Abstract
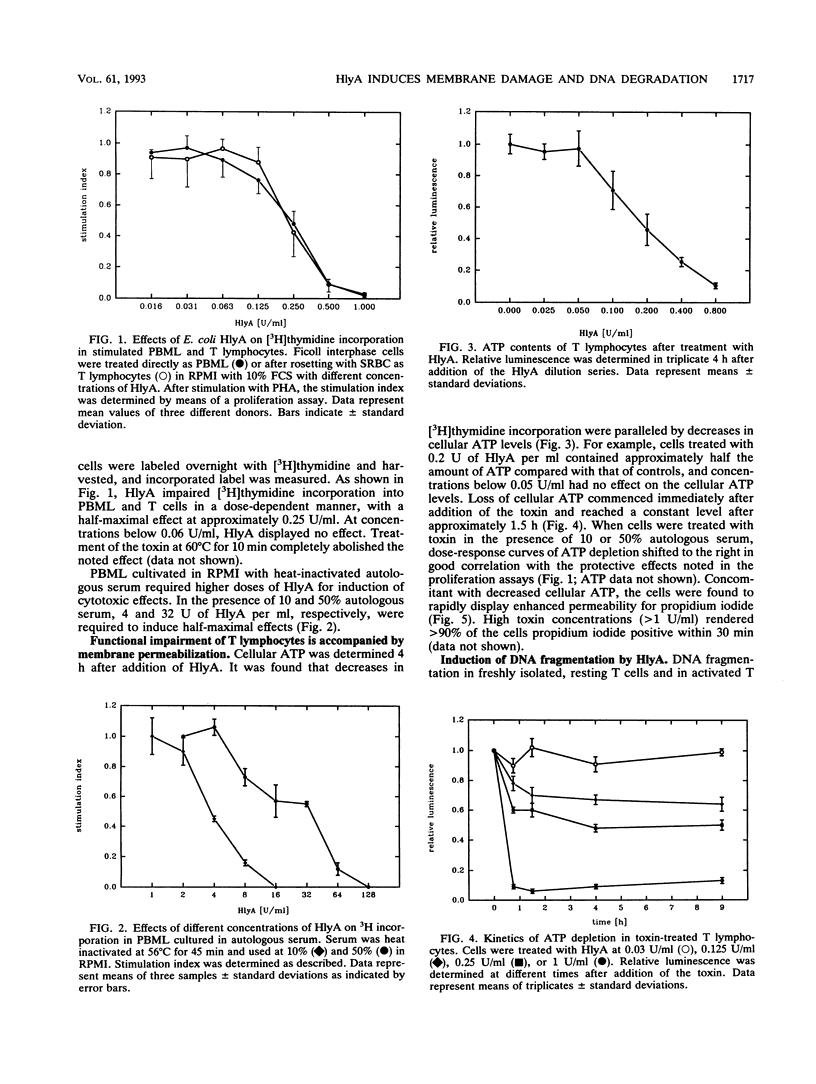
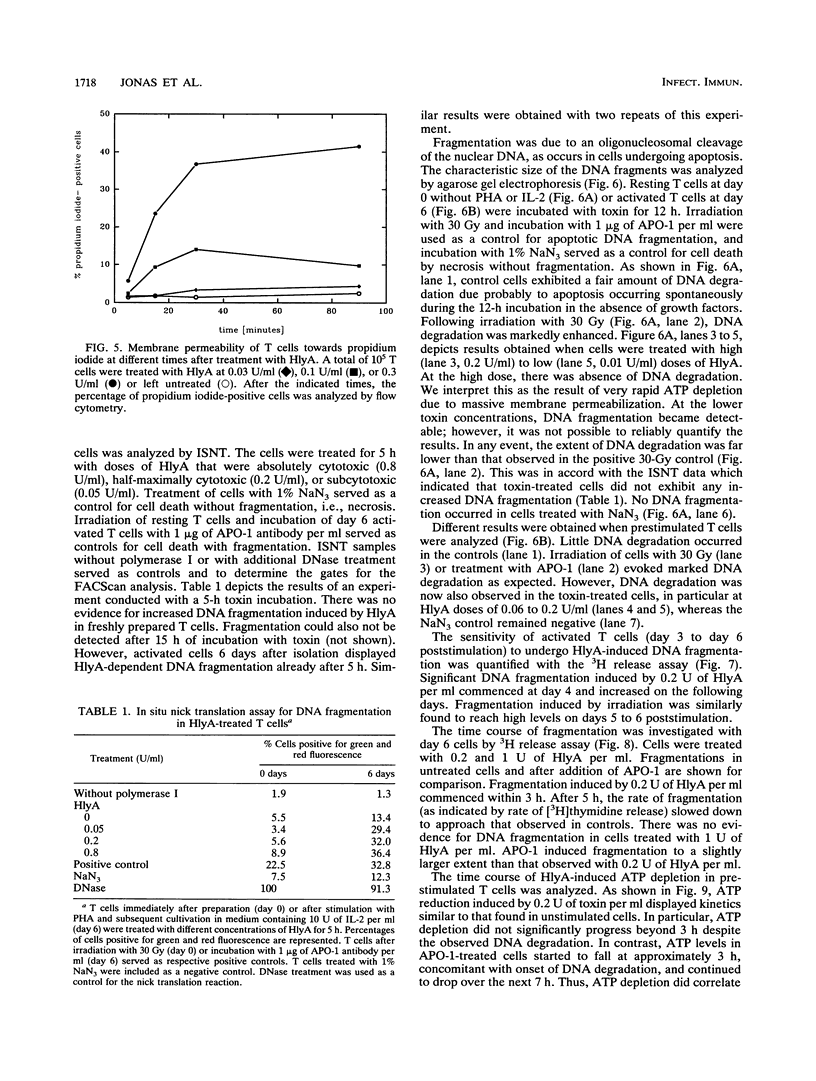
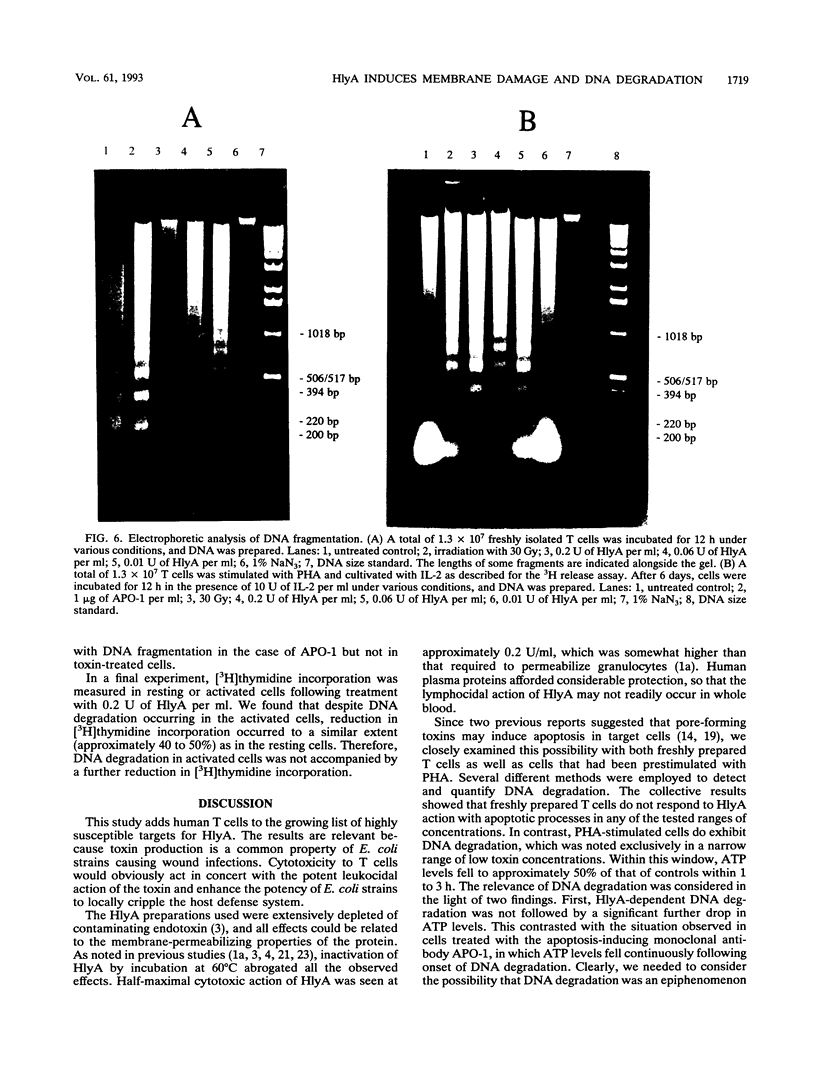
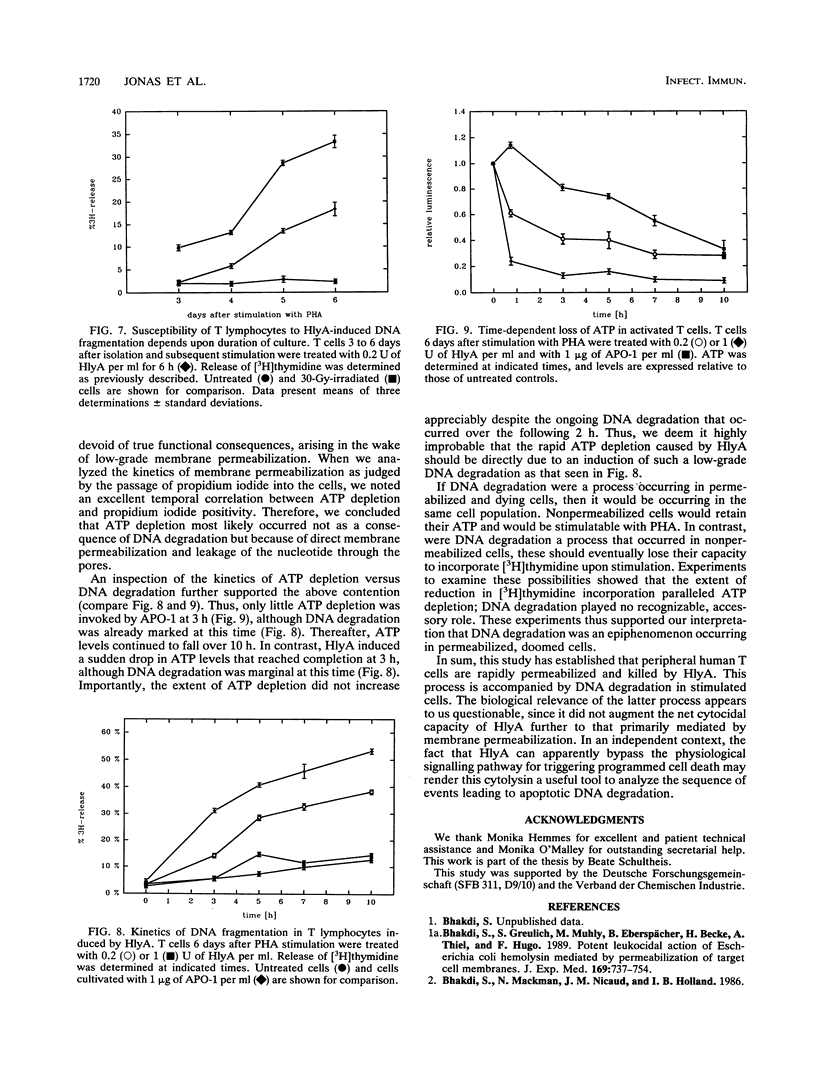
Escherichia coli hemolysin is the prototype of a large family of pore-forming toxins produced by gram-negative organisms. Besides its known cytotoxic activities against granulocytes, monocytes, endothelial cells, and renal epithelial cells, we now demonstrate that the toxin potently kills human T lymphocytes. Evidence based on different and independent approaches indicates that lymphocidal activity is due to formation of transmembrane pores. Additionally, cells prestimulated with phytohemagglutinin respond to low doses of E. coli hemolysin with DNA fragmentation similar to that observed in cells undergoing programmed cell death. Kinetic considerations lead us to conclude that DNA degradation may, however, represent an epiphenomenon. Killing of T cells is another means through which E. coli hemolysin could directly impair host defense.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Greulich S., Muhly M., Eberspächer B., Becker H., Thiele A., Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989 Mar 1;169(3):737–754. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991 Sep;59(9):2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Korom S., Schmidt G. Effects of Escherichia coli hemolysin on human monocytes. Cytocidal action and stimulation of interleukin 1 release. J Clin Invest. 1990 Jun;85(6):1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte function in vitro. Infect Immun. 1982 Sep;37(3):966–974. doi: 10.1128/iai.37.3.966-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982 May;36(2):455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debatin K. M., Goldmann C. K., Bamford R., Waldmann T. A., Krammer P. H. Monoclonal-antibody-mediated apoptosis in adult T-cell leukaemia. Lancet. 1990 Mar 3;335(8688):497–500. doi: 10.1016/0140-6736(90)90735-n. [DOI] [PubMed] [Google Scholar]
- Devenish J., Rosendal S., Johnson R., Hubler S. Immunoserological comparison of 104-kilodalton proteins associated with hemolysis and cytolysis in Actinobacillus pleuropneumoniae, Actinobacillus suis, Pasteurella haemolytica, and Escherichia coli. Infect Immun. 1989 Oct;57(10):3210–3213. doi: 10.1128/iai.57.10.3210-3213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberspächer B., Hugo F., Pohl M., Bhakdi S. Functional similarity between the haemolysins of Escherichia coli and Morganella morganii. J Med Microbiol. 1990 Nov;33(3):165–170. doi: 10.1099/00222615-33-3-165. [DOI] [PubMed] [Google Scholar]
- Gadeberg O. V., Orskov I. In vitro cytotoxic effect of alpha-hemolytic Escherichia coli on human blood granulocytes. Infect Immun. 1984 Jul;45(1):255–260. doi: 10.1128/iai.45.1.255-260.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger F., Walmrath D., Birkemeyer R. G., Bhakdi S., Seeger W. Leukotriene and hydroxyeicosatetraenoic acid generation elicited by low doses of Escherichia coli hemolysin in rabbit lungs. Infect Immun. 1990 Aug;58(8):2659–2663. doi: 10.1128/iai.58.8.2659-2663.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A., Olsen K. J., Lee M. K., Lichtenheld M. G., Podack E. R. Cytolysis by Ca-permeable transmembrane channels. Pore formation causes extensive DNA degradation and cell lysis. J Exp Med. 1989 Mar 1;169(3):765–777. doi: 10.1084/jem.169.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane W. F., Welch R., Gekker G., Peterson P. K. Mechanism of Escherichia coli alpha-hemolysin-induced injury to isolated renal tubular cells. Am J Pathol. 1987 Feb;126(2):350–357. [PMC free article] [PubMed] [Google Scholar]
- Kolodrubetz D., Dailey T., Ebersole J., Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989 May;57(5):1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Taichman N. S., Lally E. T., Wahl S. M. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect Immun. 1991 Sep;59(9):3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyaard L., Otto S. A., Jonker R. R., Mijnster M. J., Keet R. P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Seeger W., Walter H., Suttorp N., Muhly M., Bhakdi S. Thromboxane-mediated hypertension and vascular leakage evoked by low doses of Escherichia coli hemolysin in rabbit lungs. J Clin Invest. 1989 Jul;84(1):220–227. doi: 10.1172/JCI114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Flöer B., Schnittler H., Seeger W., Bhakdi S. Effects of Escherichia coli hemolysin on endothelial cell function. Infect Immun. 1990 Nov;58(11):3796–3801. doi: 10.1128/iai.58.11.3796-3801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Welch R. A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect Immun. 1987 Sep;55(9):2183–2190. doi: 10.1128/iai.55.9.2183-2190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991 Mar;5(3):521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]




