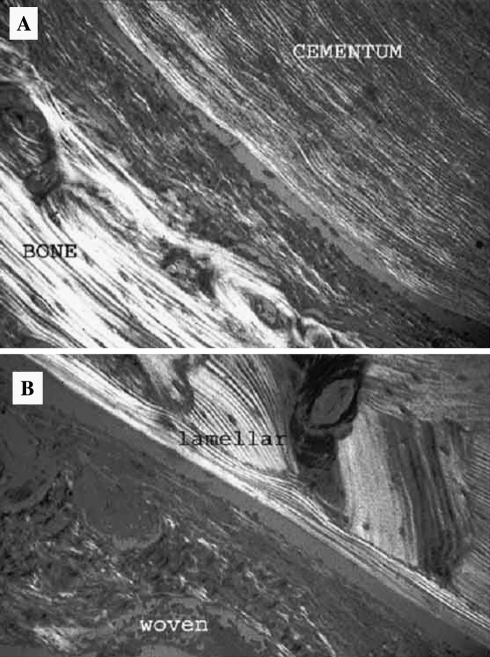
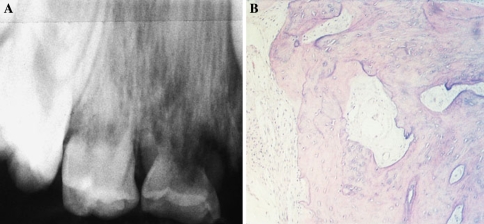
Fig. 1.
Collagen fiber patterns in normal bone and cementum. (a) Macrolamellar fiber pattern in bone, microlamellar pattern in cementum. (b) Lamellar and woven patterns in osseous trabeculae. (Crossed polars)
Table 3.
Genomic alterations in fibro-osseous lesions
| Disease | Genomic lesions |
|---|---|
| Adult osteitis deformans | Sequestosome 1 gene (SQSTM1), a scaffold protein in the NFkappaB pathway; Inactivating mutations in TNFRSF11B, which encodes osteoprotegerin (a decoy receptor for RANK ligand) |
| Childhood Paget disease (idiopathic hyper-Phosphatasemia | Insertion mutations in TNFRSF11A, a receptor activator of nuclear factor (NF)kappaB (RANK)-a critical regulator of osteoclast function |
| Hereditary inclusion body myopathy, Paget’s disease and fronto-temporal dementia | Mutation in Valosin-containing protein (VCP), targeting the inhibitor of NFkappaB for degradation by the proteasome |
| Familial expansile osteolysis/expansile skeletal Hyperphosphatasia | Tandem duplications in TNFRSF11A |
| Fibrous dysplasia, McCune Albright syndrome | Mutations of the Gsalpha gene (GNAS), the alpha-subunit of the stimulatory G protein |
| Hyperparathyroidism associated ossifying fibroma | Mutations in tumor suppressor gene HRPT2 |
| Psammomatoid Ossifying Fibroma | Chromosomal breakpoints t(X;2)(q26;q33); interstitial insertion of bands 2q24.2q33 into Xq26 |
Fibrous Dysplasia
Fibrous dysplasia is a benign dysplastic process of altered osteogenesis that may occur within a single bone (monostotic) or multiple bones (polyostotic) [2, 5, 8]. When polyostotic fibro-osseous lesions typical for fibrous dysplasia are associated with other anomalies and endocrinopathy, this variant form constitutes the McCune-Albright syndrome (MAS). The molecular underpinning of this related group of diseases is a mutation in the gene that encodes the G protein alpha-subunit (Gs-alpha) that couples cAMP to hormone receptors [11–18]. These mutations result in GTPase perturbations that lead to prolonged Gs-alpha activation and stimulation of endocrine receptors [4–9]. The lesions of fibrous dysplasia show elevated intracellular cAMP in bone marrow osteoprogenitor cells and these molecular changes probably initiate cell proliferation with differentiation defects. Gs-alpha mutation is also seen in 40% of pituitary tumors causing acromegaly. In fibrous dysplasia, these genetic lesions are postzygotic, somatic and result in cellular mosaicism, a genetic process that accounts for the fact the not all bones in polyostotic disease are affected; the normal appearing bone in affected individuals is devoid of genotypic lesions. Albright’s hereditary osteodystrophy (psuedohypoparathyroidsm) also involves a debilitating mutation in the Gs-alpha gene with a wide range of skeletal anomalies, yet fibro-osseous lesions are not extant.
Monostotic fibrous dysplasia of the craniofacial complex is often confused with other BFOL, typically ossifying fibroma and diffuse sclerosing osteomyelitis of the mandible, diseases that manifest unique clinicoradiologic features. Monostotic fibrous dysplasia occurs in the jaw, frontal, ethmoidal, temporal and calavarial bones [2–5, 8]. Clinically, the maxilla is affected more often than the mandible and lesions are first detected in the late first and early second decades without any gender or racial predilection. The disease is characterized by painless osseous expansion with facial asymmetry. Radiographic features vary depending upon the stage of the disease (Fig. 2). Early onset lesions are radiolucent and later progressively calcify, culminating in a “ground glass” or mottled mixed radiolucent/radiopaque pattern. Critical to the diagnosis is the fact that fibrous dysplasia fails to manifest any discrete margins; rather, the lesional bone subtly blends into the surrounding normal appearing bone.
Fig. 2.
Fibrous Dysplasia. (a) Expansile nonmarginated ground glass opacification in the mandible, (b). Ground glass pattern in the maxilla, (c). Clinical photograph demonstrating cortical expansion, (d). Diffuse unilateral opacification of the maxillary sinus
Histologically a fibro-osseous pattern is typically seen, yet akin to the imaging features, subtle changes are seen at various stages of the disease’s natural history. In the early formative phase, pronounced osteogenesis is seen with thin osteoid anastomosing trabeculae that are rimmed with osteoblasts. The stromal fibroblastic element is proliferative and hypercellular although no pleomorphism can be seen. With ensuing weeks, the trabeculae thicken, yet the osseous collagen pattern remains woven and the trabeculae assume the classic “Chinese figure” characteristics. The fibrous element continues to be hypercellular. In later stages of the disease woven bone is replaced by lamellar bone trabeculae; extensive remodeling may result in a mosaic pattern of resting and reversal lines (Fig. 3).
Fig. 3.
Fibrous Dysplasia. Microscopic patterns. (a) Irregular trabeculae with a fibrous element, (b) Polarization microscopy corresponding to figure A shows mature lamellar fiber orientation. (c) Similar fibro-osseous pattern. (d) Polarization microscopy corresponding to figure C depicts a less mature woven fiber pattern
Most craniofacial lesions are monostotic, yet when a diagnosis of fibrous dysplasia is rendered a skeletal imaging survey must be ordered to search out lesions in other bones. Physical examination must be performed to uncover accompaniments that support the diagnosis of McCune-Albright syndrome. Most of the anomalies in MAS can be attributed to the hormone signally that occurs as a consequence of the GNAS mutations [15–18].
Another variant of fibrous dysplasia is osteofibrous dysplasia, a pediatric disease that is typically found in the lower extremity long bones and is a fibro-osseous process in which epithelial nests and islands are found dispersed throughout the lesion [19–22]. Oftentimes the nests are quite small and are seen only with immunostaining for cytokeratins. These lesions show radiologic and histologic features typical for fibrous dysplasia and many researchers take the position that osteofibrous dysplasia is merely a variant of fibrous dysplasia. Others consider this variant to be an adamantinoma with a reactive fibro-osseous component. Genetic analyses have failed to identify any G(s) alpha mutations in osteofibrous dysplasia. Long term follow up has failed to demonstrate the emergence of classic adamantinoma from these lesions. Indeed, many cases spontaneously resolve. Osteofibrous dysplasia has not been described in the craniofacial complex although one of us (LRE) has encountered a maxillary fibro-osseous lesion with multiple epithelial nests, probably odontogenic in origin, dispersed throughout the fibrous stroma.
Fibrous dysplasia may also be associated with soft tissue myxomas, the Mazabraud syndrome [23].
Cherubism, familial fibrous dysplasia, is a benign dysplastic bone disease that is limited to the maxilla and mandible and is not included in the classification of fibro-osseous lesions. We include a brief discussion of this entity and its syndromic associations only because it was at one time considered to be a specific variant of fibrous dysplasia (which it is not). It has its onset in childhood and is inherited as an autosomal dominant trait [24, 25]. The gene for cherubism maps to chromosome 4p16, the site for adaptor protein 3BP2 which positively regulates the high affinity IgE receptor (FcepsilonRI)-mediated activation of degranulation in mast cells [26, 27]. Over-expression of 3BP2 mutant protein inhibits antigen-induced mast cell activation as well as numerous other internal signaling pathway proteins.
It affects males and females equally and the full phenotype affects both the maxilla and mandible with varying degrees of expressivity in that some cases exhibit minimal maxillary involvement. In the classic presentation, the child presents with “cherubic facies” characterized by upward gaze due to osseous expansion of the maxilla and orbital floor and bilateral osseous expansion of the posterior mandibular body and ramus. Teeth remain unerupted and imaging studies reveal bilateral multilocular radiolucencies with expanded cortices. Espansile lesions of the temporal bone have also been reported in Cherubism [28]. Microscopically cherubism is a fibrous proliferation without an osteogenic component. The collagenous network is immature with delicate areolar fibrils. Small vessels within the fibrous component exhibit a characteristic collagenous cuffing (i.e.: perivascular hyalinizing fibrosis) and randomly dispersed throughout are multinucleated giant cells with diffusely dispersed nuclei. Aggressive forms of the disease with more marked expansion occur and result in more severe functional and cosmetic deformity [29].
Cherubism has occurred in association with three syndromes: Noonan syndrome, Ramon syndrome and type I Neurofibromatosis. The Noonan syndrome is characterized by short stature, cherubic facies, congenital heart defects, chest deformity and mild mental retardation. It may be sporadic or inherited as an autosomal dominant trait and occurs between one in 1000-2500 [30–32]. The Ramon syndrome consists of mental deficiency, epilepsy, cherubism, gingival fibromatosis, hypertrichosis, stunted growth and juvenile rheumatoid arthritis [33, 34]. The rare cases with NF1 association have shown multiple mutations (NF1, SH3BP2, PTPN11) [35, 36].
Osteitis Deformans (Paget Disease)
Osteitis deformans or Paget disease of bone (PDB) is an osseous dysplasia that is characterized by rapid turnover remodeling of bone throughout the skeleton. Unlike fibrous dysplasia, osteitis deformans is a disease of the elderly although there are two Paget-like bone dysplasias that arise during childhood. Genetic alterations underscore classic Paget’s disease of the elderly as well as familial bone diseases with Pagetoid features [37–39]. All of these diseases involve defective function of the osteoprotegerin/TNFRSF11A or B/RANKL/RANK pathway, a molecular regulator of osteoclastogenesis [40–45]. The classic form of osteitis deformans is often associated with inactivation mutations in the TNFRSF11B gene that encodes osteoprotegerin. Mutations in SQSTM1 (p62), the sequestosome gene that encodes a scaffold protein for the NFKappaB signaling pathway are also often encountered in classic Paget’s disease. These mutations result in either loss of function or truncation/deletion of the ubiquitin binding-associated (UBA) domain.
Various rare heritable syndromes present with childhood onset Pagetoid bone dysplasias with overlapping clinicopathologic features [43–45]. Juvenile Paget disease or idiopathic hyperphosphatasemia is an autosomal recessive disease in which a deletion mutation within exon 3 of the TNFRSF11B gene occurs resulting in loss of aspartate in the osteoprotegerin gene product. The mutated protein fails to suppress osteoclastic resorption. In Paget disease with inclusion body myopathy and fronto-temporal dementia, mutations in the VCP gene (valosin-containing protein) which interacts with the inhibitor of NFkappaB for proteosomal degredation are identified. Familial expansile osteolysis is an autosomal dominant disorder with 18 base pair tandem duplication in TNFRSF11A, the gene that encodes the receptor activator of NFkappaB(RANK) and a related genetic disorder, expansile skeletal hyperphosphatasia, caused by a 15 base pair repeat in TNFRSF11A.
Classic Paget Disease of Bone (CPDB) is an osseous dysplasia with late adult onset. It is characterized by rapid turnover of bone resulting in osseous expansion with progressive skeletal deformities [46–56]. Tubular bones show bowing and spinal curvature with vertebral collapse occur in the later stages of the disease. Markedly elevated serum alkaline phosphatase is a constant feature, while calcium and phosphate levels are normal. All bones of the craniofacial complex can be affected to varying degrees. As facial and skull base remodeling evolves, cranial nerve neuropathies can develop as a consequence of foramina narrowing with deafness being the predominant finding in most instances. In early stages of disease, radiolucent “coin shaped” lesions appear in the flat bones of the skull, a condition termed osteitis circumscripta (Fig. 4). Microscopically, these are cellular fibro-osseous lesions with minimally calcified osteoid trabeculae exhibiting osteoblastic rimming with concomitant osteoclastic resorptive lacunae. Multinucleated cells are also found within the fibrocellular foci, without juxtaposition to the osseous elements. In addition osteoclasts are numerous, larger than normal and have increased numbers of nuclei per cell.
Fig. 4.
Osteitis deformans. (a) Osteitis circumscripta of the calvarium, (b). Cotton wool opacification in the maxilla, (c). Mosaic pattern of resting and reversal lines in sclerotic bone regions
The bones of the maxillofacial complex manifest a ground glass trabecular pattern in early stage disease, yet with progression, diffuse sclerosis is seen radiographically yielding the so-called “cotton wool” appearance of confluent nodular opacifications. Similar changes are identifiable in other bones of the craniofacial complex including the calvarium. Histologically, sclerotic craniofacial lesions show marked evidence of turnover; resting and reversal lines of lamellar compact and trabecular bone are prevalent and haphazardly arranged into a mosaic pattern (Fig. 4c). Another prominent dental finding is generalized hypercementosis which is most advanced on premolar and molar teeth.
The disease is most prevalent in the British Isles and New Zealand affecting over 1% of the population, somewhat less common in Italy, other European countries and the United States [57]. In Latin America, most cases are traced to northern European ancestry and in Japan, only 2.8 cases per million are encountered [58, 59].
There are many reports that have described viral particles and arrays in the osteoclastic cells of CPDB [60–68]. Nucleic acid sequences and microbial antigens have been observed for paramyxoviruses (measles in particular), canine distemper virus and respiratory syncytial virus. It has been shown that normal osteoclasts transduced with retroviral vectors that express Measles Virus Nuclear Protein and Measles Virus Matrix genes develop a pagetic osteoclastic phenotype with increased numbers and increased nuclei per cell. Alternatively, reverse transcriptase PCR and nested RT-PCR do not support the presence of viral transcripts. Furthermore, active virus has not been recovered from Paget bone. Therefore the presence of viral like nuclear inclusions in Paget osteoclasts remains enigmatic as far as a causal factor is concerned.
Two neoplastic processes may occur in patients with CPDB, namely giant cell tumors and sarcomas (osteogenic sarcoma, and less commonly fibrosarcoma, chondrosarcoma and undifferentiated sarcoma). Giant cell tumors are histologically identical with giant cell granulomas of the jaws and giant cell tumor of long bones [69, 70]. In the craniofacial complex they appear as multilocular radiolucencies on plane films or as multicompartmentalized lesions on CT images. Paget-associated giant cell lesions are rare and occur in the absence of hyperparathyroidism.
Osteosarcomas in CPDB are seen in the elderly with severe advanced disease and occur primarily in the pelvis., proximal femur, tibia and in the calvarium [71–82]. Most are high grade and have already metastasized at the time of discovery. Only 14% survive beyond 2.5 years. In a case study of 13 instances of head and neck osteosarcoma, only 1 patient presented with Paget disease.
Bisphosphonates (pamidronate, alendronate, and risedronate) have been shown to have significant effects on reversing many of the osseous lesions in CPDB and histologic findings following pharmacologic intervention appear more normal [83, 84].
Juvenile Paget disease (Idiopathic hyperphosphatasia) is a bone dysplasia that is inherited as an autosomal recessive trait that is characterized clinically by deformities in the long bones, kyphosis, acetabular protrusion and pathophysiologically by rapid bone turnover [43, 85, 86]. The disease begins in infancy or early childhood and is characterized by long bone widening with propensity for pathologic fracture and thickening of the skull. Serum alkaline phosphatase is elevated. Extremely rapid bone turnover is accompanied by osteopenia and skeletal deformity with bowed limbs.
Familial expansile osteolysis (FEO) was first described in a family from Northern Ireland and is inherited as an autosomal dominant trait [87–91]. The disease becomes manifest in the second decade and is characterized by osteoclastic resorption with cancellous bone expansion and elevation in serum alkaline phosphatase. Early tooth loss by external resorption and deafness attributed to ossicle agenesis are featured. Histologically the involved bone shows focal collections of multinucleated giant cells and ultrastructural analysis discloses the presence of viral-like inclusions. A fibro-osseous pattern is encountered with evidence of extensive remodeling and deposition of woven bone. Areas of radiolucency are histologically comprised of adipose and fibrovascular marrow elements with minimal osteogenesis.
Expansile skeletal hyperphosphatasia (ESH) shows overlapping features with FEO, also being inherited as an autosomal dominant trait [44, 45, 92, 93]. Accelerated bone turnover is seen along with hyperostotic expansion of the long bones with significant pain involving the phalanges, premature tooth exfoliation and deafness. Unlike FEO, ESH manifests episodic hypercalcemia and there is an absence of large osteolytic lesions with cortical thinning. The bones of the skull and appendicular skeleton show hyperostotic and sclerotic changes radiographically. Alkaline phosphatase is elevated. Extensive skeletal remodeling is seen histologically, yet no viral like inclusions have been reported in osteoclastic cells.
Segmental Odontomaxillary Dysplasia
A unique monostotic pathologic process that occurs in the maxilla is segmental odontomaxillary dysplasia [94–98]. The lesion is confined to a single segment of the maxilla, usually involving the premolar and molar teeth and associated alveolar bone. Some would elect to exclude this entity from the classification of BFOLs based on histology which is unique (vide infra). Clinically, teeth often fail to erupt and in the affected region, dental anomalies are encountered including malformed, misshapened and/or teeth of anomalous size. There may also be enamel defects. The alveolar bone in the affected segment exhibits mild expansion and radiographically, normal trabeculation is replaced by opacified streaks that resemble falling sleet (Fig. 5a). Microscopically, a fibro-osseous pattern is encountered. Both woven and lamellar irregular trabeculae are encountered with minimal osteoblastic rimming and the fibrous element is represented by small immature collagen with only a mild increase in cellularity. In many areas, more dense sclerotic bone is seen and cementicle ovoid calcifications may also be encountered. The bone is unique, showing mosaic Pagetoid resting and reversal lines with prominent osteocyte hypercellularity characterized by multiple large lacune (Fig. 5b). The hypocellularity in the stroma, unlike most other BFOLs is common, although there are microscopic fields in which the stroma is cellular.
Fig. 5.
Segmental Odontomaxillary Dysplasia. (a) Replacement of trabeculae by a “sleet storm” configuration and underupted teeth. (b) Fibroosseous microscoscopic pattern with low cellularity, prominent osteocyte hypercellularity and mosaic bone
Cemento-Osseous Dysplasias
Clarification of Terminology
Periapical cemento-osseous dysplasia (PCOD) and focal cemento-osseous dysplasia (FCOD) represent the most common fibro-osseous lesions of the jaws [6, 99–102]. PCOD or FCOD are two different terms for the same reactive lesion. Florid cemento-osseous dysplasia (Fl.COD) denotes an extensive process with multifocal involvement of the jaws by lesional tissue with the same microscopic appearance as is encountered with PCOD and FCOD. All of the cemento-osseous dysplasias occur in tooth-bearing areas.
A bewildering number of terminologies have been used for these lesions in the literature. Periapical cemento-osseous dysplasia has been previously referred to as cementoma, periapical cementoma, periapical cemental dysplasia, and periapical fibrous dysplasia [100]. Despite the profusion of appellations, PCOD is a reasonably well-defined clinical-radiological entity, predominantly involving the apical areas of vital mandibular incisors. The term FCOD was recently recommended by Summerlin and Tomich [102] and has been previously described by different investigators under the designations of “osseous dysplasia reaction of bone to injury” and “localized fibro-osseous cemental lesions—presumably reactive in nature” [99, 103, 104]. The tooth-bearing areas of the posterior jaws, particularly in sites of former extraction, are the common sites for FCOD, some of which had been previously confused with, or misdiagnosed as cemento-ossifying fibroma [102]. The observation that PCOD and FCOD share the same histopathology and show similar, if not identical, clinical profiles prompts one to consider that both are the same entity with different locations. In fact, the World Health Organization definition of periapical cemental dysplasia, is “a non-neoplastic lesion affecting the periapical tissues of one or more teeth”, which therefore does not subclassify this lesion according to any defined location (i.e.: anterior vrs posterior apical areas of jaws) [101]. We believe that the designation of “periapical cemento-osseous dysplasia” is more appropriate than that of “periapical cemental dysplasia” because both cementum-like and bony calcifications are present in this entity. PCOD or FCOD may be used interchangeably as a matter of personal preference. The authors propose to use FCOD to encompass those lesions formerly designated as either PCOD or FCOD.
Focal Cemento-Osseous dysplasia
The literature from the different groups of studies has shown that FCOD occurs predominantly in females with a mean age in the mid-thirties, and a slightly higher distribution among African Americans (64%). The majority of these lesions is asymptomatic (62%) with an average size of 1.5 cm. The mandible is the most frequent site of occurrences (86%). On gross examination, the lesion is typically comprised of multiple tan and brown tissue aggregates that appear crumbly [102, 105–112].
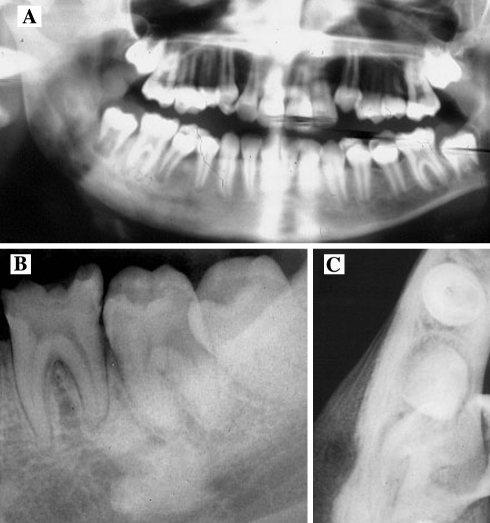
Radiographically, this condition evolves through three phases: early, intermediate and late stages. The early stage of FCOD classically presents with a well-defined radiolucency at the apices of mandibular teeth (Fig. 6). The radiographic image may be erroneously diagnosed as an endodontic infection and a pulp vitality test should clarify the clinical confusion, although some challenges may occur. The lesions of FCOD at its intermediate stage demonstrate a mixed radiolucent-opaque pattern with a well defined radiolucent rim around the radiopacity. These lesions at the late stage display diffuse radiopacity often with ill-defined borders. The microscopic features of FCOC are identical to those encountered in florid cemento-osseous dysplasia described below.
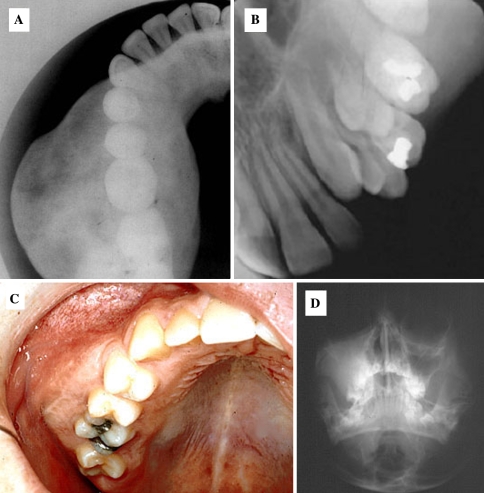
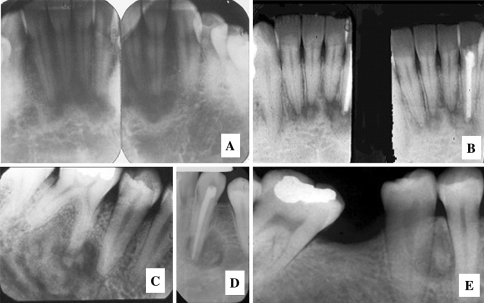
Fig. 6.
Nonexpansile cemento-osseous dysplasias. (a) Periapical Cemental Dysplasia (PACD), a subtype of focal cemento-osseous dysplasia of the anterior mandible begins as a periapical radiolucency. (b) PACD opacifies forming a radiographic “target lesion” in later stages of the disease. (c, d and e). Target lesions in focal cemento-osseous dysplasia affecting the posterior teeth. (c) A traumatic bone cyst is seen at teeth 19 and 20
It is important to differentiate lesions of FCOD from a true neoplasm, ossifying fibroma (OF). FCOD demonstrates an irregular mixed radiolucent-opaque pattern (69%) with slightly more than half of cases showing well-defined borders. However, more than half of OF present as a pure radiolucency with significantly larger size and typically cause jaw expansion clinically. Secondly, FCOD shows a close association with tooth apices (70.6%) or with previous extraction sites (21%). However, the majority of OF (86%) shows no relationship with either. These features provide a primary guidance for distinguishing these two entities clinically. FCOD represents a benign nonneoplastic process while OF is a true neoplasm [101, 102, 106]. Complete surgical removal is unnecessary after a diagnosis is made, although a periodic follow-up is recommended in order to ensure that no further enlargement or expansion occurs, features that argue in favor of an ossifying fibroma that was merely encountered in an early stage of development.
Florid Cemento-Osseous Dysplasia
When lesions with radiologic and microscopic features similar to FCOD extend to two or more quadrants of the jaw, the disease is termed florid cemento-osseous dysplasia (FlCOD) [112–120]. In most instances, the disease affects the mandible bilaterally and may or may not show concomitant maxillary involvement. Most of the patients with FlCOD are more than 45 years old, although, it has been reported in younger patients. Black females are predilected although other races may be affected. FlCOF is usually asymptomatic and symmetrically distributed. Radiographically the disease is characterized by multiple confluent and nonexpansile radio-opacities, often with a circumferential radiolucency. The lesions are most common in the mandibular molar/premolar region (Fig. 7). Pain is the most frequent complaint if patients become symptomatic. The symptomatic patients have been reported with a higher prevalence in Orientals, mainly Chinese. The diagnosis can be made based on radiographic presentation. An odontogenic infection in teeth overlying lesional tissue may result in widespread infection evolving into an acute suppurative osteomyelitis with bony sequestration. Indeed, osteomyelitis may occur as a complication of open biopsy for FlCOD.
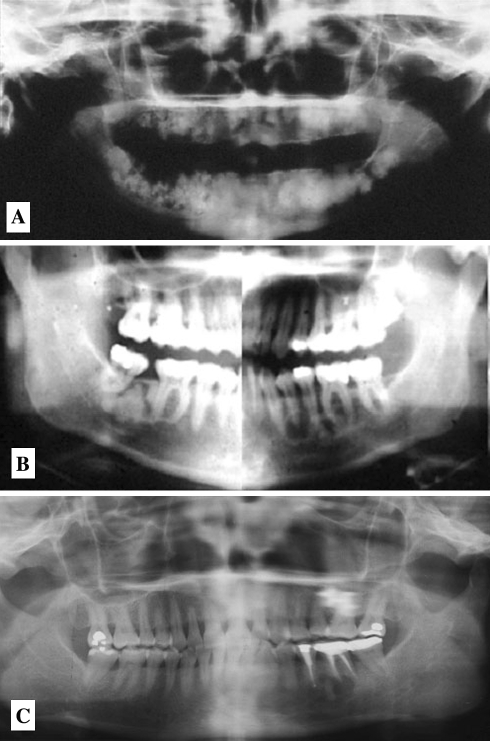
Fig. 7.
Florid Cemento-osseous Dysplasia. (a) Multiple confluent opacities in all four jaw quadrants, (b). Bilateral radiolucent and mixed lucent/opaque lesions, (c). radiolucent regions are empty bone cavities similar to traumatic bone cyst of the jaws
Concomitant cystic changes may occur in both FCOD and FlCOD, being more often associated with the latter [112, 119, 120]. These irregular or multilocular radiolucencies resemble simple bone cysts being vacant cavities in bone without a cystic epithelial lining.
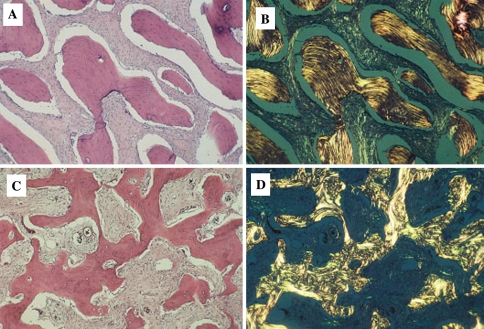
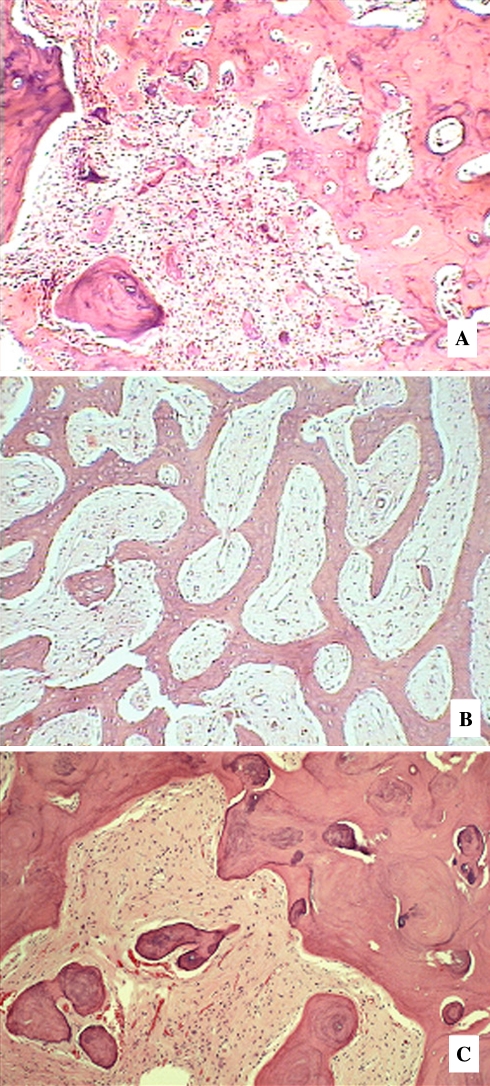
In the early stage of the lesion which is largely radiolucent, FCOD reveals multi-fragmented tissue composed of vascular fibrous stroma with scattered osteoid trabeculae. The stroma often displays characteristic cavernous-like vascularity which is almost always associated with early formation of bony trabeculae (Fig. 8a, b). Free hemorrhage is frequently interspersed in the artifactual spaces throughout the specimen. Osteoblasts are seen occasionally around the osteoid trabeculae. In the intermediate stage, the vascular stroma becomes more fibrotic with additional formation of osteoid trabeculae (Fig. 8c). Occasional cement-like calcifications are also present. These structures, often termed “cementicles” or “bonicles” by other authors are characterized as ovoid calcifications that often show radiating Sharpey fibers when viewed under polarized light. In the late state corresponding to radiographically demonstrable opacification, FCOD often displays very little fibrotic stroma with thick curvilinear trabeculae (“ginger root” pattern) or irregularly shaped cementum-like masses (Fig. 8d, e).
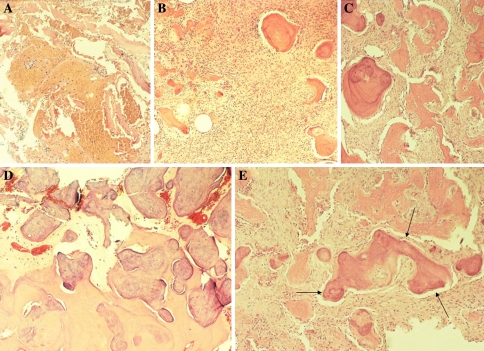
Fig. 8.
The Cemento-osseous Dysplasias. (a) Early stage lesion with hemorrhagic foci, (b). Early region with fibro-osseous pattern, (c). Mid stage lesion with progressively more trabeculae, (d). Late stage lesion with sclerotic bone. (e) Late stage lesion with “ginger root” curvilinear confluent trabeculae
The study in a pathologic spectrum of 316 cases assessed 24 histopathologic parameters for their ability to distinguish between FOCD and OF [106]. Results revealed that 92.5% of FCODs were composed of multiple small fragments of tissue while 88.0% of OFs showed a large intact specimen. Thick curvilinear trabeculae (“ginger root” pattern) or irregularly shaped cementum-like masses were typically seen in FCOD whereas thin isolated trabeculae with prominent osteoblastic rimming were more commonly observed in OF. The stroma of FCOD often displayed characteristic cavernous-like vascularity which was almost always associated with bony trabeculae. Free hemorrhage was frequently interspersed in the artifactual spaces throughout FCOD. In contrast, the cases of OF showed more cellularity in the stroma in which a storiform pattern was present in more than half the lesions studied. The features described here allowed distinction between FCOD and OF histopathologically in 94% of cases studied [10]. The histopathology of FlCOD shows mixed stages identical to FCOD depending on where the biopsy is taken. The majority of the cases reveals the characteristic late stage of FCOD (Fig. 8a, b). Simultaneous cystic changes may occur in FlCOD and the histopathology from the cystic area is identical to simple bone cyst [105, 112].
Inflammatory/Reactive Processes
Most infections of the jaws are odontogenic in origin. Both pyogenic and anaerobic bacteria are usually responsible for acute, subacute or chronic osteomyelitis (streptococci, bacteroides, actinomycetes). These infections when suppurative may develop intraoral as well as cutaneous sinus tracts, and the osseous lesions are destructive, typically portrayed as “moth-eaten” radiolucencies owing to the osteoclastic resorption induced by the infectious agents and the host inflammatory response. Anachoretic infections may also seed the jaws to cause osteolytic lesions, yet an odontogenic source of infection from dental caries is far more common.
Low grade infections may occur within the craniofacial bones, usually the mandible, and initiate osteoblastic rather than osteoclastic activity. These infections are caused by anaerobic organisms of low virulence and histologic evidence of an inflammatory response in bone marrow is typically absent. The host response to these low grade infections is reactive osteogenesis and imaging patterns show diffuse homogeneous opacification. Reactive proliferation of the periosteum is also a frequent accompaniment. In concert, facial bones that envelop the paranasal sinuses are occasionally affected in sinusitis and may show a reactive osteosclerotic reaction with microscopic features that are fibro-osseous in nature.
Focal Sclerosing Osteomyelitis (Condensing Osteitis)
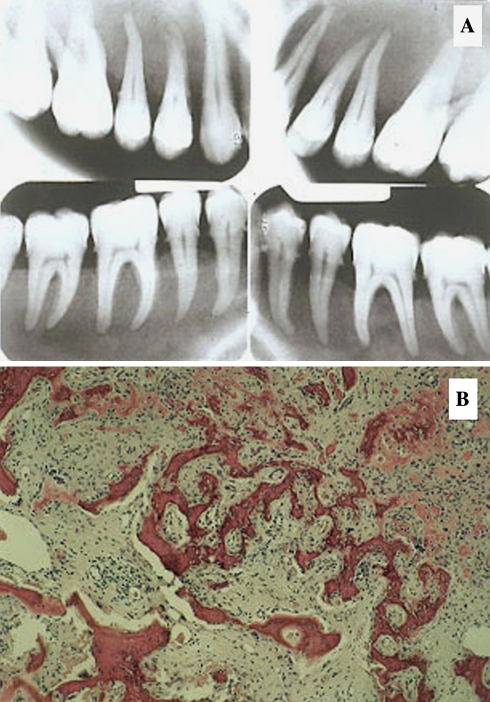
The mildest and most self-limited form of sclerosing osteomyelitis is encountered in the posterior mandible at the apices of molar teeth [121–129]. The condition is extremely rare in the maxilla. The lesion is of putative microbial origin, although extensive bacterial culture studies have not been forthcoming. A bacterial origin for the disease is teleological and empirical, based on the finding that the involved tooth affected by advanced caries that supposedly resulted in pulp exposure by cariogenic and other oral bacteria. The pathogenic bacteria are believed to be of low virulence leading to a chronic low grade pulpitis with spread of infection out the apex with resultant osteoblastic stimulation in the absence of an inflammatory response.
Condensing osteitis is characterized by an asymptomatic, nonexpansile periapical lesion associated with a tooth exhibiting deep caries. In the early stage of the infection, a radiolucency is seen at the apex, simulating a dental abscess, granuloma or cyst. If biopsied, no inflammation is encountered; rather, a benign fibro-osseous histology is encountered. With ensuing longevity, the apical lesion progressively opacifies and microscopically, dense lamellar Haversian bone is formed as the fibrous element becomes overshadowed (Figs. 9 and 10). A clinicoradiologic diagnosis is usually made without need for biopsy. Root canal therapy is the treatment of choice, yet after completed, the apical radio-opacity fails to resolve.
Fig. 9.
Chronic Sclerosing Osteomyelitis. (a) Diffuse form showing right mandibular opacification, (b). Focal Condensing osteitis with focal opacification at the root apex, (c). Proliferative periostitis showing carious associated tooth and cortical “onion skinning”
Fig. 10.
Chronic Sclerosing Osteomyelitis. (a) Fibro-osseous pattern in early stages and around the periphery of solidly opaque regions, (b). Paralleling of trabeculae in proliferative periostitis. (c) Late stage dense osteosclerosis in diffuse sclerosing osteomyelitis
Focal sclerosing osteomyelitis in its later sclerotic phase, is often confused with focal osteosclerosis or bone scar. The latter occurs at the apices or is interposed between the roots of sound noncarious teeth. It is densely radio-opaque and does not evolve from a benign fibro-osseous lesion. Another entity in the differential diagnosis is focal cemento-osseous dysplasia that is coincidentally, yet not causally, at the apex of a carious tooth.
Diffuse Sclerosing Osteomyelitis
As mentioned previously, most forms of osteomyelitis affecting the jaws derive from the spread of odontogenic infection into marrow spaces. These bacterial infections are often acute or subacute, showing motheaten radiolucencies. Conversely, diffuse sclerosing osteomyelitis (DSO), akin to its lesser cousin, focal sclerosing osteomyelitis, is characterized by a unilateral diffuse ground glass opacification without defined boundaries, of the mandibular body (Fig. 9) [130–137]. Clinically, the patient presents with cortical osseous expansion and a history of dull episodic pain that may last for weeks only to subside and later become symptomatic again. Routine culture usually yields normal oral flora. A definitive anaerobic culture must be procured from a trephine bone biopsy specimen. DSO is usually caused by gram negative anaerobic bacteria of low virulence. The source of infection may be odontogenic with a grossly carious tooth overlying the region; conversely, some cases have no identifiable odontogenic source.
Microscopically, the ground glass bone exhibits a fibro-osseous pattern with intervening foci of dense sclerotic bone (Fig. 10). The osseous elements are trabecular in the fibro-osseous regions, cementifying areas being absent.
Fibrous dysplasia and florid cemento-osseous dysplasia are the chief considerations in the differential diagnosis, however the former is a painless lesion and the latter manifests multiquadrant opaque lesions with associated radiolucencies that on biopsy show both trabecular and cementifying areas along with hollow bone cavities. In the earlier literature, florid cemento-osseous dysplasia was erroneously diagnosed as DSO, probably because the latter is occasionally found to be secondarily infected with fistulae and sequestra.
Proliferative Periostitis
When the low grade infectious progress of DSO involves the periosteum, it frequently induces neo-osteogenesis and the periosteal layer becomes redundant, yielding the classic “onion skinning” phenomenon originally subsumed under the appellation Garré’s periostitis (Fig. 9) [138–144]. In fact, it is the periosteal reaction that accounts for the clinically evident cortical expansion seen in DSO.
Biopsy of the periosteum discloses a trabecular pattern that is often reteform with a tendency for parallel orientation (Fig. 10).
DSO is managed by eliminating any odontogenic sources of infection by either extraction or root canal therapy. Culture and sensitivity must be obtained and long term antibiotic therapy can then be administered. With appropriate antibiotic therapy, the cortically expanded areas ultimately resolve autonomously.
Hyperparathyroidism
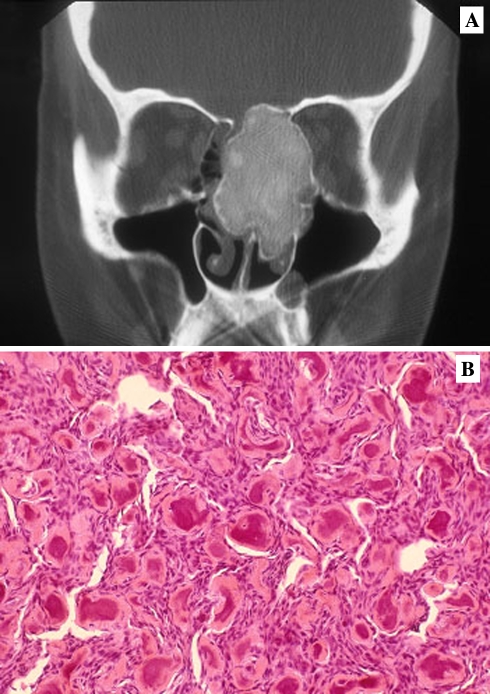
Craniofacial bone lesions may be seen in both primary and secondary hyperparathyroidism (HPT) with benign fibro-osseous lesions being more often reported among patients with renal osteodystrophy [145–152]. The “brown tumor” of hyperparathyroidism, a giant cell lesion, may be encountered anywhere in the skeleton in both primary and secondary HPT. In the early stages of renal osteodystrophy, the jaws and facial bones will exhibit a loss of normal trabeculation as seen radiographically, replaced by a nonexpansile diffuse ground glass opacification (Fig. 11). Microscopically, a BFOL characterized by trabecular bone is seen.
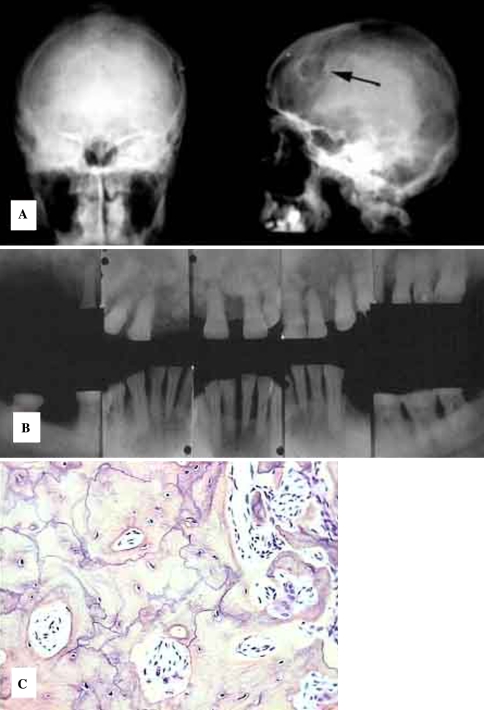
Fig. 11.
Hyperparathyroidism. (a) Ground glass diffuse opacification in renal osteodystrophy. (b) Histology is fibro-osseous
Marked facial deformity is seen in a subset of renal osteodystrophy patients with thickening of diploe and massive maxillomandibular enlargement with protrusion of the anterior teeth and wide diastemas [152–157]. Similar findings have been found in animals where the condition has been referred to simply as “big head” disease. Other terms applied to this condition include the Sagliker syndrome and the uglifying human face syndrome. In humans the enlarged regions of the facial bones, jaws and skull manifest a dense ground glass opacification not unlike that of Alber’s Schonberg (familial osteopetrosis, marble bone disease). Parathyroidectomy does not usually result in resolution of the bony enlargements. This condition has been reported in the past as Leontiasis Ossea. This clinical term has also been applied to expansile lesions of fibrous dysplasia and Paget disease of bone and should not, therefore, be restricted to secondary HPT.
Another maxillofacial fibro-osseous lesion that is seen in hyperparathyroidism is ossifying fibroma [153–160]. In this condition, referred to as hyperparathyroidism-jaw tumor syndrome, patients suffer from familial parathyroid adenomas, ossifying fibroma of the jaws, renal cysts and Wilms tumors. The HRPT2 tumor suppressor gene has been found to be mutated in this syndrome and more recent studies have found perturbations in this gene among nonsyndrome associated ossifying fibromas of the jaws. The mutated gene inactivates the parafobromin protein through truncation. The microscopic appearance of the syndrome associated ossifying fibromas is the same as that encountered in ossifying fibroma NOS (vide infra).
Ossifying Fibromas
Neoplasms with a fibro-osseous histology are represented by the ossifying fibroma group of lesions. These are neoplasms in the true sense, exhibiting progressive proliferative capabilities with boney expansion and, importantly, well defined margins radiologically. Subsumed under this diagnostic category are ossifying/cementifying fibroma not otherwise specified (NOS), implying that the clinicopathologic features do not conform to the other types of ossifying fibromas; these specific subtypes include psammomatoid variant of ossifying fibroma, trabecular variant of ossifying fibroma and gigantiform cementoma, the latter of which may show an autosomal dominant genetic or “familial” underpinning. Most ossifying fibromas are single focal lesions; however gigantiform cementoma is typically multifocal and may occur in all four jaw quadrants in a single patient. There are also reports of lesion multiplicity in the other forms of ossifying fibroma yet such occurrences are quite rare.
Ossifying/Cementifying Fibroma, NOS
The most common form of ossifying fibroma occurs in the maxilla and mandible and represents a neoplastic process that is typically painless, presenting with expansion of the buccal and lingual cortices and in larger lesions may expand the inferior aspect of the mandible [105, 106, 120, 161–164]. Teeth in the vicinity of these lesions are often displaced superiorly (Mandibular lesions) or inferiorly (Maxillary lesions) and the latter typically expand into the maxillary antrum. Radiographically, early stage lesions in their formative phase, are typically radiolucent since the osseous element is noncalcified osteoid. Over time, the tumors become progressively radioopaque as more matrix calcifies (Fig. 12). Very few molecular studies have been reported for the ossifying fibroma group of lesions, only a few case reports having appeared in the literature. As mentioned previously, there are reports that identify mutations in HRPT2 a gene that encodes parafibromin protein.
Fig. 12.
Ossifying fibroma NOS. Radiographically, the lesions are expansile and radiolucent with internal floccular to dense opacities
Ossifying fibroma NOS shows three histologic patterns and some demonstrate a mixture of these patterns. The common “ossifying” form shows a pattern that may be indistinguishable from fibrous dysplasia, with small irregular osteoid trabeculae that are typically rimmed by osteoblasts. The stromal element is hypercellular and the fibroblastic cells are devoid of atypical cytologic features. Early formative tumors show woven bone patterns when assessed under polarized light; however, when the lesions mature, osteoblastic rimming is minimal and the irregular trabeculae are often lamellar (Figs. 13, 14). The “cementifying” form is similar to the psammomatoid variant that will be described later. Most cementifying types also contain more typical osseous trabeculae in addition to the cemental structures which are ovoid or droplet in shape. These ovoid calcifications resemble normal cementicles that are present in the periodontal ligament. In previous publications the ovoid lesions have been referred to as cementifying fibromas while those with both osseous and cementoid calcifications are labeled as cemento-ossifying fibromas. Lastly, the “storiform” form of ossifying fibroma (NOS) is typified by streaming of the fibroblastic stromal elements in a pinwheel configuration similar to benign fibrous histiocytoma. Dispersed throughout are whispy calcifications that appear like dystrophic bone and many also show an ovoid configuration.
Fig. 13.
Ossifying Fibroma NOS. Microscopic variations. (a) irregular trabeculae with osteoblastic rimming, (b). corresponding to figure A, both lamellar and woven patterns are present, crossed polars, (c). mosaic bone without osteoblastic rimming, (d). corresponding to figure C, predominantly woven bone, crossed polars
Fig. 14.
Ossifying Fibroma NOS with trabecular and stromal variations. (a). dystrophic appearing calcifications in a storiform fibrous element, (b). Cementicle calcifications in a patternless stroma, (c). Cemento-ossifying pattern, (d). Whispy calcifications
Regardless of the variant forms of ossifying fibroma NOS, the behavior and natural history appear to be the same. The lesions grow slowly and expand cortices, yet simple curettage and enucleation are generally sufficient for a cure. Recurrences are uncommon.
Juvenile Ossifying Fibroma
The term juvenile ossifying fibroma (JOF) also known as Juvenile Active Ossifying Fibroma [165–167] and juvenile aggressive ossifying fibromas [165, 168, 169] is used in the literature to describe two distinct clinicopathologic entities; (1) Trabecular juvenile ossifying fibroma (TrJOF) and (2) Psammomatoid juvenile ossifying fibroma (PsJOF).
Trabecular Juvenile Ossifying Fibroma
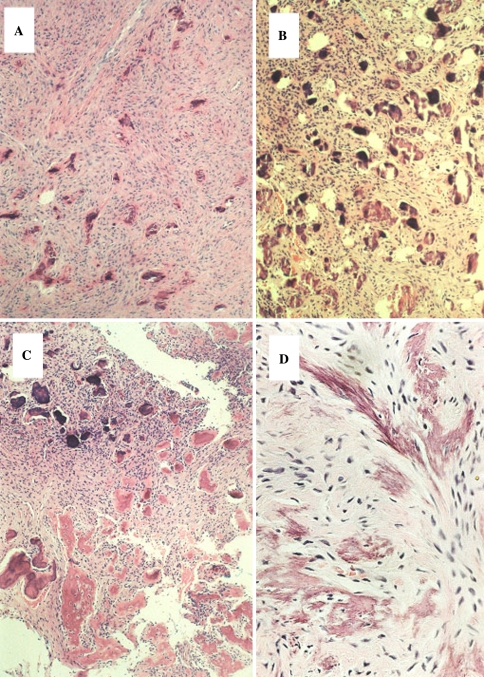
TrJOF also known as trabecular desmo-osteoblastoma is defined as a lesion affecting the jaws of children composed of a cell rich fibrous stroma containing bundles of cellular osteoid and bone trabeculae without osteoblastic rimming, and aggregates of giant cells [165, 167, 169, 170].
The great majority of the patients are children and adolescents. Only 20% of the patients are over 15 years of age. In a review of a number of case series the mean age range was found to be 8.5–12 years. Males and females are equally affected [165, 168–171]. The maxilla and the mandible are the dominant sites of incidence. Occurrence in the maxilla is slightly more frequent than in the mandible. Origin in extragnathic locations is extremely rare.
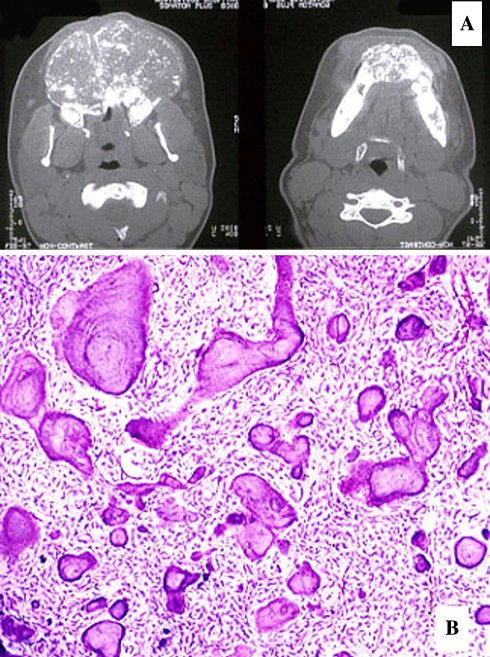
Clinically, TrJOF is often characterized by a progressive and sometimes rapid expansion of the affected area; pain is a rare symptom [165–173]. In the maxilla, obstruction of the nasal passages and epistaxis may be present. Radiographically, the tumor is expansive and may be fairly well demarcated, with cortical thinning and perforation. Depending on the amount of calcified tissue produced, the lesion will show varying degrees of radiolucency or radiodensity. Ground-glass as well as a multilocular honeycomb appearance has been described (Fig. 15).
Fig. 15.
Aggressive Juvenile Ossifying Fibroma, Trabecular Type. (a) Expansile maxillary mass, (b). Trabecular bone and hypercellular stroma, (c). ossification poor, hypercellular fibroblastic zones, (d). Focal collections of multinucleated giant cells
Microscopically, TrJOF is unencapsulated and shows infiltration of the surrounding bone. The tumor has a characteristic loose structure. The stroma is cell-rich, with spindle or polyhedral cells that produce little collagen [165, 168–171, 173]. Cellular, immature osteoid forms strands that may be long and slender or plump (Fig. 15). These structures have been likened to paint brush strokes. The immature cellular osteoid is not always easily distinguished from the cellular stroma. Irregular mineralization takes place at the center of the strands. Maturation to lamellar bone is not observed. Local aggregates of osteoclastic giant cells are invariably present in the stroma (Fig. 15). Mitotic activity of the stromal cells may be present but is never numerous. Cystic degeneration and aneurysmal bone cyst formation has been reported in a few cases.
The clinical course of JAOF is characterized by infrequent recurrence following conservative excision. One or more recurrences were observed in 3 of 10 patients reported by Slootweg et al. [169]. Eventual complete cure could be achieved in those cases without resorting to radical surgical intervention. Malignant transformation has not been reported.
Psammomatoid Juvenile Ossifying Fibroma
Unlike TrJOF, psammomatoid juvenile ossifying fibroma (PsJOF) is a lesion that affects predominantly the extragnathic craniofacial bones, particularly centered on the periorbital, frontal, and ethmoid bones [165]. PsJOF was initially described by Gogl [174] as early as 1949 under the designation psammomatoid fibroma of the nose and paranasal sinuses. Margo, in 1985 [175] described PsJOF as a distinctive solitary fibro-osseous lesion of young individuals that affects the orbit and shows distinguishing histologic features. PsJOF was also reported under the designation juvenile active ossifying fibroma by Johnson et al. [166] and juvenile ossifying fibroma with psammoma-like ossicles [169] and psammous desmo-osteoblastoma by Makek [165]. PsJOF is not classified under osseous tumors of the jaws in the Armed Forces Institute of Pathology (AFIP) Atlas of Tumor Pathology [176] nor was it considered a gnathic tumor in the WHO histologic classification of odontogenic tumors [167]. Contrary to the belief of some authors, PsJOF is a separate clinicopathologic entity than the gnathic cemento-ossifying fibroma.
Afflicted individuals tend to be young, although the average age of incidence has varied in different studies from 16 to 33 years with an age range of 3 months to 72 years [165, 169–171, 174–182]. In general, patients with PsJOF are a few years older than those with TrJOF. But, as in the case of TrJOF, there is no sex predilection. The greatest majority of the reported cases of PsJOF originated in the paranasal sinuses, particularly frontal and ethmoid. About 10% have been reported in the calvarium. Makek [173] in his review indicated that 7% of the cases occurred in the mandible. In a more recent study one of three cases originated in the mandibular ramus [165].
PsJOF is clinically manifested as bone expansion that may involve the orbital or the nasal bones and sinuses. Orbital extension of sinonasal tumors may result in proptosis, and visual complaints including blindness, nasal obstruction, ptosis, papilledema, and disturbances in ocular mobility.
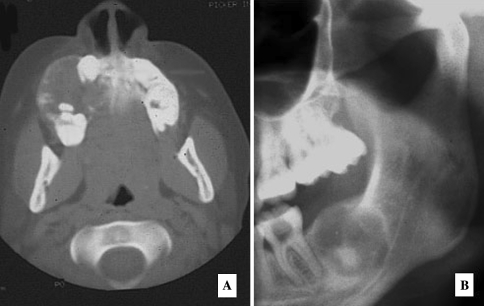
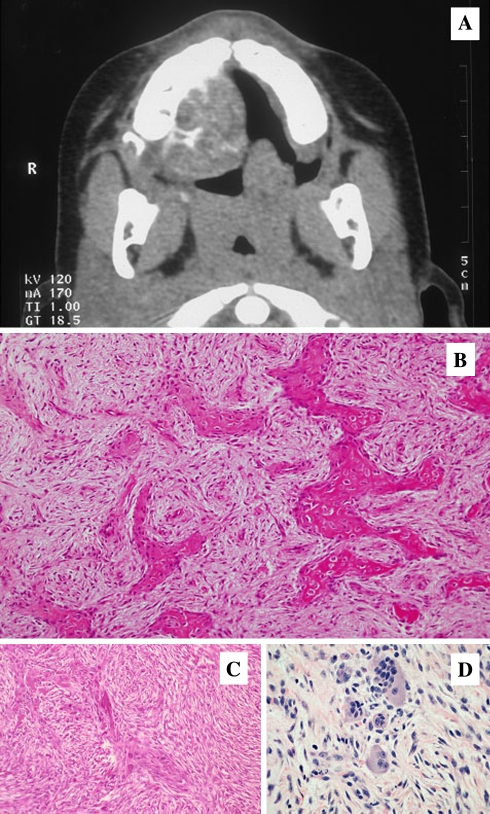
Radiographic examination shows a round, well-defined, sometimes corticated osteolytic lesion with a cystic appearance [106, 165, 172]. Sclerotic changes are evident in the lesion which may show a ground-glass appearance. In computed tomographic scans set on bone window, the lesions appear less dense than normal bone (Fig. 16a). The lesions may range in size from 2 to 8 cm in diameter. PSOF may appear multiloculated on CT scans. Areas of low CT density may be noted due to cystic changes. It is stated that in the facial skeleton a well-circumscribed expansive mass with a thick wall of bone density on CT scan and enhancement of this area on post contrast MR image is strongly suggestive of psammomatoid juvenile ossifying fibroma.
Fig. 16.
Aggressive Ossifying Fibroma, Psammomatoid Type. (a) Paraorbital well demarcated expansile radioopacity. (b) Psammomatoid calcifications resembling cementicles with a hypercellular stromal element
On gross examination, the tumor is described as yellowish, white and gritty. On light microscopic examination, the tumor is significant for multiple round uniform small ossicles (psammomatoid bodies) embedded in a relatively cellular stroma composed of uniform, stellate, and spindle shaped cells (Fig. 16b). Occasionally, shrunken cells become embedded in the calcified matrix of the ossicles. The psammomatoid bodies are basophilic and bear superficial resemblance to dental cementum, but may have an osteoid rim. Because of a superficial resemblance between these ossicles and the cementum spheres of the odontogenic ossifying fibroma, the lesion has occasionally been mislabeled as cemento-ossifying fibroma, implying an odontogenic origin which, as mentioned above, is rather unlikely in extra- gnathic bone. Mitotic activity is extremely rare in the stromal cells. At the periphery of the lesion, the ossicles seem to coalesce and form irregular thin bony trabeculae that may become thicker, with numerous reversal lines resembling Paget’s bone. A shell of normal bone is usually present and may show osteoclastic resorption endosteally associated with osteoblastic activity on the periosteal surface. Cystic degeneration and aneurysmal bone cyst formation has been reported in some cases. It is believed by some authors that fast and aggressive growth rates in some cases of JOF of both the psammomatoid and trabecular types may be related to aneurysmal bone cyst formation [165].
Confusion between TrJOF and PsJOF is mainly due to the use of the term “Juvenile ossifying fibroma” to describe these two entities, in spite of clear differences in microscopic appearance. The former is Trabecular, the later is psammomatoid. In addition PsJOF have a definite site predilection for periobital bones while TrJOF favors the gnathic bones. TrJOF also has a younger average age of incidence.
Surgical excision is the treatment of choice, although recurrence even after definitive surgery is not unusual. Recurrence rates of >30% have been reported. In some cases, multiple recurrences over a long follow-up period are reported. No malignant change has been observed.
Cytogenetic analysis was done in only a few cases of ossifying fibroma. In one case of COF of the mandible, deletions were detected in 2q31-32 q35-36 [183]. A study of 3 cases of PsJOF of the orbit demonstrated non random chromosome break points at Xq26 and 2q33 resulting in (X;2) translocations [184].
Gigantiform Cementoma
An extremely rare form of ossifying fibroma is multifocal with tumors that are often massive. Autosomal dominant inheritance is seen among some cases whereas others are “familial”. Among the few cases that have been reported, the gene appears to have a high level of penetrance with variable expressivity [185–198]. Lesions arise during childhood and progressively expand to cause facial deformity during early adult years. Yet other instances within a kindred may manifest minimal involvement without progressive aggressive behavior and the lesions are confined failing to expand the cortices. Sporadic cases without any heritable features have been also been recorded.
Gigantiform Cementomas are restricted to the jaws and may arise in two, three or all four quadrants. Expansile masses of the maxilla and mandible are readily apparent in most instances and radiographically, marked expansion is seen with a radiolucent mass containing floccular calcifications (Fig. 17a). In the forme frusta, the clinicoradiologic features are similar to those seen in florid osseous dysplasia, being multiple confluent opacities that are minimally expansile.
Fig. 17.
Gigantiform Cementoma. (a) Massive mixed radiolucent/radioopaque expansile lesions in both jaws. (b) Fibro-osseous pattern with cementicles and boney trabecuae, the former oftern appearing much larger that those seen in cemento-ossifying fibroma
Microscopically a benign hypercellular stroma is observed with monomorphic appearing fibroblasts and mature collagen fibers [185, 186], Mitotic figures are absent. Dispersed throughout are ovoid, often laminated, psammomatoid calcifications that are variable in size. Many of these spheroidal calcifications are large, much larger that those seen in the psammomatoid variant of ossifying fibroma (Fig. 17b). After all, everything is big with gigantiform cementoma. Under polarized light, Sharpy’s fibers are seen to project radially from these larger spheroidal deposits that resemble cementicles normally encountered in the periodontal ligament.
Treatment for gigantiform cementoma is resection with immediate or staged reconstruction (Table 3).
Discussion
There has been considerable controversy concerning the nosology of benign fibro-osseous lesions, due in part to the varied histomorphologic patterns of stroma and bone in these lesions and the fact that similar or even identical microscopic features can be shared among two or more different entities. We have classified these diseases according to pathogenetic mechanisms yet the most practical, utilitarian approach is to consider BFOL in the context of combined clinical, radiologic and pathologic characteristics (Table 4). It is axiomatic that a definitive diagnosis can rarely be rendered on the basis of microscopic features alone; although there are subtle changes that may lead the pathologist to favor one entitiy over others. Age sex and race are variables that carry weight in the formulation of a final diagnosis. The lesional boundaries as seen in radiographic imaging studies are also of utmost importance since some BFOL are associated with teeth; some show diffuse ill-defined borders while others are well delineated. And finally, there are indeed certain histologic patterns that may be restricted to only one disease process.
Table 4.
Clinical, radiographic and microscopic parameters that distinguish among the benign fibro-osseous lesions
| Disease | Clinical features | Radiologic findings | Histopathology |
|---|---|---|---|
| Fibrous dysplasia | Expansion of bone Unilateral, painless Alk phosphatase Mono or Polyostotic |
Diffuse radiolucent or ground glass | Trabecular “Chinese/Hebrew” |
| Osteitis deformans | Expansile lesions Alk phosphatase Cranial neuropathies Polyostotic |
Ground glass Cotton wool |
Mosaic bone Rare sarcoma & giant cell lesions |
| Hyperparathyroidism | Parathormone, Ca Renal disease “Big head disease” |
Ground glass Multilocular Brown tumors Massive opaque jaw lesions |
Trabecular Giant cell lesions |
| Focal cemento-osseous Dysplasia |
Non-expansile Painless |
Focal circumscribed apical Lucent or target lesions |
Trabecular Cementifying |
| Florid cement-osseous Dysplasia |
Non-expansile African descent |
Multiquadrant opacities Associated bone cavities |
Trabecular Sclerotic |
| Focal sclerosing Osteomyelitis |
Non-expansile Painless Carious tooth |
Apical well delineated Lucent, target or opaque |
Trabecular Sclerotic |
| Diffuse sclerosing Osteomyelitis |
Expansile Painful Dental source of infection + or – |
Diffuse ground glass Proliferative periostitis |
Trabecular Sclerotic |
| Ossifying fibroma Not otherwise specified |
Expansile, painless Rarely multifocal Jaws |
Circumscribed lucent or target lesion Root divergence |
Trabecular Cementifying |
| Ossifying fibroma Trabecular variant |
Expansile, painless Root divergence Aggressive |
Circumscribed lucent Floccular opacities |
Trabecular Giant cell foci Fibroplasia |
| Ossifying fibroma Psammomatoid |
Expansile Facial bones Aggressive |
Circumscribed Dense floccular opacities |
Psammoma |
| Gigantiform cementoma | Massive, expansile Multiquadrant Often familial |
Well delineated lucent with floccular opacities | Trabecular Cementifying |
The diagnostic criteria that are most controversial, at least as gleaned from the earlier literature are those that distinguish (1) fibrous dysplasia from ossifying fibromas and chronic sclerosing osteomyelitis, (2) florid cementoosseous dysplasia from diffuse sclerosing osteomyelitis and gigantiform cementoma and (3) focal cementoosseous dysplasia from ossifying fibromas.
The microscopic features outlined by Reed [199] Zimmerman et al. [200] and vanHorn et al. [201] for fibrous dysplasia refer to the presence of “metaplastic” bone, characterized by trabeculae that are morphed from simple mesenchymal connective tissue in the absence of osteoblasts and also represented a maturation arrest with deposition of woven bone only. In the craniofacial bones these criteria do not apply. Documented followup with periodic rebiopsy discloses that early formative lesions of fibrous dysplasia lay down woven bone trabeculae with extentive osteoblastic rimming of trabeculae and marked stromal hypercellularity whereas “older, mature” lesions show both woven and lamellar bone with trabeculae that lack juxtaposed osteoblasts. The socalled “chinese or Hebrew” letter figures remain a classis feature, yet these atypical trabeculae may be seen in other BFOL. The major criteria for the diagnosis of fibrous dysplasia in the craniofacial complex are (1) a fibroosseous histology with trabecular rather than cemental bone, (2) onset in childhood or teenage years, (3) unilateral distribution with polyostotic forms being uncommon, (4) a ground glass radiographic pattern in the maxilla and facial bones or a mottled mixed radiolucent/radioopaque pattern in the mandible, all with poorly defined lesional boundaries.
While fibrous dysplasia and ossifying fibromas may share microscopic features, the clinicoradiologic differences are now widely accepted. Recently, Toyosawa et al. [164] demonstrated that the two entities can be distinguished from one another on the basis of molecular detection of Gs-alpha mutations in fibrous dysplasia of the jaws while ossifying fibromas are found lacking.
The confusion between fibrous dysplasia and chronic sclerosing osteomyelitis is common. Both show osseous expansion, occur in youngsters and manifest a ground glass radiographic pattern, clinico radiologic findings that may be almost identical. The major differentiating factors include symptomatology and histology. FD is painless while CSO patients complain of episodic dull pain and it is always the mandible that is affected in CSO whereas the maxilla is more often involved than the mandible in FD. Histologically, FD never shows islands of dense cortical bone while CSO does along with concomitant fibro-osseous features. Lastly, bone biopsy culture will often reveal low virulence anaerobic bacteria in CSO and proliferative periostitis is a frequent accompaniment.
The second areas of controversy involve confusion over diagnostic features for florid cementoosseous dysplasia, diffuse sclerosing osteomyelitis and gigantiform cementoma. This confusion began with a paper by Shafer [202], in which a series of cases were published under the rubric of CSO when in fact, it was later realized that the lesions he was referring to were, in reality, classic examples of florid cemento-osseous dysplasia, an entity that was clearly delineated 19 years after Shafer’s publication, by Melrose et al. [111]. In defense of Shafer’s original interpretation, many cases of FlCOD become secondarily infected, fistulated and sequestrated, signs of bone fide osteomyelitis. Yet later in the course of events, Scandanavian papers appeared that presented cases of sclerosing bone lesions in children that could be attributed to low virulence bacterial organisms, many of which are anaerobic. [130–137]. The characteristic microscopic features outlined by Melrose et al. [111] and by Su et al. [105, 106] clearly define an entity that differs from sclerosing osteomyelitis with cementicle formation, “ginger root” trabeculae and large osteosclerotic islands. Furthermore, FlCOD is more often reported among African descent subjects, tends to be multifocal and at surgery, empty bone cavities are encountered.
Another point of controversy involves confusing FlCOD with gigantiform cementoma. There are many papers in the older literature that report cases of gigantiform cementoma that are in reality instances of FlCOD. Yet other reports have been referred to as multiple enostoses. Gigantiform cementoma as originally reported by Agazzi and Beloni [203] and more recently by Young et al. [185] and Abdulsayed et al. [186] exhibits the classic clinicoradiologic/pathologic features that have already been detailed. One caveat, in familial cases of gigantiform cementoma, there are family members who present with radiologic changes that are mild (incomplete penetrance and/or espressivity) and do in fact resemble FlCOD.
Lastly, focal cementoosseous dysplasia is often confused with ossifying fibromas. Importantly, the former is an endosseous nonneoplastic process that occurs around the roots of mandibular teeth and fails to expand bone. Alternatively, ossifying fibromas are potentially aggressive lesions that cause cortical expansion and often cause divergence of contiguous teeth. Both lesions may show similar histologic features with trabecular bone and cementifying areas. Older lesions of FCOD may show dense corticated bone islands, a finding that is not present in ossifying fibromas.
It is noteworthy that molecular studies are finally being performed and well defined DNA lesions are being uncovered in osteitis deformans, fibrous dysplasia and ossifying fibromas. Surely BFOLs of the jaws with familial or ethnic features such as gigantiform cementoma, florid cementoosseous dysplaisia, periapical cemental dysplasia and focal cementoosseous dysplasia will be subjected to rigorous analysis in the search for an undercurrent of molecular disease.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Waldron CA, Giansanti JS, Browand BC. Sclerotic cemental masses of the jaws (so-called chronic sclerosing osteomyelitis, sclerosing osteitis, multiple enostosis, and gigantiform cementoma. Oral Surg Oral Med Oral Pathol. 1975;39:590–604. doi: 10.1016/0030-4220(75)90201-7. [DOI] [PubMed] [Google Scholar]
- 2.Waldron CA, Giansanti JS. Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. Oral Surg Oral Med Oral Pathol. 1973;35:190–201. doi: 10.1016/0030-4220(73)90285-5. [DOI] [PubMed] [Google Scholar]
- 3.Waldron CA, Giansanti JS. Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. II. Benign fibro-osseous lesions of periodontal ligament origin. Oral Surg Oral Med Oral Pathol. 1973;35:340–50. doi: 10.1016/0030-4220(73)90072-8. [DOI] [PubMed] [Google Scholar]
- 4.Hamner JE, 3rd, Scofield HH, Cornyn J. Benign fibro-osseous jaw lesions of periodontal membrane origin. An analysis of 249 cases. Cancer. 1968;22:861–78. doi: 10.1002/1097-0142(196810)22:4<861::aid-cncr2820220425>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Eversole LR, Sabes WR, Rovin S. Fibrous dysplasia: a nosologic problem in the diagnosis of fibro-osseous lesions of the jaws. J Oral Pathol. 1972;1:189–220. doi: 10.1111/j.1600-0714.1972.tb01659.x. [DOI] [PubMed] [Google Scholar]
- 6.Slootweg PJ. Maxillofacial fibro-osseous lesions: classification and differential diagnosis. Semin Diagn Pathol. 1996;13:104–12. [PubMed] [Google Scholar]
- 7.Alawi F. Benign fibro-osseous diseases of the maxillofacial bones. A review and differential diagnosis. Am J Clin Pathol. 2002;118(Suppl):S50–70. doi: 10.1309/NUXA-JUT9-HA09-WKMV. [DOI] [PubMed] [Google Scholar]
- 8.Brannon RB, Fowler CB. Benign fibro-osseous lesions: a review of current concepts. Adv Anat Pathol. 2001;8:126–43. doi: 10.1097/00125480-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Yoon JH, Kim J, Lee CK, Choi IJ. Clinical and histopathological study of fibro-osseous lesions of the jaws. Yonsei Med J. 1989;30:133–43. doi: 10.3349/ymj.1989.30.2.133. [DOI] [PubMed] [Google Scholar]
- 10.Edwards PA, Corio RL. Benign fibro-osseous lesions of the jaws. Ear Nose Throat J. 1984;63:383–92. [PubMed] [Google Scholar]
- 11.Cohen MM., Jr. The new bone biology: pathologic, molecular, clinical correlates. Am J Med Genet A. 2006;140:2646–706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein LS. G(s)alpha mutations in fibrous dysplasia and McCune-Albright syndrome. J Bone Miner Res. 2006;21(Suppl 2):120–4. doi: 10.1359/jbmr.06s223. [DOI] [PubMed] [Google Scholar]
- 13.Kalfa N, Philibert P, Audran F, Ecochard A, Hannon T, Lumbroso S, Sultan C. Searching for somatic mutations in McCune-Albright syndrome: a comparative study of the peptidic nucleic acid versus the nested PCR method based on 148 DNA samples. Eur J Endocrinol. 2006;155:839–43. doi: 10.1530/eje.1.02301. [DOI] [PubMed] [Google Scholar]
- 14.Sanctis L, Delmastro L, Russo MC, Matarazzo P, Lala R, Sanctis C. Genetics of McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2006;19(Suppl 2):577–82. doi: 10.1515/jpem.2006.19.s2.577. [DOI] [PubMed] [Google Scholar]
- 15.Lietman SA, Ding C, Levine MA. A highly sensitive polymerase chain reaction method detects activating mutations of the GNAS gene in peripheral blood cells in McCune-Albright syndrome or isolated fibrous dysplasia. J Bone Joint Surg Am. 2005;87:2489–94. doi: 10.2106/JBJS.E.00160. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M. Minireview: GNAS: normal and abnormal functions. Endocrinology. 2004;145:5459–64. doi: 10.1210/en.2004-0865. [DOI] [PubMed] [Google Scholar]
- 17.Perdigao PF, Pimenta FJ, Castro WH, Marco L, Gomez RS. Investigation of the GSalpha gene in the diagnosis of fibrous dysplasia. Int J Oral Maxillofac Surg. 2004;33:498–501. doi: 10.1016/j.ijom.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Lumbroso S, Paris F, Sultan C, , European Collaborative Study Activating Gsalpha mutations: analysis of 113 patients with signs of McCune-Albright syndrome–a European collaborative study. J Clin Endocrinol Metab. 2004;89:2107–13. doi: 10.1210/jc.2003-031225. [DOI] [PubMed] [Google Scholar]
- 19.Maki M, Athanasou N. Osteofibrous dysplasia and adamantinoma: correlation of proto-oncogene product and matrix protein expression. Hum Pathol. 2004;35:69–74. doi: 10.1016/j.humpath.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto A, Oda Y, Iwamoto Y, Tsuneyoshi M. A comparative study of fibrous dysplasia and osteofibrous dysplasia with regard to Gsalpha mutation at the Arg201 codon: polymerase chain reaction-restriction fragment length polymorphism analysis of paraffin-embedded tissues. J Mol Diagn. 2000;2:67–72. doi: 10.1016/s1525-1578(10)60618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto A, Oda Y, Iwamoto Y, Tsuneyoshi M. A comparative study of fibrous dysplasia and osteofibrous dysplasia with regard to expressions of c-fos and c-jun products and bone matrix proteins: a clinicopathologic review and immunohistochemical study of c-fos, c-jun, type I collagen, osteonectin, osteopontin, and osteocalcin. Hum Pathol. 1999;30:1418–26. doi: 10.1016/s0046-8177(99)90162-4. [DOI] [PubMed] [Google Scholar]
- 22.Campanacci M. Osteofibrous dysplasia of long bones a new clinical entity. Ital J Orthop Traumatol. 1976;2:221–37. [PubMed] [Google Scholar]
- 23.Faivre L, Nivelon-Chevallier A, Kottler ML, Robinet C, Khau Kien P, Lorcerie B, Munnich A, Maroteaux P, Cormier-Daire V, LeMerrer M. Mazabraud syndrome in two patients: clinical overlap with McCune-Albright syndrome. Am J Med Genet. 2001;99:132–6. doi: 10.1002/1096-8628(2000)9999:999<00::aid-ajmg1135>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Meng XM, Yu SF, Yu GY. Clinicopathologic study of 24 cases of cherubism. Int J Oral Maxillofac Surg. 2005;34:350–6. doi: 10.1016/j.ijom.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Lannon DA, Earley MJ. Cherubism and its charlatans. Br J Plast Surg. 2001;54:708–11. doi: 10.1054/bjps.2001.3701. [DOI] [PubMed] [Google Scholar]
- 26.Imai Y, Kanno K, Moriya T, Kayano S, Seino H, Matsubara Y, Yamada A. A missense mutation in the SH3BP2 gene on chromosome 4p16.3 found in a case of nonfamilial cherubism. Cleft Palate Craniofac J. 2003;40:632–8. doi: 10.1597/1545-1569_2003_040_0632_ammits_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 27.Tiziani V, Reichenberger E, Buzzo CL, Niazi S, Fukai N, Stiller M, Peters H, Salzano FM, Raposo do Amaral CM, Olsen BR. The gene for cherubism maps to chromosome 4p16. Am J Hum Genet. 1999;65:158–66. doi: 10.1086/302456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca LC, Freitas JB, Maciel PH, Cavalcanti MG. Temporal bone involvement in Cherubism: case report. Braz Dent J. 2004;15:75–8. doi: 10.1590/s0103-64402004000100014. [DOI] [PubMed] [Google Scholar]
- 29.Wang CN, Song YL, Peng B, Lu DH, Fan MW, Li J, Ye XQ, Fan HL, Bian Z. The aggressive form of cherubism: report of two cases in unrelated families. Br J Oral Maxillofac Surg. 2006;44:322–4. doi: 10.1016/j.bjoms.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Jafarov T, Ferimazova N, Reichenberger E. Noonan-like syndrome mutations in PTPN11 in patients diagnosed with cherubism. Clin Genet. 2005;68:190–1. doi: 10.1111/j.1399-0004.2005.00475.x. [DOI] [PubMed] [Google Scholar]
- 31.Betts NJ, Stewart JC, Fonseca RJ, Scott RF. Multiple central giant cell lesions with a Noonan-like phenotype. Oral Surg Oral Med Oral Pathol. 1993;76:601–7. doi: 10.1016/0030-4220(93)90069-g. [DOI] [PubMed] [Google Scholar]
- 32.Dunlap C, Neville B, Vickers RA, O’Neil D, Barker B. The Noonan syndrome/cherubism association. Oral Surg Oral Med Oral Pathol. 1989;67:698–705. doi: 10.1016/0030-4220(89)90012-1. [DOI] [PubMed] [Google Scholar]
- 33.Ramon Y, Berman W, Bubis JJ. Gingival fibromatosis combined with cherubism. Oral Surg Oral Med Oral Pathol. 1967;24:435–48. doi: 10.1016/0030-4220(67)90416-1. [DOI] [PubMed] [Google Scholar]
- 34.van Capelle CI, Hogeman PH, van der Sijs-Bos CJ, Heggelman BG, Idowu B, Slootweg PJ, Wittkampf AR, Flanagan AM. Neurofibromatosis presenting with a cherubism phenotype. Eur J Pediatr. 2006; Nov 21 [Epub ahead of print]. [DOI] [PubMed]
- 35.Martinez-Tello FJ, Manjon-Luengo P, Martin-Perez M, Montes-Moreno S. Cherubism associated with neurofibromatosis type 1, and multiple osteolytic lesions of both femurs: a previously undescribed association of findings. Skeletal Radiol. 2005;34:793–8. doi: 10.1007/s00256-005-0938-3. [DOI] [PubMed] [Google Scholar]
- 36.Daroszewska A, Ralston SH. Mechanisms of disease: genetics of Paget’s disease of bone and related disorders. Nat Clin Pract Rheumatol. 2006;2:270–7. doi: 10.1038/ncprheum0172. [DOI] [PubMed] [Google Scholar]
- 37.Daroszewska A, Ralston SH. Genetics of Paget’s disease of bone. Clin Sci (Lond). 2005;109:257–63. doi: 10.1042/CS20050053. [DOI] [PubMed] [Google Scholar]
- 38.Michou L, Collet C, Laplanche JL, Orcel P, Cornelis F. Genetics of Paget’s disease of bone. Joint Bone Spine. 2006;73:243–8. doi: 10.1016/j.jbspin.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Reddy SV. Etiologic factors in Paget’s disease of bone. Cell Mol Life Sci. 2006;63:391–8. doi: 10.1007/s00018-005-5473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yip KH, Feng H, Pavlos NJ, Zheng MH, Xu J. p62 ubiquitin binding-associated domain mediated the receptor activator of nuclear factor-kappaB ligand-induced osteoclast formation: a new insight into the pathogenesis of Paget’s disease of bone. Am J Pathol. 2006;169:503–14. doi: 10.2353/ajpath.2006.050960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whyte MP. Paget’s disease of bone and genetic disorders of RANKL/OPG/RANK/NF-kappaB signaling. Ann NY Acad Sci. 2006;1068:143–64. doi: 10.1196/annals.1346.016. [DOI] [PubMed] [Google Scholar]
- 42.Cavey JR, Ralston SH, Sheppard PW, Ciani B, Gallagher TR, Long JE, Searle MS, Layfield R. Loss of ubiquitin binding is a unifying mechanism by which mutations of SQSTM1 cause Paget’s disease of bone. Calcif Tissue Int. 2006;78:271–7. doi: 10.1007/s00223-005-1299-6. [DOI] [PubMed] [Google Scholar]
- 43.Janssens K, Vernejoul MC, Freitas F, Vanhoenacker F, Hul W. An intermediate form of juvenile Paget’s disease caused by a truncating TNFRSF11B mutation. Bone. 2005;36:542–8. doi: 10.1016/j.bone.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Chong B, Hegde M, Fawkner M, Simonet S, Cassinelli H, Coker M, Kanis J, Seidel J, Tau C, Tuysuz B, Yuksel B, Love D, , International Hyperphosphatasia Collaborative Group Idiopathic hyperphosphatasia and TNFRSF11B mutations: relationships between phenotype and genotype. J Bone Miner Res. 2003;18:2095–104. doi: 10.1359/jbmr.2003.18.12.2095. [DOI] [PubMed] [Google Scholar]
- 45.Whyte MP, Hughes AE. Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J Bone Miner Res. 2002;17:26–9. doi: 10.1359/jbmr.2002.17.1.26. [DOI] [PubMed] [Google Scholar]
- 46.Siris ES. Paget’s disease of bone. J Bone Miner Res. 1998;13:1061–5. doi: 10.1359/jbmr.1998.13.7.1061. [DOI] [PubMed] [Google Scholar]
- 47.Fraser WD. Paget’s disease of bone. Curr Opin Rheumatol. 1997;9:347–54. doi: 10.1097/00002281-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Stappen A, Degryse H, Hauwe L. Paget disease of the skull and temporal bone. JBR-BTR. 2005;88:156–7. [PubMed] [Google Scholar]
- 49.Ellis GL, Connole PW. Diffuse mandibular enlargement caused by osteitis deformans. Ear Nose Throat J. 1985;64:466–72. [PubMed] [Google Scholar]
- 50.Murphy JB, Segelman A, Doku C. Osteitis deformans. Report of a long-standing case with extensive oral involvement. Oral Surg Oral Med Oral Pathol. 1978;46:765–71. doi: 10.1016/0030-4220(78)90305-5. [DOI] [PubMed] [Google Scholar]
- 51.Rao VM, Karasick D. Hypercementosis—an important clue to Paget disease of the maxilla. Skeletal Radiol. 1982;9:126–8. doi: 10.1007/BF00360497. [DOI] [PubMed] [Google Scholar]
- 52.Helfrich MH. Osteoclast diseases and dental abnormalities. Arch Oral Biol. 2005;50:115–22. doi: 10.1016/j.archoralbio.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Fisher EW. Rhinological manifestations of Paget’s disease of bone (Osteitis deformans) J Craniomaxillofac Surg. 1990;18:169–72. doi: 10.1016/s1010-5182(05)80513-x. [DOI] [PubMed] [Google Scholar]
- 54.Russo A, Dell’Aquila A, Gargiulo M, Sica G. Paget disease of the jaws. Review of the subject. Clinical-therapeutic considerations. Minerva Stomatol. 1996;45:349–54. [PubMed] [Google Scholar]
- 55.McGowan DA. Clinical problems in Paget’s disease affecting the jaws. Br J Oral Surg. 1974;11:230–5. doi: 10.1016/0007-117x(74)90106-1. [DOI] [PubMed] [Google Scholar]
- 56.Morgan GA, Morgan PR. Oral and skull manifestations of Paget’s disease. J Can Dent Assoc. 1969;35:208–12. [PubMed] [Google Scholar]
- 57.Gennari L, Stefano M, Merlotti D, Giordano N, Martini G, Tamone C, Zatteri R, Lucchi R, Baldi C, Vattimo A, Capoccia S, Burroni L, Geraci S, Paola V, Calabro A, Avanzati A, Isaia G, Nuti R. Prevalence of Paget’s disease of bone in Italy. J Bone Miner Res. 2005;20:1845–50. doi: 10.1359/JBMR.050518. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto J, Ohno I, Nakatsuka K, Yoshimura N, Takata S, Zamma M, Yabe H, Abe S, Terada M, Yoh K, Fukunaga M, Cooper C, Morii H, Yoshikawa H, Japanese Committee on Clinical Guidelines of Diagnosis and Treatment of Paget’s Disease of Bone of the Japan Osteoporosis Society Prevalence and clinical features of Paget’s disease of bone in Japan. J Bone Miner Metab. 2006;24:186–90. doi: 10.1007/s00774-005-0670-z. [DOI] [PubMed] [Google Scholar]
- 59.Rojas-Villarraga A, Patarroyo PA, Contreras AS, Restrepo JF, Iglesias-Gamarra A. Paget disease of bone in Colombia and Latin America. J Clin Rheumatol. 2006;12:57–60. doi: 10.1097/01.rhu.0000208491.78501.71. [DOI] [PubMed] [Google Scholar]
- 60.Hoyland JA, Dixon JA, Berry JL, Davies M, Selby PL, Mee AP. A comparison of in situ hybridisation, reverse transcriptase-polymerase chain reaction (RT-PCR) and in situ-RT-PCR for the detection of canine distemper virus RNA in Paget’s disease. J Virol Methods. 2003;109:253–9. doi: 10.1016/s0166-0934(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 61.Kurihara N, Reddy SV, Menaa C, Anderson D, Roodman GD. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest. 2000;105:607–14. doi: 10.1172/JCI8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birch MA, Taylor W, Fraser WD, Ralston SH, Hart CA, Gallagher JA. Absence of paramyxovirus RNA in cultures of pagetic bone cells and in pagetic bone. J Bone Miner Res. 1994;9:11–6. doi: 10.1002/jbmr.5650090103. [DOI] [PubMed] [Google Scholar]
- 63.Gordon MT, Mee AP, Anderson DC, Sharpe PT. Canine distemper virus transcripts sequenced from pagetic bone. Bone Miner. 1992;19:159–74. doi: 10.1016/0169-6009(92)90923-2. [DOI] [PubMed] [Google Scholar]
- 64.O’Driscoll JB, Buckler HM, Jeacock J, Anderson DC. Dogs, distemper and osteitis deformans: a further epidemiological study. Bone Miner. 1990;11:209–16. doi: 10.1016/0169-6009(90)90060-s. [DOI] [PubMed] [Google Scholar]
- 65.Basle MF, Fournier JG, Rozenblatt S, Rebel A, Bouteille M. Measles virus RNA detected in Paget’s disease bone tissue by in situ hybridization. J Gen Virol. 1986;67:907–13. doi: 10.1099/0022-1317-67-5-907. [DOI] [PubMed] [Google Scholar]
- 66.Basle MF, Russell WC, Goswami KK, Rebel A, Giraudon P, Wild F, Filmon R. Paramyxovirus antigens in osteoclasts from Paget’s bone tissue detected by monoclonal antibodies. J Gen Virol. 1985;66:2103–10. doi: 10.1099/0022-1317-66-10-2103. [DOI] [PubMed] [Google Scholar]
- 67.Harvey L, Gray T, Beneton MN, Douglas DL, Kanis JA, Russell RG. Ultrastructural features of the osteoclasts from Paget’s disease of bone in relation to a viral aetiology. J Clin Pathol. 1982;35:771–9. doi: 10.1136/jcp.35.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gherardi G, Lo Cascio V, Bonucci E. Fine structure of nuclei and cytoplasm of osteoclasts in Paget’s disease of bone. Histopathology. 1980;4:63–74. doi: 10.1111/j.1365-2559.1980.tb02898.x. [DOI] [PubMed] [Google Scholar]
- 69.Miller AS, Cuttino CL, Elzay RP, Levy WM, Harwick RD. Giant cell tumor of the jaws associated with Paget disease of bone. Report of two cases and review of the literature. Arch Otolaryngol. 1974;100:233–6. doi: 10.1001/archotol.1974.00780040241018. [DOI] [PubMed] [Google Scholar]
- 70.Bhambhani M, Lamberty BG, Clements MR, Skingle SJ, Crisp AJ. Giant cell tumours in mandible and spine: a rare complication of Paget’s disease of bone. Ann Rheum Dis. 1992;51:1335–7. doi: 10.1136/ard.51.12.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mankin HJ, Hornicek FJ. Paget’s sarcoma: a historical and outcome review. Clin Orthop Relat Res. 2005;438:97–102. doi: 10.1097/01.blo.0000180053.99840.27. [DOI] [PubMed] [Google Scholar]
- 72.Cheng YS, Wright JM, Walstad WR, Finn MD. Osteosarcoma arising in Paget’s disease of the mandible. Oral Oncol. 2002;38:785–92. doi: 10.1016/s1368-8375(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg S, Slamovits TL, Dorfman HD, Rosenbaum PS. Sarcomatous transformation of the orbit in a patient with Paget’s disease. Ophthalmology. 2000;107:1464–7. doi: 10.1016/s0161-6420(00)00180-9. [DOI] [PubMed] [Google Scholar]
- 74.Mooy CM, Naus NC, Klein A, Bosch WA, Paridaens DA. Orbital chondrosarcoma developing in a patient with Paget disease. Am J Ophthalmol. 1999;127:619–21. doi: 10.1016/s0002-9394(98)00405-x. [DOI] [PubMed] [Google Scholar]
- 75.Fransen P, Mestdagh C, Dardenne G. Pagetic sarcoma of the calvarium: report of two cases. Acta Neurol Belg. 1998;;98:352–5. [PubMed] [Google Scholar]
- 76.Fransen P, Mestdagh C, Dardenne G. Pagetic sarcoma of the calvarium: report of two cases. Acta Neurol Belg. 1998;98:352–5. [PubMed] [Google Scholar]
- 77.Jattiot F, Goupille P, Azais I, Roulot B, Alcalay M, Jeannou J, Bontoux D, Valat JP. Fourteen cases of sarcomatous degeneration in Paget’s disease. J Rheumatol. 1999;26:150–5. [PubMed] [Google Scholar]
- 78.Mancebo-Aragoneses L, Lacambra-Calvet C, Jorge-Blanco A, Coarasa-Cerdan A, Guadano-Salvadores V. Paget’s disease of the skull with osteosarcoma and neurological symptoms associated. Eur Radiol. 1998;8:1145–7. doi: 10.1007/s003300050524. [DOI] [PubMed] [Google Scholar]
- 79.Oda D, Bavisotto LM, Schmidt RA, McNutt M, Bruckner JD, Conrad EU, 3rd, Weymuller EA., Jr Head and neck osteosarcoma at the University of Washington. Head Neck. 1997;19:513–23. doi: 10.1002/(sici)1097-0347(199709)19:6<513::aid-hed9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 80.Salvati M, Cervoni L, Raguso M, Raco A. Post-Paget osteosarcomas of the skull. Remarks on five cases. Tumori. 1993;79:363–6. doi: 10.1177/030089169307900517. [DOI] [PubMed] [Google Scholar]
- 81.Pfleiderer AG, Molyneux AJ, Tee MK. Metastatic spread of osteosarcoma to the temporal bone in a patient with Paget’s disease: a case report. J Otolaryngol. 1992;21:112–4. [PubMed] [Google Scholar]
- 82.Moore TE, King AR, Kathol MH, el-Khoury GY, Palmer R, Downey PR. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199–203. doi: 10.2214/ajr.156.6.2028867. [DOI] [PubMed] [Google Scholar]
- 83.Hosking D. Pharmacological therapy of Paget’s and other metabolic bone diseases. Bone. 2006;38(2 Suppl 2):S3–7. doi: 10.1016/j.bone.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 84.Peris P, Alvarez L, Vidal S, Kasper D, Leeming DJ, Monegal A, Angeles Martinez M, Pons F, Guanabens N. Biochemical response to bisphosphonate therapy in pagetic patients with skull involvement. Calcif Tissue Int. 2006;79:22–6. doi: 10.1007/s00223-005-0247-9. [DOI] [PubMed] [Google Scholar]
- 85.Hofbauer LC, Schoppet M. Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med. 2002;347:1622–3. doi: 10.1056/NEJM200211143472015. [DOI] [PubMed] [Google Scholar]
- 86.Antoniades K, Karakasis D, Kapetanos G, Lasaridis N, Tzarou V. Chronic idiopathic hyperphosphatasemia. Case report. Oral Surg Oral Med Oral Pathol. 1993;76:200–4. doi: 10.1016/0030-4220(93)90205-i. [DOI] [PubMed] [Google Scholar]
- 87.Osterberg PH, Wallace RG, Adams DA, Crone RS, Dickson GR, Kanis JA, Mollan RA, Nevin NC, Sloan J, Toner PG. Familial expansile osteolysis. A new dysplasia. J Bone Joint Surg Br. 1988;70:255–60. doi: 10.1302/0301-620X.70B2.3346299. [DOI] [PubMed] [Google Scholar]
- 88.Wallace RG, Barr RJ, Osterberg PH, Mollan RA. Familial expansile osteolysis. Clin Orthop Relat Res. 1989;248:265–77. [PubMed] [Google Scholar]
- 89.Mitchell CA, Kennedy JG, Owens PD. Dental histology in familial expansile osteolysis. J Oral Pathol Med. 1990;19:65–70. doi: 10.1111/j.1600-0714.1990.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell CA, Kennedy JG, Wallace RG. Dental abnormalities associated with familial expansile osteolysis: a clinical and radiographic study. Oral Surg Oral Med Oral Pathol. 1990;70:301–7. doi: 10.1016/0030-4220(90)90145-i. [DOI] [PubMed] [Google Scholar]
- 91.Daneshi A, Shafeghati Y, Karimi-Nejad MH, Khosravi A, Farhang F. Hereditary bilateral conductive hearing loss caused by total loss of ossicles: a report of familial expansile osteolysis. Otol Neurotol. 2005;26:237–40. doi: 10.1097/00129492-200503000-00018. [DOI] [PubMed] [Google Scholar]
- 92.Whyte MP, Mills BG, Reinus WR, Podgornik MN, Roodman GD, Gannon FH, Eddy MC, McAlister WH. Expansile skeletal hyperphosphatasia: a new familial metabolic bone disease. J Bone Miner Res. 2000;15:2330–44. doi: 10.1359/jbmr.2000.15.12.2330. [DOI] [PubMed] [Google Scholar]
- 93.Hughes AE, Shearman AM, Weber JL, Barr RJ, Wallace RG, Osterberg PH, Nevin NC, Mollan RA. Genetic linkage of familial expansile osteolysis to chromosome 18q. Hum Mol Genet. 1994;3:359–61. doi: 10.1093/hmg/3.2.359. [DOI] [PubMed] [Google Scholar]
- 94.Danforth RA, Melrose RJ, Abrams AM, Handlers JP. Segmental odontomaxillary dysplasia. Report of eight cases and comparison with hemimaxillofacial dysplasia. Oral Surg Oral Med Oral Pathol. 1990;70:81–5. doi: 10.1016/0030-4220(90)90183-s. [DOI] [PubMed] [Google Scholar]
- 95.Packota GV, Pharoah MJ, Petrikowski CG. Radiographic features of segmental odontomaxillary dysplasia: a study of 12 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:577–84. doi: 10.1016/s1079-2104(96)80206-x. [DOI] [PubMed] [Google Scholar]
- 96.Becktor KB, Reibel J, Vedel B, Kjaer I. Segmental odontomaxillary dysplasia: clinical, radiological and histological aspects of four cases. Oral Dis. 2002;8:106–10. doi: 10.1034/j.1601-0825.2002.1c773.x. [DOI] [PubMed] [Google Scholar]
- 97.Velez I, Vedrenne D, Cralle P, Yap S. Segmental odontomaxillary dysplasia. Report of two cases and review of the literature. Todays FDA. 2002;14:20–1. [PubMed] [Google Scholar]
- 98.Armstrong C, Napier SS, Boyd RC, Gregg TA. Histopathology of the teeth in segmental odontomaxillary dysplasia: new findings. J Oral Pathol Med. 2004;33:246–8. doi: 10.1111/j.0904-2512.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- 99.Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1985;43:249–62. doi: 10.1016/0278-2391(85)90283-6. [DOI] [PubMed] [Google Scholar]
- 100.Waldron CA. Fibro-osseous lesions of jaws. J Oral Maxillofac Sur. 1993;51:828–35. doi: 10.1016/s0278-2391(10)80097-7. [DOI] [PubMed] [Google Scholar]
- 101.Kramer IRH, Pindborg JJ, Shear M. Neoplasm and other lesions related to bone. In: WHO, editors. Histologic typing of odontogenic tumours. Berlin: Springer-Verlag; 1992.
- 102.Summerlin DJ, Tomich CE. Focal cemento-osseous dysplasia: A clinicopathologic study of 221 cases. Oral Surg Oral Med Oral Pathol. 1994;78:611–20. doi: 10.1016/0030-4220(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 103.Waldron CA. Chapter 14: Bone Pathology. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral & maxillofacial pathology. Philadelphia: W.B. Saunders Company; 1995. [Google Scholar]
- 104.Robinson HBG. Osseous dysplasia: reaction of bone to injury. J Oral Surg. 1956;14:3. [PubMed] [Google Scholar]
- 105.Su L, Dwight Weathers D, Charles Waldron C. Distinguishing features of cemento-osseous dysplasias and cemento-ossifying fibromas I. A pathologic spectrum of 316 cases. Oral Surg., Oral Med., Oral Pathol. 1997;84:301–9. doi: 10.1016/s1079-2104(97)90348-6. [DOI] [PubMed] [Google Scholar]
- 106.Su L, Weathers D, Waldron C. Distinguishing features of cemento-osseous dysplasias and cemento-ossifying fibromas II. A clinical and radiographic spectrum of 316 cases. Oral Surg., Oral Med., Oral Pathol. 1997;84:540–9. doi: 10.1016/s1079-2104(97)90271-7. [DOI] [PubMed] [Google Scholar]
- 107.Mohammadi-Araghi H, Haery C. Fibro-osseous lesions of craniofacial bones: the role of imaging. Radiol Clin North Am. 1993;31:121–34. [PubMed] [Google Scholar]
- 108.Galgano C, Samson J, Kuffer R, Lombardi T. Focal cemento-osseous dysplasia involving a mandibular lateral incisor. Int Endod J. 2003;36(12):907–11. doi: 10.1111/j.1365-2591.2003.00736.x. [DOI] [PubMed] [Google Scholar]
- 109.Kawai T, Hiranuma H, Kishino M, Jikko A, Sakuda M. Cemento-osseous dysplasia of the jaws in 54 Japanese patients: a radiographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(1):107–14. doi: 10.1016/s1079-2104(99)70303-3. [DOI] [PubMed] [Google Scholar]
- 110.Eversole LR, Leider AS, Nelson K. Ossifying fibroma: a clinicopathologic study of sixty-four cases. Oral Surg Oral Med Oral Pathol. 1985;60:505–11. doi: 10.1016/0030-4220(85)90239-7. [DOI] [PubMed] [Google Scholar]
- 111.Melrose RJ, Abrams AM, Mills BG. Florid osseous dysplasia. A clinical-pathologic study of thirty-four cases. Oral Surg Oral Med Oral Pathol. 1976;41:62–82. doi: 10.1016/0030-4220(76)90254-1. [DOI] [PubMed] [Google Scholar]
- 112.Neville BW, Albenesius RJ. The prevalence of benign fibro-osseous lesions of periodontal ligament origin in black women: a radiographic survey. Oral Surg Oral Med Oral Pathol. 1986;62:340–4. doi: 10.1016/0030-4220(86)90018-6. [DOI] [PubMed] [Google Scholar]
- 113.Miyauchi M, Ogawa I, Takata T, Ito H, Nikai H, Ijuhin N, Tanimoto K. Florid cemento-osseous dysplasia with concomitant simple bone cysts: a case in a Japanese woman. J Oral Pathol Med. 1995;24(6):285–7. doi: 10.1111/j.1600-0714.1995.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 114.Ong ST, Siar CH. Florid cemento-osseous dysplasia in a young Chinese man. Case report. Aust Dent J. 1997;42(6):404–8. doi: 10.1111/j.1834-7819.1997.tb06086.x. [DOI] [PubMed] [Google Scholar]
- 115.Ariji Y, Ariji E, Higuchi Y, Kubo S, Nakayama E, Kanda S. Florid cemento-osseous dysplasia. Radiographic study with special emphasis on computed tomography. Oral Surg Oral Med Oral Pathol. 1994;78(3):391–6. doi: 10.1016/0030-4220(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 116.Singer SR, Mupparapu M, Rinaggio J. Florid cemento-osseous dysplasia and chronic diffuse osteomyelitis. Report of a simultaneous presentation and review of the literature. J Am Dent Assoc. 2005;136:927–31. doi: 10.14219/jada.archive.2005.0294. [DOI] [PubMed] [Google Scholar]
- 117.Jerjes W, Banu B, Swinson B, Hopper C. Florid cemento-osseous dysplasia in a young Indian woman. A case report. Br Dent J. 2005;198:477–8. doi: 10.1038/sj.bdj.4812251. [DOI] [PubMed] [Google Scholar]
- 118.MacDonald-Jankowski DS. Florid cemento-osseous dysplasia: a systematic review. Dentomaxillofac Radiol. 2003;32:141–9. doi: 10.1259/dmfr/32988764. [DOI] [PubMed] [Google Scholar]
- 119.Higuchi Y, Nakamura N, Tashiro H. Clinicopathologic study of cemento-osseous dysplasia producing cysts of the mandible. Report of four cases. Oral Surg Oral Med Oral Pathol. 1988;65:339–42. doi: 10.1016/0030-4220(88)90119-3. [DOI] [PubMed] [Google Scholar]
- 120.Mahomed F, Altini M, Meer S, Coleman H. Cemento-osseous dysplasia with associated simple bone cysts. J Oral Maxillofac Surg. 2005;63:1549–54. doi: 10.1016/j.joms.2005.05.322. [DOI] [PubMed] [Google Scholar]
- 121.Wallace JA, Tyler CJ, Donne DD, Schmutz J. Should we or shouldn’t we treat condensing osteitis with root canal therapy? Ohio Dent J. 1992; Spring-Summer;66:65–8. Review. [PubMed]
- 122.Buch B, Matthee MJ. Radiological diagnosis V. Focal sclerosing osteomyelitis. J Dent Assoc S Afr. 1984;39:641. [PubMed] [Google Scholar]
- 123.Eversole LR, Stone CE, Strub D. Focal sclerosing osteomyelitis/focal periapical osteopetrosis: radiographic patterns. Oral Surg Oral Med Oral Pathol. 1984;58:456–60. doi: 10.1016/0030-4220(84)90344-x. [DOI] [PubMed] [Google Scholar]
- 124.al-Sebaei MO, Kahn MA, Papageorge MB. A clinico-pathologic correlation. Condensing osteitis (focal sclerosing osteomyelitis) J Mass Dent Soc. 2003;52:52–4. [PubMed] [Google Scholar]
- 125.Douglass GD, Trowbridge HO. Chronic focal sclerosing osteomyelitis associated with a cracked tooth. Report of a case. Oral Surg Oral Med Oral Pathol. 1993;76:351–5. doi: 10.1016/0030-4220(93)90267-8. [DOI] [PubMed] [Google Scholar]
- 126.Ludlow JB, Brooks SL. Idiopathic focal sclerosing osteomyelitis mimicking retained root tip. Oral Surg Oral Med Oral Pathol. 1990;70:241–2. doi: 10.1016/0030-4220(90)90129-g. [DOI] [PubMed] [Google Scholar]
- 127.Kessler HP. Oral and maxillofacial pathology case of the month. Condensing osteitis. Tex Dent J. 2003;120:178–188–9. [PubMed] [Google Scholar]
- 128.Williams TP, Brooks SL. longitudinal study of idiopathic osteosclerosis and condensing osteitis. Dentomaxillofac Radiol. 1998;27:275–8. doi: 10.1038/sj/dmfr/4600362. [DOI] [PubMed] [Google Scholar]
- 129.Eliasson S, Halvarsson C, Ljungheimer C. Periapical condensing osteitis and endodontic treatment. Oral Surg Oral Med Oral Pathol. 1984;57:195–9. doi: 10.1016/0030-4220(84)90211-1. [DOI] [PubMed] [Google Scholar]
- 130.Jacobsson S, Hollender L, Lindberg S, Larsson A. Chronic sclerosing osteomyelitis of the mandible. Scintigraphic and radiographic findings. Oral Surg Oral Med Oral Pathol. 1978;45:167–74. doi: 10.1016/0030-4220(78)90080-4. [DOI] [PubMed] [Google Scholar]
- 131.Jacobsson S. Diffuse sclerosing ostemyelitis of the mandible. Acta Otolaryngol Suppl. 1979;360:61–3. doi: 10.3109/00016487809123474. [DOI] [PubMed] [Google Scholar]
- 132.Johannsen A. Chronic sclerosing osteomyelitis of the mandible. Radiographic differential diagnosis from fibrous dysplasia. Acta Radiol Diagn (Stockh) 1977;18:360–8. doi: 10.1177/028418517701800313. [DOI] [PubMed] [Google Scholar]
- 133.Schneider LC, Mesa ML. Differences between florid osseous dysplasia and chronic diffuse sclerosing osteomyelitis. Oral Surg Oral Med Oral Pathol. 1990;70:308–12. doi: 10.1016/0030-4220(90)90146-j. [DOI] [PubMed] [Google Scholar]
- 134.Rabe WC, Angelillo JC, Leipert DW. Chronic sclerosing osteomyelitis: treatment considerations in an atypical case. Oral Surg Oral Med Oral Pathol. 1980;49:117–21. doi: 10.1016/0030-4220(80)90302-3. [DOI] [PubMed] [Google Scholar]
- 135.Groot RH, Merkesteyn JP, Bras J. Diffuse sclerosing osteomyelitis and florid osseous dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:333–42. doi: 10.1016/s1079-2104(96)80334-9. [DOI] [PubMed] [Google Scholar]
- 136.Orpe EC, Lee L, Pharoah MJ. A radiological analysis of chronic sclerosing osteomyelitis of the mandible. Dentomaxillofac Radiol. 1996;25:125–9. doi: 10.1259/dmfr.25.3.9084260. [DOI] [PubMed] [Google Scholar]
- 137.Suei Y, Tanimoto K, Miyauchi M, Ishikawa T. Partial resection of the mandible for the treatment of diffuse sclerosing osteomyelitis: report of four cases. J Oral Maxillofac Surg. 1997;55:410–4. doi: 10.1016/s0278-2391(97)90138-5. [DOI] [PubMed] [Google Scholar]
- 138.Eversole LR, Leider AS, Corwin JO, Karian BK. Proliferative periostitis of Garre: its differentiation from other neoperiostoses. J Oral Surg. 1979;37:725–31. [PubMed] [Google Scholar]
- 139.Kawai T, Murakami S, Sakuda M, Fuchihata H. Radiographic investigation of mandibular periostitis ossificans in 55 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:704–12. doi: 10.1016/s1079-2104(96)80447-1. [DOI] [PubMed] [Google Scholar]
- 140.Kawai T, Hiranuma H, Kishino M, Murakami S, Sakuda M, Fuchihata H. Gross periostitis ossificans in mandibular osteomyelitis. Review of the English literature and radiographic variation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:376–81. doi: 10.1016/s1079-2104(98)90188-3. [DOI] [PubMed] [Google Scholar]
- 141.Nortje CJ, Wood RE, Grotepass F. Periostitis ossificans versus Garre’s osteomyelitis. Part II: Radiologic analysis of 93 cases in the jaws. Oral Surg Oral Med Oral Pathol. 1988;66:249–60. doi: 10.1016/0030-4220(88)90102-8. [DOI] [PubMed] [Google Scholar]
- 142.Larheim TA, Aspestrand F, Trebo S. Periostitis ossificans of the mandible. The value of computed tomography. Dentomaxillofac Radiol. 1993;22:93–6. doi: 10.1259/dmfr.22.2.8375561. [DOI] [PubMed] [Google Scholar]
- 143.Jacobson HL, Baumgartner JC, Marshall JG, Beeler WJ. Proliferative periostitis of Garre: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:111–4. doi: 10.1067/moe.2002.124861. [DOI] [PubMed] [Google Scholar]
- 144.Belli E, Matteini C, Andreano T. Sclerosing osteomyelitis of Garre periostitis ossificans. J Craniofac Surg. 2002;13:765–8. doi: 10.1097/00001665-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 145.Ganibegovic M. Dental radiographic changes in chronic renal disease. Med Arh. 2000;54(2):115–8. [PubMed] [Google Scholar]
- 146.Warnakulasuriya S, Markwell BD, Williams DM. Familial hyperparathyroidism associated with cementifying fibromas of the jaws in two siblings. Oral Surg Oral Med Oral Pathol. 1985;59(3):269–74. doi: 10.1016/0030-4220(85)90165-3. [DOI] [PubMed] [Google Scholar]
- 147.Kelly WH, Mirahmadi MK, Simon JH, Gorman JT. Radiographic changes of the jawbones in end stage renal disease. Oral Surg Oral Med Oral Pathol. 1980;50(4):372–81. doi: 10.1016/0030-4220(80)90423-5. [DOI] [PubMed] [Google Scholar]
- 148.Maxwell DR, Spolnik KJ, Cockerill EM, Patterson SS, Kleit SA. Roentgenographic manifestations of maxillomandibular renal osteodystrophy. Nephron. 1985;41(3):223–9. doi: 10.1159/000183587. [DOI] [PubMed] [Google Scholar]
- 149.Syrjanen S, Lampainen E. Mandibular changes in panoramic radiographs of patients with end stage renal disease. Dentomaxillofac Radiol. 1983;12(1):51–6. doi: 10.1259/dmfr.1983.0010. [DOI] [PubMed] [Google Scholar]
- 150.Bras J, Ooij CP, Abraham-Inpijn L, Wilmink JM, Kusen GJ. Radiographic interpretation of the mandibular angular cortex: a diagnostic tool in metabolic bone loss. Part II. Renal osteodystrophy. Oral Surg Oral Med Oral Pathol. 1982;53:647–50. doi: 10.1016/0030-4220(82)90356-5. [DOI] [PubMed] [Google Scholar]
- 151.Lautenbach E, Dockhorn R. Osteodystrophia fibrosa generalisata (Recklinghausen’s disease; hyperparathyroidism) and its effects on the jaws. Oral Surg Oral Med Oral Pathol. 1968;25(3):479–84. doi: 10.1016/0030-4220(68)90024-8. [DOI] [PubMed] [Google Scholar]
- 152.Michiwaki Y, Michi K, Yamaguchi A. Marked enlargement of the jaws in secondary hyperparathyroidism–a case report. Int J Oral Maxillofac Surg. 1996;25(1):54–6. doi: 10.1016/s0901-5027(96)80012-9. [DOI] [PubMed] [Google Scholar]
- 153.Phelps KR, Bansal M, Twersky J. Jaw enlargement complicating secondary hyperparathyroidism in three hemodialysis patients. Clin Nephrol. 1994;41:173–9. [PubMed] [Google Scholar]
- 154.Ghadour K, Yates C. Primary hyperparathyroidism presenting as a massive maxillary swelling. Br Dent J. 1990;168:112–5. doi: 10.1038/sj.bdj.4807097. [DOI] [PubMed] [Google Scholar]
- 155.Sagliker Y, Balal M, Sagliker Ozkaynak P, Paydas S, Sagliker C, Sabit Sagliker H, Kiralp N, Mumin Adam S, Tuncer I, Gonlusen G, Esenturk M, Gocmez E, Taskapan H, Yeksan M, Kobaner E, Ozkaya O, Yuksekgonul M, Emir I, Cengiz N, Onder Isik I, Bilginer O, Guler T, Yakar H, Sarsmaz N, Dilaver S, Akoglu B, Basgumus M, Chirik E. Sagliker syndrome: uglifying human face appearance in late and severe secondary hyperparathyroidism in chronic renal failure. Semin Nephrol. 2004;24:449–55. doi: 10.1016/j.semnephrol.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 156.Aggunlu L, Akpek S, Coskun B. Leontiasis ossea in a patient with hyperparathyroidism secondary to chronic renal failure. Pediatr Radiol. 2004;34:630–2. doi: 10.1007/s00247-004-1188-6. [DOI] [PubMed] [Google Scholar]
- 157.Lee VS, Martinez S. Leontiasis ossea in secondary hyperparathyroidism. J Bone Miner Res. 1997;12:1952–3. doi: 10.1359/jbmr.1997.12.11.1952. [DOI] [PubMed] [Google Scholar]
- 158.Haven CJ, Wong FK, Dam EW, Juijt R, Asperen C, Jansen J, Rosenberg C, Wit M, Roijers J, Hoppener J, Lips CJ, Larsson C, Teh BT, Morreau H. A genotypic and histopathological study of a large Dutch kindred with hyperparathyroidism-jaw tumor syndrome. J Clin Endocrinol Metab. 2000;85:1449–54. doi: 10.1210/jcem.85.4.6518. [DOI] [PubMed] [Google Scholar]
- 159.Fujikawa M, Okamura K, Sato K, Mizokami T, Tamaki K, Yanagida T, Fujishima M. Familial isolated hyperparathyroidism due to multiple adenomas associated with ossifying jaw fibroma and multiple uterine adenomyomatous polyps. Eur J Endocrinol. 1998;138:557–61. doi: 10.1530/eje.0.1380557. [DOI] [PubMed] [Google Scholar]
- 160.Aldred MJ, Talacko AA, Savarirayan R. Jaw lesions and hyperparathyroidism. J Oral Maxillofac Surg. 2004;62:519. doi: 10.1016/j.joms.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 161.Mintz S, Velez I. Central ossifying fibroma: an analysis of 20 cases and review of the literature. Quintessence Int. 2007;38:221–7. [PubMed] [Google Scholar]
- 162.Goh EK, Cho KS, Lee IW, Chon KM. A case of isolated ossifying fibroma of the mastoid cavity of the temporal bones. Am J Otolaryngol. 2006;27:358–61. doi: 10.1016/j.amjoto.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 163.Canger EM, Celenk P, Kayipmaz S, Alkant A, Gunhan O. Familial ossifying fibromas: report of two cases. J Oral Sci. 2004;46:61–4. doi: 10.2334/josnusd.46.61. [DOI] [PubMed] [Google Scholar]
- 164.Toyosawa S, Yuki M, Kishino M, Ogawa Y, Ueda T, Murakami S, Konishi E, Iida S, Kogo M, Komori T, Tomita Y. Ossifying fibroma vs fibrous dysplasia of the jaw: molecular and immunological characterization. Mod Pathol. 2007;20:389–96. doi: 10.1038/modpathol.3800753. [DOI] [PubMed] [Google Scholar]
- 165.El-Mofty S. Psammomatoid and trabecular juvenile ossifying fibroma of the craniofacial skeleton: two distinct clinicopathologic entities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:296–304. doi: 10.1067/moe.2002.121545. [DOI] [PubMed] [Google Scholar]
- 166.Johnson LC, Yousefi M, Vinh TN, Heffner DK, Hyams VJ, Hartman KS. Juvenile active ossifying fibroma. Its nature, dynamics and origin. Acta Otolaryngol Suppl. 1991;488:1–40. [PubMed] [Google Scholar]
- 167.Kramer IR, Pindborg JJ, Shear M. Histopathologic typing of odontogenic tumors. 2. Berlin: Springer Verlag; 1992. [Google Scholar]
- 168.Slootweg PJ, El-Mofty Sk. Ossifying Fibroma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology & genetics head and neck tumours. Lyon, France: IARC Press; 2005. pp. 319–20. [Google Scholar]
- 169.Slootweg PJ, Panders AK, Koopmans R, Nikkels PG. Juvenile ossifying fibroma. An analysis of 33 cases with emphasis on histopathological aspects. J Oral Pathol Med. 1994;23:385–9. doi: 10.1111/j.1600-0714.1994.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 170.Khoury NJ, Naffaa LN, Shabb NS, Haddad MC. Juvenile ossifying fibroma: CT and MR findings. Eur Radiol. 2002;12(Suppl 3):S109–13. doi: 10.1007/s00330-002-1412-4. [DOI] [PubMed] [Google Scholar]
- 171.Slootweg PJ, Muller H. Juvenile ossifying fibroma. Report of four cases. J Craniomaxillofac Surg. 1990;18:125–9. doi: 10.1016/s1010-5182(05)80329-4. [DOI] [PubMed] [Google Scholar]
- 172.Wenig BM, Vinh TN, Smirniotopoulos JG, Fowler CB, Houston GD, Heffner DK. Aggressive psammomatoid ossifying fibromas of the sinonasal region: a clinicopathologic study of a distinct group of fibro-osseous lesions. Cancer. 1995;76:1155–65. doi: 10.1002/1097-0142(19951001)76:7<1155::aid-cncr2820760710>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 173.Makek M. Clinical pathology of fibro-osteo-cemental lesions of the craniofacial and jaw bones. Basel: Karger; 1983.
- 174.Gogl H. Das Psammo-osteoid-fibroma der Nase und ihrer Nebenh<um>ohlen. Monatsschr F Ohrenheilk Lar Rhin. 1949;83:1–10. [PubMed] [Google Scholar]
- 175.Margo CE, Ragsdale BD, Perman KI, Zimmerman LE, Sweet DE. Psammomatoid (juvenile) ossifying fibroma of the orbit. Ophthalmology. 1985;92:150–9. doi: 10.1016/s0161-6420(85)34070-8. [DOI] [PubMed] [Google Scholar]
- 176.Hoffman S, Jacoby J, Kroll SO. Intraosseus and parosteal tumors of the jaws. In: Atlas of tumor pathology. 2nd ed. Washington, DC: Armed Forces Institute of Pathology; 1987.
- 177.Bertrand B, Eloy P, Cornelis JP, Gosseye S, Clotuche J, Gilliard C. Juvenile aggressive cemento-ossifying fibroma: case report and review of the literature. Laryngoscope. 1993;103:1385–90. doi: 10.1288/00005537-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 178.Margo CE, Weiss A, Habal MB. Psammomatoid ossifying fibroma. Arch Ophthalmol. 1986;104:1347–51. doi: 10.1001/archopht.1986.01050210101034. [DOI] [PubMed] [Google Scholar]
- 179.Slootweg PJ, Panders AK, Nikkels PG. Psammomatoid ossifying fibroma of the paranasal sinuses. An extragnathic variant of cemento-ossifying fibroma. Report of three cases. J Craniomaxillofac Surg. 1993;21:294–7. doi: 10.1016/s1010-5182(05)80350-6. [DOI] [PubMed] [Google Scholar]
- 180.Shields JA, Nelson LB, Brown JF, Dolinskas C. Clinical, computed tomographic, and histopathologic characteristics of juvenile ossifying fibroma with orbital involvement. Am J Ophthalmol. 1983;96:650–3. doi: 10.1016/s0002-9394(14)73424-5. [DOI] [PubMed] [Google Scholar]
- 181.Han MH, Chang KH, Lee CH, Seo JW, Han MC, Kim CW. Sinonasal psammomatoid ossifying fibromas: CT and MR manifestations. AJNR Am J Neuroradiol. 1991;12:25–30. [PMC free article] [PubMed] [Google Scholar]
- 182.Storkel S, Wagner W, Makek MS. Psammous desmo-osteoblastoma. Ultrastructural and immunohistochemical evidence for an osteogenic histogenesis. Virchows Arch A Pathol Anat Histopathol. 1987;411:561–8. doi: 10.1007/BF00713287. [DOI] [PubMed] [Google Scholar]
- 183.Dal Cin P, Sciot R, Fossion E, Damme B, Berghe H. Chromosome abnormalities in cementifying fibroma. Cancer Genet Cytogenet. 1993;71:170–2. doi: 10.1016/0165-4608(93)90025-h. [DOI] [PubMed] [Google Scholar]
- 184.Sawyer JR, Tryka AF, Bell JM, Boop FA. Nonrandom chromosome breakpoints at Xq26 and 2q33 characterize cemento-ossifying fibromas of the orbit. Cancer. 1995;76:1853–9. doi: 10.1002/1097-0142(19951115)76:10<1853::aid-cncr2820761026>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 185.Young SK, Markowitz NR, Sullivan S, Seale TW, Hirschi R. Familial gigantiform cementoma: classification and presentation of a large pedigree. Oral Surg Oral Med Oral Pathol. 1989;68:740–7. doi: 10.1016/0030-4220(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 186.Abdelsayed RA, Eversole LR, Singh BS, Scarbrough FE. Gigantiform cementoma: clinicopathologic presentation of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:438–44. doi: 10.1067/moe.2001.113108. [DOI] [PubMed] [Google Scholar]
- 187.Winer HJ, Goepp RA, Olson RE. Gigantiform cementoma resembling Paget’s disease: report of case. J Oral Surg. 1972;30:517–9. [PubMed] [Google Scholar]
- 188.Waal I, Kwast WA. A case of gigantiform cementoma. Int J Oral Surg. 1974;3:440–4. doi: 10.1016/S0300-9785(74)80010-4. [DOI] [PubMed] [Google Scholar]
- 189.Cannon JS, Keller EE, Dahlin DC. Gigantiform cementoma: report of two cases (mother and son) J Oral Surg. 1980;38:65–70. [PubMed] [Google Scholar]
- 190.Punniamoorthy A. Gigantiform cementoma: review of the literature and a case report. Br J Oral Surg. 1980;18:221–9. doi: 10.1016/0007-117x(80)90066-9. [DOI] [PubMed] [Google Scholar]
- 191.Chen CH. A case of gigantiform cementoma associated with multiple unerupted teeth. Gaoxiong Yi Xue Ke Xue Za Zhi. 1989;5:299–303. [PubMed] [Google Scholar]
- 192.Thompson SH, Altini M. Gigantiform cementoma of the jaws. Head Neck. 1989;11:538–44. doi: 10.1002/hed.2880110612. [DOI] [PubMed] [Google Scholar]
- 193.Millet DT. Gigantiform cementoma showing apparent activity on a bone scan. Dentomaxillofac Radiol. 1990;19:137–8. doi: 10.1259/dmfr.19.3.2088788. [DOI] [PubMed] [Google Scholar]
- 194.Oikarinen K, Altonen M, Happonen RP. Gigantiform cementoma affecting a Caucasian family. Br J Oral Maxillofac Surg. 1991;29:194–7. doi: 10.1016/0266-4356(91)90038-7. [DOI] [PubMed] [Google Scholar]
- 195.MacDonald-Jankowski DS. Gigantiform cementoma occurring in two populations, London and Hong Kong. Clin Radiol. 1992;45:316–8. doi: 10.1016/s0009-9260(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 196.Finical SJ, Kane WJ, Clay RP, Bite U. Familial gigantiform cementoma. Plast Reconstr Surg. 1999;103:949–54. doi: 10.1097/00006534-199903000-00027. [DOI] [PubMed] [Google Scholar]
- 197.Rea G, Nuzzo L, Pietto F, Cangiano G, Ciccarelli R. Gigantiform cementoma of the jaw. A case report. Radiol Med (Torino) 2002;104:377–81. [PubMed] [Google Scholar]
- 198.Rossbach HC, Letson D, Lacson A, Ruas E, Salazar P. Familial gigantiform cementoma with brittle bone disease, pathologic fractures, and osteosarcoma: a possible explanation of an ancient mystery. Pediatr Blood Cancer. 2005;44:390–6. doi: 10.1002/pbc.20253. [DOI] [PubMed] [Google Scholar]
- 199.Reed RJ. Fibrous dysplasia of bone. A review of 25 cases. Arch Pathol. 1963;75:480–95. [PubMed] [Google Scholar]
- 200.Zimmerman DC, Dahlin Staphne DC EC. Fibrous dysplasia of the maxilla and mandible. Oral Surg Oral Med Oral Pathol. 1958;11:55–61. doi: 10.1016/0030-4220(58)90222-6. [DOI] [PubMed] [Google Scholar]
- 201.Horn PE, Jr, Dahlin DC, Bickel WH. Fibrous dysplasia: a clinical pathologic study of orthopedic surgical cases. Proc Staff Meet Mayo Clin. 1963;38:175–89. [PubMed] [Google Scholar]
- 202.Shafer WG. Chronic sclerosing osteomyelitis. J Oral Surg. 1957;15:138–42. [PubMed] [Google Scholar]
- 203.Agazzi, C, Belloni L. Gli odontomi duri dei mascellari contributo clinico-rontgenologico e antomicomicroscopico con particolare riguardo alle formle ad ampia estensione e alla comparsa familiare. Arch Ital Otol 1953;64(Suppl 16):1–10. [PubMed]