Abstract
Solitary fibrous tumour (SFT) is a rare tumour principally found in adults in the pleural cavity. Extrapleural occurrences are rare. Two cases of SFT of the thyroid gland are described in this paper showing their distinctive microscopical architecture, namely “patternless growth pattern”. It is characterized by a bland spindle-cell proliferation alternating hyper- and hypo-cellular areas, keloid-like hyalinization and a focal hemangiopericytoma-like vascular pattern. Tumour cells revealed a diffuse strong positivity for CD34, CD99, bcl-2 and Vimentin, but negativity for Desmin, EMA, AE1/AE3, SMA, S-100 and CD31 antibodies. The differential diagnosis of thyroid SFT includes different types of spindle cell proliferation, benign and malignant mesenchymal tumours, medullary thyroid carcinoma, fasciitis-like papillary carcinoma, and undifferentiated (anaplastic) carcinoma. However, the morphologic and immunohistochemical findings of SFT are so characteristic that this diagnosis seldom represent a difficulty.
Keywords: Solitary fibrous tumour, Thyroid, Immunohistochemistry, Review
Introduction
Solitary fibrous tumour (SFT) usually is a soft tissue neoplasm. It was initially described in the pleura by Klemperer and Rabin [1] as a form of localized fibrous mesothelioma. Subsequent studies have reported sporadic cases of SFT in various extrapleural sites, such as the mediastinum, pericardium, nasal cavity, peritoneum, retroperitoneum and liver have been increasing [2–6].
With regard to the thyroid gland, only 19 cases have been reported to date [7–17].
In the current paper, two case of a solitary fibrous tumour of thyroid gland with their immunohistochemical features and a literature review were presented.
Materials and Methods
Surgical specimen were fixed in 10% neutral buffered formaldehyde and embedded in paraffin. Routine haematoxylin and eosin staining was performed on the microtomic sections for histopathologic examination.
For each case, a paraffin block for immunohistochemical study was chosen, based on the quality of the morphologic preservation of all available haematoxylin and eosin stained slides.
Immunohistochemical evaluations were carried out using the avidin-biotin-peroxidase complex method. All antibodies were purchased from Dako Cytomation (Milano, Italy). The antibodies employed are shown in Table 1.
Table 1.
Antibodies employed
| Antigen | Antibody/Clone | Dilution | Pre-treatment |
|---|---|---|---|
| CD34 | Monoclonal mouse/Clone Q Bend-10 | 1:50 | Citrate buffer |
| CD99 | Monoclonal mouse/Clone 12E7 | 1:100 | No pre-treatment |
| Bcl-2 | Monoclonal mouse/Clone 124 | 1:80 | Citrate buffer |
| Desmin | Monoclonal mouse/Clone DE-R-11 | 1:100 | Citrate buffer |
| Vimentin | Monoclonal mouse/Clone V9 | 1:200 | Citrate buffer |
| S-100 | Polyclonal (rabbit) | 1:2,000 | Citrate buffer |
| EMA | Monoclonal mouse/Clone GP1.4 | 1:50 | Citrate buffer |
| AE1/AE3 | Monoclonal mouse/Clone AE1/AE3 | 1:100 | Citrate buffer |
| CD31 | Monoclonal mouse/Clone JC/70A | 1:20 | EDTA buffer |
| SMA (smooth muscle actin) | Monoclonal mouse/Clone 1A4 | 1:1,000 | Citrate buffer |
| Calcitonin | Polyclonal (rabbit) | 1:50 | No pre-treatment |
| Thyreoblobulin | Polyclonal (rabbit) | 1:600 | No pre-treatment |
| Ki-67 | Monoclonal mouse/Clone MIB-1 | 1:150 | Citrate buffer |
Results
Case 1
A 61-year-old man was admitted to the Sant’Eugenio Hospital of Rome with a right cervical lump. Neither dysphagia, dysphonia nor pain were reported. Ultrasonographic examination and computerized tomography revealed a solid intrathyroid nodule in the right lobe. No fine-needle aspiration biopsy was carried out. At surgery, the thyroid appeared enlarged and contained a well-defined nodule in the right lobe. A total thyroidectomy was performed.
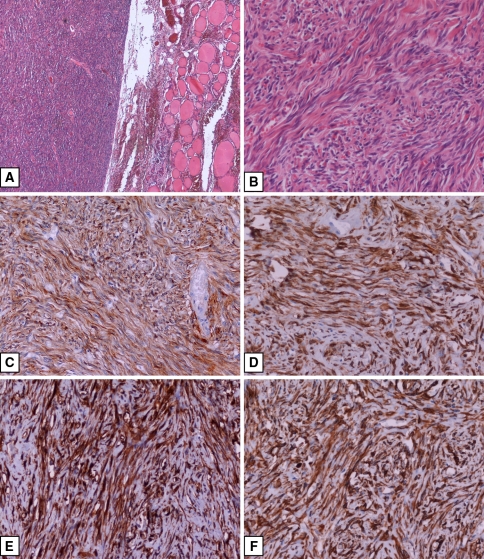
The resected tumour was 3.5 × 3 × 2.5 cm in size, well circumscribed, rounded and yellow in colour. Cystic walls had a smooth surface. Histologically, the tumour showed high cellularity and rich vascularization with hemangiopericytoma-like pattern. Most of the lesion was composed of spindle cells, with a regular, oval or round nuclei, with dispersed chromatin and small nucleoli. These cells were arranged in interlacing thin collagen fascicles that in some areas became more abundant with amianthoid-body-like appearance. The cystic walls were composed by fibrous tissue without an epithelial lining and exhibited deposits of hemosiderin and erythrocyte extravasation. There was no evidence of necrosis and mitotic figures were rare. No evidence of local recurrence or distant metastases after five years of follow-up is recorded (Fig. 1a and b).
Fig. 1.
(a) Hematoxylin and Eosin (H&E), 4× magnification; (b) H&E, 20× magnification; (c) Bcl-2 immunostaining, DAB chromogen, 20× magnification; (d) CD 34 immunostaining, DAB chromogen, 20× magnification; (e) CD 99 immunostaining, DAB chromogen, 20× magnification; (f) Vimentin immunostaining, DAB chromogen, 20× magnification
Case 2
A 42-year-old woman was admitted to the Hospital of the Catholic University of Rome because an ultrasonographic examination had revealed a solid nodule in her right thyroid lobe. Neither dysphagia, dysphonia nor pain were reported. A fine-needle biopsy under sonographic guidance was performed but resulted inadequate for a diagnosis because of poor cellularity. A right hemithyroidectomy with isthmusectomy was performed. Grossly, the tumour measured 4.7 × 4 × 3.5 cm in size and occupied the majority of the lobe. The cut surface was pale and firm and had a whorled appearance. Histologically, the lesion was well circumscribed by a thick fibrous capsule and was composed of a patternless proliferation of bland spindle cells in a collagenous and well-vascularized stroma. Neither necrosis nor mitotic activity were noted. There is no evidence of local recurrence or distant metastases after 7 years of follow-up.
Immunohistochemical Findings
By immunohistochemistry, tumour cells of both lesions revealed diffusely strong positivity for CD34, CD99, Bcl-2 and Vimentin [18, 19], but negativity for desmin, EMA, AE1/AE3, SMA, S-100 and CD31 antibodies (Fig. 1c–f). The immunostaining patterns and immunohistochemical differential diagnosis of SFT are summarized in Table 2 (data reported in Table 2 were taken from Immunoquery Database [http://www.ipox.org]). Ki-67 (MIB-1) was positive in less of 1% of the tumour cells in both lesions.
Table 2.
Immunohistochemical features (*) of lesion that morphologically are in differential diagnosis with SFT
| Lesion | CD99 | CD34 | Bcl-2 | VIM | S-100 | EMA | AE1/AE3 | CD31 | DES | SMA | CT | TG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solitary fibrous tumour | + | + | + | + | − | − | − | − | − | − | − | − |
| Hemangiopericytoma | +/− | −/+ | − | + | − | −/+ | − | − | − | +/− | − | − |
| Leiomyomatous tumour | −/+ | −/+ | −/+ | + | −/+ | − | − | − | +/− | + | − | − |
| Spindle-cell medullary carcinoma | n.r. | − | +/− | +/− | −/+ | − | + | − | − | n.r. | + | − |
| Undifferentiated (anaplastic) carcinoma | − | − | n.r. | + | − | −/+ | +/− | − | − | −/+ | − | − |
| Fasciitis-like papillary carcinoma | n.r. | −/+ | n.r. | + | +/− | +/− | + | − | n.r. | n.r | − | + |
| Schwannoma | + | −/+ | + | + | + | −/+ | − | − | − | − | − | − |
| Spindle-cell follicular adenoma | n.r. | − | n.r. | + | −/+ | −/+ | + | − | − | − | − | + |
| Liposarcoma | − | −/+ | + | + | −/+ | − | −/+ | − | −/+ | −/+ | − | − |
(+): >90% of tumours positive (*); (+/−): 50–90% of tumours positive (*); (−/+): 10–50% of tumours positive (*); (−): <10% of tumours positive (*); (n.r): not reported
(*): Data from immunoquery database (http://www.ipox.org)
Discussion
SFT is a rare tumour in adults principally found in the pleural cavity. However, reports of this tumour type occurring in other sites such as the mediastinum, pericardium, nasal cavity, peritoneum and liver are increasingly being described [2–6].
This tumour has many synonyms based on their histologic features, including localized benign mesothelioma, submesothelioma, localized fibrous tumour, fibroma and fibromyxoma. Its controversial histogenesis is reflected by the different nomenclature used to designate this tumour.
It is uncertain if this tumour arises from mesothelial or mesenchymal cells, although the latter is now preferred [18, 20] on the basis of immunohistochemical studies which identified the tumour cells with fibroblastic or myofibroblastic features as of mesenchymal origin [5, 6].
Some authors have attempted to classify extraserosal SFT into benign and malignant neoplasms based on the criteria applied to their pleural counterpart, such as high cellularity, cellular pleomorphism, high mitotic activity (cut-off point at 4/10 HPF), necrosis and haemorrhage [21]. As a matter of fact the most reliable prognostic indicators seem to be the gross appearance of the tumours and their surgical resectability [22]. The malignant cases present as poorly circumscribed infiltrative masses. Although most extrapleural SFTs are associated with a good prognosis, many authors believe that these criteria do not correlate perfectly with the clinical outcome. Therefore, a complete surgical excision with careful long-term follow-up is recommended because of the possibility of recurrencies up to several decades after surgery [13, 23].
A review of the literature regarding primary thyroid SFT, showed only 19 cases being reported to date ([7–17] and Table 3). The morphological and immunohistochemical features of SFT of thyroid, including our cases, are identical to pleural-based solitary fibrous tumours. All solitary fibrous tumours exhibit a characteristic microscopical architecture, namely “patternless growth pattern” with bland spindle-cell morphology, alternating hyper- and hypo-cellular areas, keloid-like hyalinization and a prominent but often focal haemangiopericytoma-like vascular pattern.
Table 3.
Review of the literature of SFT of the thyroid
| Author | Year | No. | Age | Sex | Size | Side | Cellularity | Atypia | Mitosis | Necrosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Present study | 2006 | 2 | 68 | F | 4.7 | L | + | No | No | No |
| 61 | M | 3.5 | R | + | No | No | No | |||
| Babouk [7] | 2004 | 1 | 45 | M | 5.0 | L | n.r. | No | No | No |
| Bohorquez [8] | 2003 | 1 | 68 | M | 9.5 | L | n.r. | No | Rare | No |
| Cameselle [9] | 2003 | 1 | 36 | M | 6.0 | L | n.r. | No | No | No |
| Parwani [10] | 2003 | 1 | 61 | M | 5.0 | L | +/− | No | No | No |
| Deshmukh [11] | 2001 | 1 | 56 | M | 8.0 | R | +/− | No | No | No |
| Rodriguez [12] | 2001 | 7 | 43 | F | 3.5 | L | ++ | No | 2 | No |
| 52 | M | 2.5 | L | + | No | No | No | |||
| 44 | M | 2.0 | L | + | Yes (Mild) | 1 | No | |||
| 64 | F | 4.5 | R | + | No | 2 | No | |||
| 53 | M | 6.0 | L | + | No | 1 | No | |||
| 47 | F | 4.5 | R | + | No | No | No | |||
| 64 | F | 3.0 | L | ++/+++ | No | No | No | |||
| Brunneman [13] | 1999 | 1 | 28 | F | 2.5 | n.r. | +++ | n.r. | >4 | n.r. |
| Kie [14] | 1997 | 1 | 48 | F | 8.0 | R | +/− | No | No | No |
| Villaschi [15] | 1996 | 1 | 51 | M | 5.0 | R | +/− | No | No | No |
| Cameselle [16] | 1994 | 1 | 43 | F | 4.0 | n.r. | + | No | No | No |
| Taccagni [17] | 1993 | 3 | 32 | F | 3.5 | R | +/− | Yes | Rare | n.r. |
| 44 | F | 6.5 | R | + | No | Rare | n.r. | |||
| 61 | M | 6.0 | L | +++ | No | No | n.r. |
No.: number of cases reported; M: male; F: female; L: left; R: right; n.r: not reported
According to cumulative data, including the present cases, mean age is 50 years (range 28–68 years), and there is almost an equivalence of incidence between men (11 cases; 52.4%) and women (10 cases; 47.6%). Tumour size ranged from 2 to 9.5 cm (mean 4.9 cm). Eleven tumours (57.9%) were located in the left lobe and 8 (42.1%) in the right. In two cases, location of lesion were not reported. Histologically, all except one case displayed the characteristic lack of architectural pattern and the distinctive cytologic findings. One exceptional case had an extensive lipomatous component [17]. No evidence of local recurrence or distant metastases were reported. This benign clinical outcome is in contrast to that observed in patients with soft tissue and pleural lesions, in which 10% of cases pursue an aggressive course [24].
The differential diagnosis of thyroid SFT includes different types of spindle cell proliferation, benign and malignant mesenchymal tumours, medullary carcinoma, fasciitis-like papillary carcinoma, and undifferentiated (anaplastic) carcinoma (Table 2).
The morphologic distinction of SFT from other spindle cell malignancies may be difficult, especially in the thyroid gland which may be the site of metastatic spread from other districts (mostly lung, kidney and larynx). An important diagnostic clue for a metastatic tumor is the presence of abundant necrosis and multifocality of the neoplastic foci, the detection of more than one cellular line (e.g. spindle and squamoid in carcinoma, multinucleated anaplastic cells in malignant histiocytoma) and of heterologous tissues in mesenchymal malignancies. The differentiation of SFT from medullary carcinoma is based on the detection of areas with trabecular pattern and with amyloid tissue within the C-cell tumor.
The immunohistochemical findings (CD34, CD99, Bcl-2, vimentin positive and S-100, SMA, cytokeratins, desmin negative) of SFT are so characteristic that they very rarely represent a difficult task. By these immunohistochemical findings, mesenchymal tumours of the thyroid reported in previous studies as leiomyoma [25, 26], neurilemmoma [25, 27, 28], and in some cases as hemangiopericytoma [29, 30] should probably be classified as SFT.
Acknowledgments
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Giuseppe Santeusanio, Email: santeusanio@med.uniroma2.it.
Guido Fadda, Email: guidofadda@rm.unicatt.it.
References
- 1.Klemperer P, Rabin CB. Primary neoplasms of the pleura: a report of five cases. Arch Pathol (Chic) 1931;11:385–412. [Google Scholar]
- 2.Weidner N. Solitary fibrous tumor of the mediastinum. Ultrastruct Pathol. 1991;15:489–92. doi: 10.3109/01913129109016254. [DOI] [PubMed] [Google Scholar]
- 3.Goto Y, Sakurada T, Suzuki I, et al. A localized fibrous tumor (mesothelioma) in the mediastinum: report of a case. Surg Today. 1997;27:871–3. doi: 10.1007/BF02385282. [DOI] [PubMed] [Google Scholar]
- 4.Bortolotti U, Calabro F, Loy M, et al. Giant intrapericardial solitary fibrous tumor. Ann Thorac Surg. 1992;54:1219–20. doi: 10.1016/0003-4975(92)90106-e. [DOI] [PubMed] [Google Scholar]
- 5.Mentzel T, Bainbridge TC, Katenkamp D. Solitary fibrous tumour: clinicopathological, immunohistochemical, and ultrastructural analysis of 12 cases arising in soft tissues, nasal cavity and nasopharynx, urinary bladder and prostate. Virchows Arch. 1997;430:445–53. doi: 10.1007/s004280050054. [DOI] [PubMed] [Google Scholar]
- 6.Kubota Y, Kawai N, Tozawa K, Hayashi Y, Sasaki S, Kohri K. Solitary fibrous tumor of the peritoneum found in the prevesical space. Urol Int. 2000;65:53–6. doi: 10.1159/000064836. [DOI] [PubMed] [Google Scholar]
- 7.Babouk NL. Solitary fibrous tumor of the thyroid gland. Saudi Med J. 2004;25:805–7. [PubMed] [Google Scholar]
- 8.Bohórquez CL, González-Cámpora R, Loscertales CL, et al. Solitary fibrous tumor of the thyroid with capsular invasion. Pathol Res Pract. 2003;199:687–90. doi: 10.1078/0344-0338-00481. [DOI] [PubMed] [Google Scholar]
- 9.Cameselle-Teijeiro J, Manuel Lopes J, Villanueva JP, et al. Lipomatous haemangiopericytoma (adipocytic variant of solitary fibrous tumour) of the thyroid. Histopathology. 2003;43:406–8. doi: 10.1046/j.1365-2559.2003.01696.x. [DOI] [PubMed] [Google Scholar]
- 10.Parwani AV, Galindo R, Steinberg DM, et al. Solitary fibrous tumor of the thyroid: cytopathologic findings and differential diagnosis. Diagn Cytopathol. 2003;28:213–6. doi: 10.1002/dc.10264. [DOI] [PubMed] [Google Scholar]
- 11.Deshmukh NS, Mangham DC, Warfield AT, Watkinson JC. Solitary fibrous tumor of the thyroid gland. J Laryngol Otol. 2001;115:940–2. doi: 10.1258/0022215011909440. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez I, Ayala E, Caballero C, Miguel C, Matías-Guiu X, Cubilla AL, Rosai J. Solitary fibrous tumor of the thyroid gland. Am J Surg Pathol. 2001;25:1424–8. doi: 10.1097/00000478-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Brunnemann RB, Ro JY, Ordonez NG, et al. Extrapleural solitary fibrous tumor: a clinicopathologic study of 24 cases. Mod Pathol. 1999;12:1034–42. [PubMed] [Google Scholar]
- 14.Kie JH, Kim JY, Park YN, Lee MK, Yang WI, Park JS. Solitary fibrous tumor of the thyroid. Histopathology. 1997;30:365–8. doi: 10.1046/j.1365-2559.1997.d01-618.x. [DOI] [PubMed] [Google Scholar]
- 15.Villaschi S, Macciomei MC. Solitary fibrous tumor of the perithyroid soft tissue. Report of a case simulating a thyroid nodule. Ann Ital Chir. 1996;1:89–91. [PubMed] [Google Scholar]
- 16.Camesselle-Teijeiro J, Varela-Durán J, Fonseca E, Villanueva JP, Sobrinho-Simoes M. Solitary fibrous tumor of the thyroid. Am J Clin Pathol. 1994;101:535–8. doi: 10.1093/ajcp/101.4.535. [DOI] [PubMed] [Google Scholar]
- 17.Taccagni G, Sambade C, Nesland J, Sobrinho-Simoes M. Solitary fibrous tumor of the thyroid: clinicopathological, immunohistochemical and ultrastructural study of three cases. Virchows Arch A Pathol Anat Histopathol. 1993;422:491–7. doi: 10.1007/BF01606459. [DOI] [PubMed] [Google Scholar]
- 18.Dervan PA, Tibin B, O’Connor M. Solitary (localized) fibrous mesothelioma: evidence against mesothelial cell origin. Histopathology. 1986;10:867–75. doi: 10.1111/j.1365-2559.1986.tb02584.x. [DOI] [PubMed] [Google Scholar]
- 19.Pizzolitto S, Falconieri G, DeMaglio G. Solitary fibrous tumor of the spinal cord: a clinicopathologic study of two cases. Ann Diagn Pathol. 2004;8:268–75. doi: 10.1016/j.anndiagpath.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Said JW, Nash G, Bank-Schlegel S, Sassoon AF, Shintaku IP. Localized fibrous mesothelioma: an immunohistochemical and electron microscopic study. Hum Pathol. 1984;15:440–3. doi: 10.1016/S0046-8177(84)80077-5. [DOI] [PubMed] [Google Scholar]
- 21.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–8. doi:10.1097/00000478-198908000-00003 [DOI] [PubMed]
- 22.Moran CA, Suster S, Koss MN. The spectrum of histologic growth patterns in benign and malignant fibrous tumors of the pleura. Semin Diagn Pathol. 1992;9:169–80. [PubMed] [Google Scholar]
- 23.Morimitsu Y, Nakajima M, Hisaoka M, Hashimoto H. Extrapleural solitary fibrous tumor: clinicopathologic study of 17 cases and molecular analysis of the p53 pathway. APMIS. 2000;108:617–25. doi: 10.1034/j.1600-0463.2000.d01-105.x. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher CDM. Soft tissue tumors. In: Fletcher CDM, editor. Diagnostic histopathology of tumors. 2nd ed. London: Churchill Livingstone; 2000. p. 1473–540.
- 25.Andrion A, Bellis D, Delsedime L, et al. Leiomyoma and neurilemmoma: report of two unusual nonepithelial tumors of the thyroid gland. Virchows Arch A. 1988;413:367–72. doi: 10.1007/BF00783030. [DOI] [PubMed] [Google Scholar]
- 26.Hendrick JW. Leiomyoma of the thyroid gland. Surgery. 1957;42:597–9. [PubMed] [Google Scholar]
- 27.Delaney WE, Fry KE. Neurilemmoma of the thyroid gland. Ann Surg. 1964;160:1014–6. doi: 10.1097/00000658-196412000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein J, Tovi F, Sidi J. Primary schwannoma of the thyroid gland. Int Surg. 1982;67:433–4. [PubMed] [Google Scholar]
- 29.Justrabo E, Michiels JF, Mairie J, Jacquot JP, Levillain P. Hemangiopericytome de la glande thyroide. Ann Endocrinol (Paris) 1989;50:26–30. [PubMed] [Google Scholar]
- 30.Kallemberg F, Anagnostaki L. Hemangiopericytoma in the thyroid gland. Ugeskr Laeger. 1979;141:3530–1. [PubMed] [Google Scholar]