Abstract
An unusual case of mucoepidermoid carcinoma (MEC) of intraoral minor salivary gland origin with spindled morphology was presented. A fascicular stream of bland spindle cells from typical MEC areas amounted to more than 70% of the tumor. Spindle population had features consistent with immunohistochemical and ultrastructural profiles of intermediate cells and lacked overexpression of p53 and cyclin D1. There was no difference in the Ki-67 index between two distinct components. Awareness of extensive spindle cell change replacing a significant portion of low-grade MEC is crucial to avoid an erroneous interpretation of dedifferentiated sarcomatoid transformation.
Keywords: Intermediate cell, Low-grade malignancy, Mucoepidermoid carcinoma, Spindle cell change
Introduction
Mucoepidremoid carcinoma (MEC) is histologically composed of mucous, epidermoid, intermediate and clear cells, each proliferating alone or in varying combinations, in a cystic or solid form [1, 2]. In addition to the multiplicity of cell types, complex and varied architectural patterns are also the hallmark of MEC [2]. Although several histologic variants have been proposed [1–4], MEC seldom consists of a predominant population of spindled cells [2].
The extensive areas of spindle cell change within MEC were formally first noted and illustrated in 1964 by Lucas [5] and subsequently mentioned in the second series of the AFIP monograph [6]; however, detailed information could not be available. In 1986, Love and Sarma [7] described a highly malignant sarcomatoid tumor of the submandibular gland under the designation of spindle cell MEC. Based on this case and own observation, Luna [2] subclassified the spindle-cell type as a high-grade variant. At this time, it probably represents an example of sarcomatoid dedifferentiated carcinomas [8]. We present herein a diagnostically challenging lesion of the minor salivary gland characterized by the coexistence of low-grade MEC and bland spindle cell component.
Case Report
A 65-year-old woman presented with a 2-month history of a slow-growing painless mass in the right posterior maxilla. Oral examination disclosed a well-circumscribed, non-ulcerated, 15-mm tumor localized to the maxillary gingiva in the retromolar region. Radiographs showed slight erosion of the underlying bone. Since resection margins were free for tumor and metastatic work-up was negative, she did not receive any adjuvant chemo- or radio-therapy. The patient is well 7-year after surgery.
The operative material was fixed in 10% phosphate-buffered formalin. Sections were routinely stained with hematoxylin-eosin, periodic acid-Schiff, Alcian blue and mucicarmine. Immunohistochemical examination was curried out on paraffin-embedded sections by the avidin-biotin-peroxidase complex method. The primary antibodies used were as follows: epithelial membrane antigen (EMA, E29; DakoCytomation, Glostrup, Denmark; 1:20), cytokeratin (CK, AE1/AE3; DakoCytomation; 1:200), anti-mitochondrial antibody (113-1; Biogenex, San Rammon, CA; 1:100), vimentin (V9; DakoCytomation; 1:50), S-100 protein (polyclonal; DakoCytomation; 1:800), α-smooth muscle actin (SMA, 1A4; DakoCytomation; 1:150), p53 (DO7; DakoCytomation; 1:200), cyclin D1 (P2D11F11; Novocastra, Newcastle, UK; 1:50) and Ki-67 (MIB-1; DakoCytomation; 1:400). For ultrastructural analysis, fresh tissues were fixed in 2% glutaraldehyde and post-fixed in 1% osmium tetraoxide. Epon-embedded sections were stained with uranyl acetate-lead citrate and examined in an electron microscopy (JEM 100B; JEOL Ltd, Tokyo, Japan).
Histologically, a well-demarcated, but minimally invasive, solid-cystic tumor had two distinctive morphological patterns which were seen to blend with each other in varying proportions (Fig. 1a). Microcystic tumor area was composed of a variable admixture of goblet-type mucous, epidermoid, intermediate and mucin-negative clear cells. This element was readily diagnosed as a classic low-grade MEC (Fig. 1b). Cellular spindle component made up at least 70% of the tumor volume (Fig. 1c) and had elongated fusiform nuclei and bipolar cytoplasmic extensions mimicking a mesenchymal tumor (Fig. 1d). Their cytologic features remained low-grade, with neither significant nuclear atypia nor cellular pleomorphism. No necrosis at all was seen.
Fig. 1.
(a) Transitional zone between typical mucoepidermoid carcinoma (lower) and solid spindle component (upper) (b) Low-grade mucoepidermoid carcinoma area (c) Continuous streaming of spindle cells from peripheral microcystic mucoepidermoid carcinoma (d) Bland spindle proliferation with mesenchymal features (Hematoxylin-Eosin, a and c, ×100; b and d, ×200) Diffuse reactivity for epithelial membrane antigen (e) and focal expression of anti-mitochondrial antibody (f) in spindled cells (ABC method, ×400)
Immunohistochemically, spindled areas showed diffuse expression of EMA (Fig. 1e) and CK and up to 20% were positive with anti-mitochondrial antibody (Fig. 1f). Sporadic tumor cells (<0.5% of spindle cells) were reactive for vimentin and SMA. In all areas, no overexpression of p53 and cyclin D1 was detected. The Ki-67 index was 5.7% and 4.5% for spindle and classic MEC components, respectively.
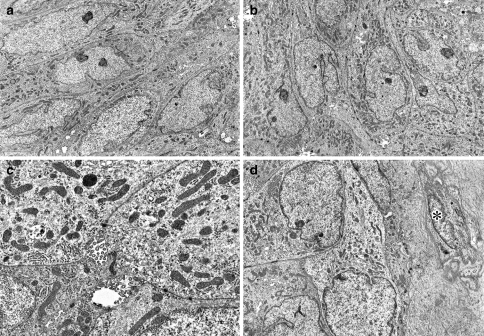
Ultrastructurally, spindled tumor cells showed increased numbers of mitochondria and free ribosome (Fig. 2a, b). Mitochondria varied in shape from round to elongate to irregular and contained tubulovesicular cristae rather than usual lamellar form [9]. Fine tonofilaments were identified but mucous granules were absent. Neighboring cells were tightly connected by desmosomes. Microglandular luminae with numerous microvilli were found in slightly widened intercellular spaces (Fig. 2c). Small numbers of classical myoepithelial cells, recognized by cytoplasmic microfilament arrays with focal densities, pinocytotic vesicles and reduplicated basal lamina, immediately surrounded the tumor nests (Fig. 2d).
Fig. 2.
(a, b) Spindle intermediate cells containing abundant mitochondria and numerous free ribosomes (×3000). (c) Mitochondria with tubulovesicular cristae, desmosomes and microglandular luminae with microvilli (×10500) (d) Myoepithelial cell (asterisk) surrounded by thick basal lamina (×3500)
Discussion
Dedifferentiation in the form of high-grade sarcomatoid tumor has been documented in nearly every type of salivary gland carcinomas [3, 8, 10–13]. Our case must be distinguished cautiously from dedifferentiated MEC [7, 14]. Although spindled areas in the present MEC lost their original well-differentiated cytoarchitectural identity, uniformity of nuclei, minimum degree of cellular pleomorphism and complete absence of tumor necrosis and brisk mitotic rate all signify its low-grade malignancy. No difference in the staining pattern of p53, cyclin D1 and Ki-67 between unmistakable MEC and spindle components further help confirmation of our interpretation. Consequently, this spindle cell growth does not warrant morphologic progression and should not be misconstrued as a dedifferentiated process [3, 8].
In salivary gland tumors, neoplastic myoepithelium is prone to assume a spindle cell configuration [3, 8, 15] and has been reported to undergo dedifferentiation [10, 11, 13]. Although spindled portion of our MEC is morphologically superimposable to myoepithelial proliferation and involvement of myoepithelial lineage in MEC has been well established [16–18], spindle cells in our MEC showed a reactivity profile characteristically different from neoplastic myoepithelium [3, 8, 15]. Moreover, ultrastructural features conclusively precluded their ‘bona fide’ myoepithelial line of differentiation [15]. Combining the present findings and the previous information [19], it is likely that spindle cell clone could have arisen from the mitochondria-rich intermediate component of MEC.
In summary, this case broadens a morphologic spectrum of MEC, emphasizing a potential for the diagnostic confusion with sarcomatoid tumor progression. The molecular mechanism of prominent spindle transformation of intermediate cells within MEC is worth exploring.
References
- 1.Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma. A clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835–45. doi: 10.1097/00000478-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Luna MA. Salivary mucoepidermoid carcinoma: Revisited. Adv Anat Pathol. 2006;13:293–307. doi: 10.1097/01.pap.0000213058.74509.d3. [DOI] [PubMed] [Google Scholar]
- 3.Cheuk W, Chan JKC. Advances in salivary gland pathology. Histopathology. 2007;51:1–20. doi: 10.1111/j.1365-2559.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 4.Ide F, Obara K, Enatsu K, Mishima K, Saito I. Sclerosing mucoepidermoid carcinoma of the oral cavity. J Oral Pathol Med. 2005;34:187–9. doi: 10.1111/j.1600-0714.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- 5.Lucas RB. Pathology of tumours of the oral tissues. London: Churchill; 1964. pp. 228–32. [Google Scholar]
- 6.Thackray AC, Lucas RB. Tumors of the major salivary glands. Washington DC: Armed Forces Institute of Pathology; 1974. pp. 69–80. [Google Scholar]
- 7.Love GL, Sarma DP. Spindle cell mucoepidermoid carcinoma of submandibular gland. J Surg Oncol. 1986;31:66–8. doi: 10.1002/jso.2930310116. [DOI] [PubMed] [Google Scholar]
- 8.Zarbo RJ. Salivary gland neoplasia: A review for the practicing pathologist. Mod Pathol. 2002;15:298–323. doi: 10.1038/modpathol.3880525. [DOI] [PubMed] [Google Scholar]
- 9.Latham B, Dickersin GR, Oliva E. Subtypes of chromophobe cell renal carcinoma. An ultrastructural and histochemical study of 13 cases. Am J Surg Pathol. 1999;23:530–5. doi: 10.1097/00000478-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cheuk W, Chan JKC, Ngan RKC. Dedifferentiation in adenoid cystic carcinoma of salivary gland. An uncommon complication associated with an accelerated clinical course. Am J Surg Pathol. 1999;23:465–72. doi: 10.1097/00000478-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Piana S, Cavazza A, Pedroni C, Scotti R, Serra L, Gardini G. Dedifferentiated acinic cell carcinoma of the parotid gland with myoepithelial features. Arch Pathol Lab Med. 2002;126:1104–5. doi: 10.5858/2002-126-1104-DACCOT. [DOI] [PubMed] [Google Scholar]
- 12.Ide F, Mishima K, Saito I. Sarcomatoid salivary duct carcinoma of the oral cavity. Virchows Arch. 2003;443:686–9. doi: 10.1007/s00428-003-0876-1. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa I, Nishida T, Miyauchi M, Sato S, Takata T. Dedifferentiated malignant myoepithelioma of the parotid gland. Pathol Int. 2003;53:704–9. doi: 10.1046/j.1440-1827.2003.01536.x. [DOI] [PubMed] [Google Scholar]
- 14.Nagao T, Gaffey TA, Kay PA, et al. Dedifferentiation in low-grade mucoepidermoid carcinoma of the parotid gland. Hum Pathol. 2003;34:1068–72. doi: 10.1053/S0046-8177(03)00418-0. [DOI] [PubMed] [Google Scholar]
- 15.Savera AT, Zarbo RJ. Defining the role of myoepithelium in salivary gland neoplasia. Adv Anat Pathol. 2004;11:69–85. doi: 10.1097/00125480-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhry AP, Cutler LS, Liefer C, Labay G, Satchidanand S, Yamame GM. Ultrastructural study of the histogenesis of salivary gland mucoepidermoid carcinoma. J Oral Pathol Med. 1989;18:400–9. doi: 10.1111/j.1600-0714.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 17.Dardick I, Gliniecki MR, Heathcote JG, Buford-Mason A. Comparative histogenesis and morphogenesis of mucoepidermoid carcinoma and pleomorphic adenoma. An ultrastructural study. Virchows Arch A Pathol Anat. 1990;417:405–17. doi: 10.1007/BF01606029. [DOI] [PubMed] [Google Scholar]
- 18.Yook JI, Lee SA, Chun YC, Huh J, Cha IH, Kim J. The myoepithelial cell differentiation of mucoepidermoid carcinoma in a collagen gel-based coculture model. J Oral Pathol Med. 2004;33:237–42. doi: 10.1111/j.0904-2512.2004.00056.x. [DOI] [PubMed] [Google Scholar]
- 19.Nicolatou O, Harwick RD, Putong P, Leifer C. Ultrastructural characterization of intermediate cells of mucoepidermoid carcinoma of the parotid. Oral Surg Oral Med Oral Pathol. 1979;48:324–36. doi: 10.1016/0030-4220(79)90032-X. [DOI] [PubMed] [Google Scholar]