Abstract
Objective Patients with head and neck squamous cell carcinoma (SCC) often present with cervical lymph node metastasis. Occasionally the primary tumor site remains unknown even after thorough investigation. Management of such cases is problematic and may result in over-treatment and consequent increased morbidity. High risk HPV has been advocated recently as an important etiologic factor for a subset of head and neck SCC. These are believed to have a special predilection for the oropharyngeal tonsils and are characterized by nonkeratinizing basaloid morphology, and a strong reactivity to p16 immunostain. Identifying HPV-related SCC in the lymph nodes may thus provide a means for localizing the primary tumor site. Design Ninety-three cases of SCC metastatic to the neck from known primary tumors were classified morphologically into conventional keratinizing SCC (KSCC) and non-keratinizing SCC (NKCa). In situ hybridization (ISH) for high risk HPV as well as immunostaining for p16 were performed on all metastsatic and primary tumors. Results Of the 93 cases of metastatic carcinomas 32 were oropharyngeal, 35 oral, and 26 arose in the laryx/hypopharynx. Twenty-three cases were found to be HPV+ by ISH, of which 22/23 had oropharyngeal origin (P < 0.0001), with 95.7% sensitivity and 85.7% specificity. Twenty-one of these HPV+ oropharyngeal tumors were NKCa (P < 0.0001). The remaining case showed overlapping NKCa/KSCC hybrid morphology. All NKCa were HPV+ and stained diffusely and strongly with p16 antibodies. Conclusion We have demonstrated that HPV status of the lymph node metastasis can be assessed not only by ISH and p16 immunoreactivity but also histomorphologically. In addition, a positive microscopic identification of HPV-related carcinoma is a reliable predictor of oropharyngeal origin.
Keywords: Oropharynx, Nonkeratinizing squamous cell carcinoma, HPV, Occult head and neck carcinoma, p16, ISH
Introduction
In most cases of squamous cell carcinoma of the upper aerodigestive tract (UADT), metastasis to the cervical lymph nodes is a relatively late event occurring often after the primary tumors are well established and clinically identifiable. However, in up to 10% of cases, patients with cervical metastasis will reveal no evidence of a primary carcinoma even after thorough clinical and radiographic examination, and multiple targeted endoscopic biopsies [1, 2]. The management of this group of patients is problematic and commonly involves wide field irradiation that includes the entire pharyngeal axis and larynx with impending serious morbidity [3–6]. It is therefore of importance to exert all possible efforts to identify the primary tumor site in order to target it for therapy.
Clinical observations by us as well as others, have indicated that a subset of oropharyngeal squamous cell carcinomas of the tonsils and base of tongue, have a propensity for early metastasis [7]. In addition, it is observed that in several cases of cervical metastasis with occult primary tumor, the subsequent emerging tumor is most commonly located in the oropharynx [8].
About twenty years ago, high risk HPV (HR-HPV) was identified in squamous cell carcinoma of the head and neck [9]. A multitude of studies using a variety of techniques including ISH, PCR, and Southern blots have since been able to demonstrate the presence of HPV DNA in some UADT carcinomas, most commonly in the oropharynx, and specifically in the tonsils and base of tongue [9–16]. We and others have shown that HPV related squamous cell carcinoma of the oropharynx is microscopically and molecularly distinct [10, 12, 13, 15, 16]. The tumors are morphologically characterized by a non-keratinizing basaloid cell morphology, high mitotic activity, and comedo type necrosis. The tumor cells also have a characteristic immunophenotype distinguished by a strong and diffuse reactivity to p16INK4a (p16) antibodies, a negative or weak staining for p53 protein and high Ki67 staining scores [10, 12]. Identification of HPV by ISH and p16 immunostaining in SCC metastatic to cervical lymph nodes was shown to be a reliable way to establish origin from the oropharynx [17].
The purpose of this study is first, to ascertain the utility of using microscopic features in identifying HPV-related cervical metastasis, and second, to determine the reliability of predicting the site of the primary carcinoma of such metastasis.
Materials and Methods
Identification of HPV-related Carcinoma in Lymph Node Metastasis
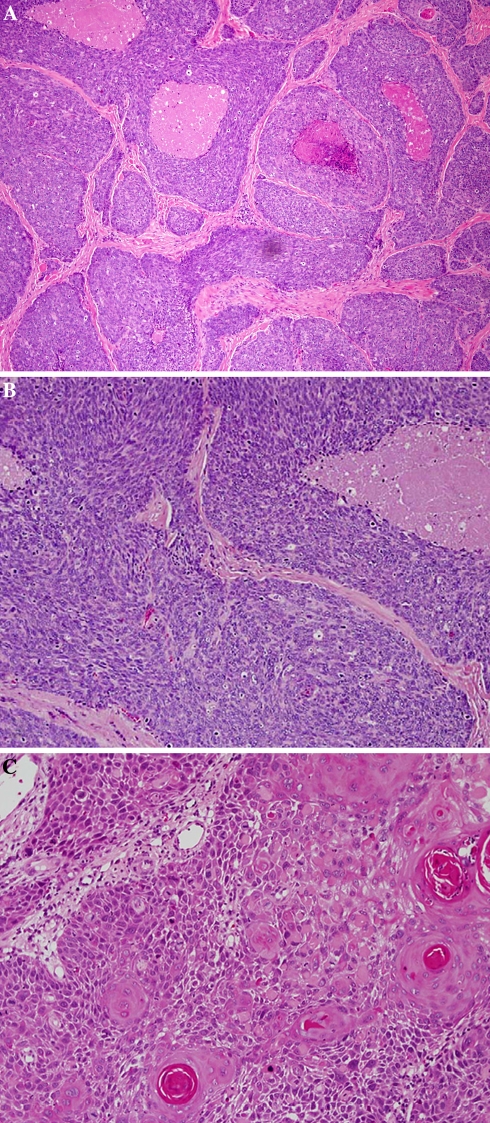
Ninety-three cases of squamous cell carcinoma of the head and neck with known primary tumor sites and lymph node metastasis accessioned during a five year period were retrieved from departmental files. Routine sections of the primary tumors and the positive lymph nodes were examined with light microscopy and the tumors were classified according to their microscopic features into two groups: Non-keratinizing SCC carcinoma (NKCa) and conventional keratinizing squamous cell carcinoma (KSCC). HPV-related NKCa was described previously [10, 12] and is characterized by relatively monomorphic, ovoid and spindle shaped basaloid cells with hyperchromatic nuclei, inconspicuous cytoplasm and indistinct cell borders. The tumor cells form sheets, cords and nests, with rather well defined margins and occasional peripheral palisading. Comedo type necrosis and excessive mitosis are characteristic features (Fig. 1a, b). Each primary tumor and its metastasis were phenotypically identical in all cases. For confirmation of HPV-relationship, additional sections from the lymph nodes and the primary tumors were used to identify high risk HPV by ISH, and p16 immunostaining.
Fig. 1.
(a) HPV-related non-keratinizing carcinoma (NKCa) of oropharyngeal tonsil. Basaloid small cells with dark basophilic nuclei, inconspicuous cytoplasm and indistinct cell borders. Areas of necrosis are seen. (b) Higher magnification of a showing mitotic figures. (c) Conventional keratinizing SCC showing characteristic “keratin pearls”. The cells are keratinocytic with abundant eosinophilic cytoplasm, prominent cell borders and intercellular bridges
ISH for HPV
ISH was performed on formalin fixed paraffin embedded sections using ISH I View Blue Plus Detection Kit (Ventana Medical System, Inc., Tucson, AZ.) according to the manufacturer’s instructions. The probes used hybridized with high risk HPV (HR-HPV) genotypes including type 16, 18, 33, 35, 45, 51, 52, 56, and 66. Ventana Red Counterstain II (Ventana Medical System, Inc., Tucson, AZ) was used. Positive staining was identified as blue nuclear dots.
Immunohistochemistry for p16
Immunoperoxidase staining was done on 4-mm cut tissue sections using the DAKO LSAB2 horseradish peroxidase system (DAKO Corp., Carpinteria, California) following the manufacturer’s instructions. Antigen retrieval was done by microwave heating for 10 minutes in 10 mM citrate buffer (pH 6.0); p16 monoclonal antibodies 1:40 were used (Novocastro Labs Ltd., United Kingdom). Staining was considered strong and diffuse when the nuclei and cytoplasm of >80% of the cells were positive. Other patterns of staining were designated focal and weak or negative.
Results
Identification of HPV: Related Carcinomas in Cervical Lymph Nodes
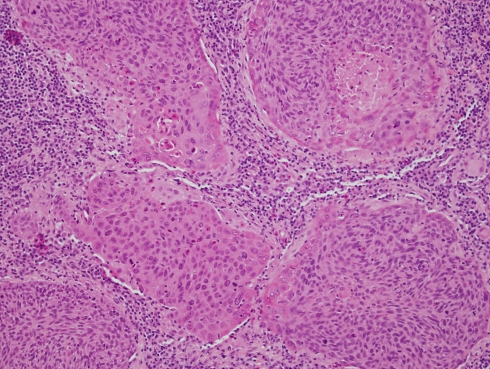
Of the 93 metastatic carcinomas, 32 cases originated in the oropharynx, 35 in the oral cavity, and 26 in the larynx/hypopharynx. The age range of the patients was 34–80 years. The oropharyngeal tumors originated exclusively from the palatine tonsils and base of tongue. The latter were commonly centered on the lingual tonsils. A male dominance was observed in all cases. The male to female ratio was 4:1 for the oral tumors, 7:1 for larynx/hypopharynx, and 9:1 for the oropharyngeal tumors. The distribution of the morphologic subtypes for each primary tumor site is shown in Table 1. Of the 32 oropharyngeal carcinomas, 21 (66%) were non-keratinizing (Fig. 1a, b), six were keratinizing (Fig. 1c) and five tumors showed overlapping morphology. In these hybrid cases, the basaloid epithelial cell of the non-keratinizing carcinomas showed focal areas of maturation throughout the tumor. In these areas the cells had keratinocytic rather than basal cell appearance with more prominent cytoplasm, distinct cell membrane and intercellular bridges (Fig. 2).
Table 1.
Distribution of 93 cases of neck metastasis by site and histologic type of primary tumors
| Primary site | Histologic types | ||
|---|---|---|---|
| (Number of cases) | KSCC | NK Ca(%) | Hybrid |
| Oral cavity (35) | 35 | 0 (0%) | 0 |
| Larynx/Hypopharynx (26) | 24 | 0 (0%) | 2 |
| Oropharynx (32) | 6 | 21(65%) | 5 |
Fig. 2.
Hybrid NKCa/KSCC with overlapping morphology. Basaloid cells showing a trend towards differentiation to a keratinizing phenotype, at the periphery of the cell nests and trabeculae
All of the 35 carcinomas of the oral cavity were of the keratinizing type and none had hybrid morphology. Carcinomas of the larynx and hypopharynx were also predominantly keratinizing type (24/26), two were hybrid type, and none were non-keratinizing.
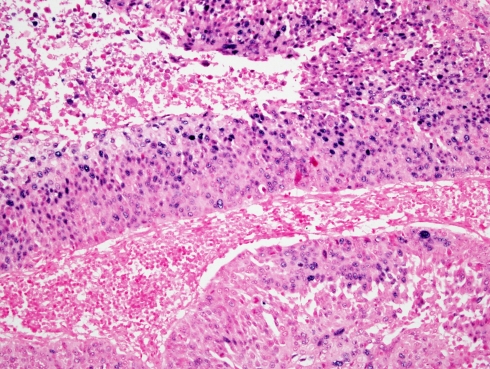
HPV was identified by ISH in 23 of the 93 metastatic lymph nodes (24.7%) (Fig. 3). In 22 of the 23 HPV positive lymph nodes (95.6%) the tumors originated in the oropharynx (P < 0.0001), with 95.7% sensitivity, 85.7% specificity as determined by the Chi-square and Fisher’s Exact tests. Only one HPV positive case was from non-oropharyngeal site; it arose in the larynx/hypopharynx.
Fig. 3.
ISH for HR-HPV. Positive staining is seen as blue nuclear dots in several cells of NKCa of an oropharyngeal tonsil
Of the 23 HPV positive carcinomas, 21 (91.3%) were non-keratinizing (P < 0.0001), and 2 showed hybrid features (Table 2). Twenty-one of the HPV positive NKCa were oropharyngeal in origin. In contrast, of the 70 HPV negative lesions 65 (92.8%) were predominantly keratinizing type and overwhelmingly non-oropharyngeal in origin (Table 2). The remaining 5 cases showed hybrid morphology. Ten HPV negative carcinomas were derived from the oropharynx. Of these, 6 were keratinizing carcinomas and four showed hybrid morphology (Table 2).
Table 2.
Microscopic features of HPV + and HPV− metastasis from different anatomic sites
| Primary site | HPV+ | HPV− | ||||
|---|---|---|---|---|---|---|
| K | NK | Hyb | K | NK | Hyb | |
| Oral | 0 | 0 | 0 | 35 | 0 | 0 |
| Larynx/hypopharynx | 0 | 0 | 1 | 24 | 0 | 1 |
| Oropharynx | 0 | 21 | 1 | 6 | 0 | 4 |
K = keratinizing SCC, NK = non-keratinizing SCC, Hyb = hybrid carcinoma
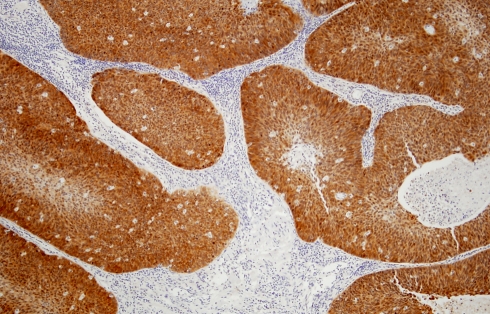
The results of p16 immunostaining are shown in Table 3. All of the 23 HPV positive tumors reacted strongly and diffusely with the antibodies (Fig. 4), while only five of the 70 HPV negative cases stained positively. Four of these five were oropharyngeal and one originated in the larynx/hypopharynx. All five tumors showed hybrid morphology. Their staining pattern was focal and weak in 3 cases and diffuse and strong in two. Table 4 summarizes the HPV status and sites of origin of all 7 tumors with hybrid morphology.
Table 3.
p16 Immunostain of HPV positive and negative metastatic carcinomas
| Primary site | HPV+ | HPV− | ||
|---|---|---|---|---|
| p16+ | p16− | p16+ | p16− | |
| Oral cavity | 0 | 0 | 0 | 35 |
| Larynx/hypopharynx | 1 | 0 | 1* | 24 |
| Oropharynx | 22 | 0 | 4** | 6 |
* Focal weak staining and hybrid morphology
** Two with focal weak and two diffuse and strong. All four had hybrid morphology
Fig. 4.
Strong and diffuse reactivity to p16 antibodies in NKCa
Table 4.
HPV status and site of tumors with hybrid morphology (7 cases)
| HPV negative (5) | HPV positive (2) | ||
|---|---|---|---|
| Oropharynx | Larynx/hypopharynx | Oropharynx | Larynx/hypopharynx |
| 4 | 1 | 1 | 1 |
Discussion
As stated above, metastasis to the cervical lymph nodes in the absence of a known primary tumor is a challenging patient management problem. A variety of techniques are routinely used, often unsuccessfully, in the search for the primary tumor site. These include imaging procedures such as MRI, CT and PET scans, as well as systematic biopsies of suspect regions and directed biopsies of endoscopically inconspicuous areas. Identification of the source of metastasis can lead to targeted treatment with reduced morbidity and more favorable outcome [18]. An alternate more direct and less invasive strategy is to identify tumor markers in the metastatic cells that can be used as a guide in determining the origin of the metastatic disease. Since the discovery that HR-HPV, particularly type 16, is associated with a subset of head and neck squamous cell carcinomas, it has been recognized that these HPV related tumors have distinct clinical and pathologic features [9–13, 15, 16, 19]. In particular, these tumors are site specific and overwhelmingly centered on the palatine and lingual tonsils [10, 12]. As stated above, these tumors are also characterized by a distinct non-keratinizing morphology and strong reactivity to p16 immunostain. In this study we have attempted first, to show that HPV related tumors in metastatic lymph nodes can be identified microscopically by their characteristic morphology, and second, to determine the reliability of predicting the primary tumor sites of these morphologically distinct metastatic carcinomas.
It should be emphasized that HPV-related NKCa differs from basaloid squamous carcinoma (BSC) of the upper aerodigestive tract (UADT), microscopically, clinically and molecularly. BSC, as originally defined by Wain et al. [20] is characterized by having biphasic morphologic features; a basaloid pattern which is intimately associated with keratinizing squamous cell carcinoma. Additional features that distinguish BSC include small gland like cystic spaces, stromal hyalinization and intercellular hyaline deposits. BSC of the UADT arise more commonly in the hypopharynx. The majority of BSC, Unlike NKCa show high labeling scores for p53 [21]. HPV has not been detected in BSC either by PCR or ISH techniques [22, 23]. More significantly HPV-related squamous cell carcinoma of the oropharynx is more responsive to therapeutic modalities and shows better prognosis [12].
The non-keratinizing morphology, which is the hallmark of HPV positive carcinomas, was evident in 21/93 lymph nodes as well as in their primary tumors. A few cases, 7/93 (7.5%) showed hybrid morphology. These will be further discussed below. All non-keratinizing carcinomas and two hybrid tumors were HPV positive by ISH (Table 2). None of the keratinizing tumors was positive for HPV.
p16 immunoreactivity is considered a surrogate marker for HPV positive carcinomas of the anogenital tract as well as those of the upper aerodigestive tract [10–12, 24–26].The paradoxical over-expression of cell cycle inhibitory protein in actively replicating neoplastic cells is thought to result from feedback control, secondary to deregulation of the tumor suppressor protein Rb. The latter is functionally inactivated by HPV oncoprotein E7 [25, 27]. p16 reactivity was diffuse and strong in all HPV positive tumors (Fig. 4). Five HPV negative carcinomas also reacted positively for p16 (Table 3). In these five HPV negative cases, p16 staining was focal and weak in three and diffuse and strong in two. All five cases showed hybrid morphology. As shown in Table 4, the two HPV-positive carcinomas with overlapping morphology, one was oropharyngeal and the other was from the larynx/hypopharynx. The five HPV negative hybrid carcinomas were predominantly (4 cases) oropharyngeal. Only one case was laryngeal/hypopharyngeal in origin.
The relationship between the ISH negative, p16 positive hybrid carcinomas to HPV infection is currently unknown. However, because these tumors are predominantly (4/5) oropharyngeal, a site with high prevalence of HPV carcinomas, it is conceivable that these tumors are HPV related. Using a more sensitive technique like PCR it may be possible to identify the viral genome in these cases. In a recent study, about 6% of ISH negative tumors were found to be HPV positive by PCR techniques [17]. Alternatively, the virus may have been shed at a later stage of progression of the tumor. Such an event could be facilitated by an unintegrated episomal status of the virus which has been documented in HPV positive tonsillar carcinomas [28, 29].
Of the 23 HPV positive metastatic carcinomas, 22 were oropharyngeal, 21 were nonkeratinizing, and one had hybrid morphology. The predictive value of HPV-related cervical metastasis for an oropharyngeal primary tumor is highly significant with a P-value of 0.0001, a sensitivity of 95.7%, and specificity of 85.7%.
In this study, we have demonstrated that HPV positive metastasis can be identified not only by ISH for HR-HPV and p16 immunoreactivity but also by their strong correlation to a distinct non-keratinizing morphology, with a high degree of statistical significance. We have also shown that identification, in cervical metastasis, of NKCa is a reliable predictor of a primary source, in the oropharyngeal palatine or lingual tonsils. Based on these observations we recommend that in cases of metastatic cervical lymph nodes, with occult primary carcinoma, HPV status of the metastasis may be simply determined by identifying non-keratinizing morphology in routine light microscopic sections. In ambiguous or hybrid cases with overlapping keratinizing and non-keratinizing morphology, p16 immunostaining and/or ISH for high risk HPV may be used. If a viral relationship is identified in the cervical metastasis a primary oropharyngeal carcinoma is strongly suspected and must be confirmed. An ipsilateral palatine tonsillectomy may be performed and microscopically examined by frozen sections. If the palatine tonsil is negative for carcinoma a lingual tonsillectomy may follow. In the event that both of the pharyngeal tonsils biopsies are negative, it is recommended that individual institutional protocol, for managing cervical metastasis with unknown primary carcinoma, should be implemented.
We have recently shown that HPV positive carcinomas in metastatic cervical lymph nodes could also be reliably identified, in fine needle aspiration (FNA) biopsies, by characteristic non-keratinizing cytologic features [30].
References
- 1.Braud F, al-Sarraf M. Diagnosis and management of squamous cell carcinoma of unknown primary tumor site of the neck. Semin Oncol. 1993;20:273–8. [PubMed] [Google Scholar]
- 2.Weber A, Schmoz S, Bootz F. CUP (carcinoma of unknown primary) syndrome in head and neck: clinic, diagnostic, and therapy. Onkologie. 2001;24:38–43. doi: 10.1159/000050280. [DOI] [PubMed] [Google Scholar]
- 3.Davidson BJ, Spiro RH, Patel S, et al. Cervical metastases of occult origin: the impact of combined modality therapy. Am J Surg. 1994;168:739–44. doi: 10.1016/S0002-9610(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 4.Grau C, Johansen LV, Jakobsen J, et al. Cervical lymph node metastases from unknown primary tumours: results from a national survey by the Danish society for head and neck oncology. Radiother Oncol. 2000;55:121–9. doi: 10.1016/S0167-8140(00)00172-9. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall WM, Mancuso AA, Parsons JT, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Head Neck. 1998;20:739–44. doi: 10.1002/(SICI)1097-0347(199812)20:8<739::AID-HED13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Nieder C, Gregoire V, Ang KK. Cervical lymph node metastases from occult squamous cell carcinoma: cut down a tree to get an apple? Int J Rad Oncol Biol Phy. 2001;50:727–33. [DOI] [PubMed]
- 7.Beaty MM, Funk GF, Karnell LH, et al. Risk factors for malignancy in adult tonsils. Head Neck. 1998;20:399–403. doi: 10.1002/(SICI)1097-0347(199808)20:5<399::AID-HED7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Guntinas-Lichius O, Klussmann P, Dinh S, et al. Diagnostic work-up and outcome of cervical metastases from an unknown primary. Acta Otolaryngol. 2006;126:536–44. doi: 10.1080/00016480500417304. [DOI] [PubMed] [Google Scholar]
- 9.Loning T, Ikenberg H, Becker J, et al. Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papillomavirus type related DNA. J Invest Dermatol. 1985;84:417–20. doi: 10.1111/1523-1747.ep12265517. [DOI] [PubMed] [Google Scholar]
- 10.El-Mofty SK, Lu DW. Prevalence of human papilloma virus type16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disese entity. Am J Surg Pathol. 2003;27:1463–70. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 11.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–72. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 12.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:339–45. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 14.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the international agency for research on cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 15.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 16.Houten VMM, Snijders PJF, Brekel MWM, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93:232–5. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 17.Begum S, Gillison ML, Ansari-Lari MA, et al. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469–75. [PubMed] [Google Scholar]
- 18.Haas I, Hoffmann TK, Engers R, et al. Diagnostic strategies in cervical carcinoma of an unknown primary (CUP) Eur Arch Otorhinolaryngol. 2002;259:325–33. doi: 10.1007/s004050100408. [DOI] [PubMed] [Google Scholar]
- 19.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 20.Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid squamous cell carcinoma of the tongue, hypopharynx and larynx: report of 10 cases. Hum Pathol. 1986;17:1158–66. doi: 10.1016/S0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez Tojo MJ, Garcia Cono FJ, Infante Sanchez JC, et al. Immunoexpression of p53, Ki-67 and E-Cadherin in basaloid squamous cell carcinoma of the larynx. Clin Transl Oncol. 2005;7:110–4. doi: 10.1007/BF02708743. [DOI] [PubMed] [Google Scholar]
- 22.Cabanillas R, Rodrigo JP, Ferlito A, et al. Is there an epidemiological link between human papillomavirus DNA and basaloid squamous cell carcinoma of the pharynx? Oral Oncol. 2007;43:327–32. doi: 10.1016/j.oraloncology.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Cho KJ, Lee SS. et al. Clinicopathologic study of basaloid squamous carcinoma of the upper aerodigeative tract. J Korean Med Sci. 1998;13:269–74. [DOI] [PMC free article] [PubMed]
- 24.Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884–91. doi: 10.1097/00000478-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–84. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 26.Lu DW, El-Mofty SK, Wang HL. Expression of p16, Rb, and p53 proteins in squamous cell carcinomas of the anorectal region harboring human papillomavirus DNA. Mod Pathol. 2003;16:692–9. doi: 10.1097/01.MP.0000077417.08371.CE. [DOI] [PubMed] [Google Scholar]
- 27.Wilczynski SP, Lin BT, Xie Y, et al. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol. 1998;152:145–56. [PMC free article] [PubMed] [Google Scholar]
- 28.Mellin H, Dahlgren L, Munck-Wikland E, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–8. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 29.Snijders PJ, Meijer CJ, Brule AJ, et al. Human papillomavirus (HPV) type 16 and 33 E6/E7 region transcripts in tonsillar carcinomas can originate from integrated and episomal HPV DNA. J Gen Virol. 1992;73:2059–66. doi: 10.1099/0022-1317-73-8-2059. [DOI] [PubMed] [Google Scholar]
- 30.Zhang MQ, El-Mofty SK, Davila RM. Detection of human papillomavirus (HPV)-related squamous cell carcinoma cytologically and by in situ hybridization (ISH) in fine needle aspiration (FNA) biopsies of cervical metastasis: a tool for identifying the site of an occult head and neck primary. Cancer. 2008;114:118–23. doi: 10.1002/cncr.23348. [DOI] [PubMed] [Google Scholar]