Abstract
Mucoepidermoid carcinoma is the most common malignant salivary gland tumor, composed of several different cell types, with controversial histogenesis. The aim of this study was to assess the expression of cytokeratins in mucoepidermoid carcinoma, comparing to cytokeratin expression in normal salivary glands, in order to establish a possible correlation between tumor cells immunostaining and mucoepidermoid carcinoma histogenesis and differentiation. Eighty cases of salivary gland mucoepidermoid carcinoma were immunohistochemically examined with the use of antibodies against cytokeratins 6, 7, 8, 13, 14, 18, and 19. Cytokeratin expression varied according to the cellular type: squamous cells presented high expression of cytokeratins 6, 7, 8, 14, 18, and 19; intermediate and mucous cells of cytokeratin 7; clear and columnar cells of cytokeratins 6, 7, 8 and the latter also expressed cytokeratin 18. Cytokeratin 13 expression was low in all cell types. Cytokeratin immunoexpression in mucoepidermoid carcinoma was variable according to the cellular type; but regardless of the cellular type studied, cytokeratins 7 and 13 were, respectively, constantly high and low expressed. The immunoprofile of the normal salivary glands was variable according to the component but, in general, cytokeratin profile in mucoepidermoid carcinoma showed similarity to the immunoexpression on the excretory duct unit of normal salivary glands.
Keywords: Mucoepidermoid carcinoma, Mucous cell, Intermediate cell, Squamous cell, Clear cell, Columnar cell, Oncocyitic cell, Histogenesis, Differentiation, Cytokeratin, Salivary glands, Immunohistochemistry
Introduction
Mucoepidermoid carcinoma (MEC) is the most common malignant salivary gland tumor, comprising about 15% of all salivary gland tumors and 30% of all salivary malignancies [1–4]. It is an epithelial neoplasm composed of variable proportions of squamous, mucous, intermediate, clear, columnar, and other uncommon cell types, such as oncocytes, organized in solid and cystic growth patterns [1, 5–7]. Its histogenesis is thought to be derived from pluripotential reserve cells located in the excretory ducts [8–10]. However, due to the great diversity in its cellular components, the histogenesis of MEC remains controversial and incompletely elucidated [1, 10–14].
Cytokeratins (CK) are intermediate filaments mostly expressed by epithelial cells, which includes a wide range of proteins, varying in molecular weight, isoelectric pH values, and affinity [15–17]. CK expression varies among different types of epithelia in their different stages of development, and they may be used as an adjunctive tool for epithelial classification and histological diagnosis [15, 18–20]. Some studies have evaluated the pattern of CK expression in MEC [10, 13, 14, 21–24], but there is scarce information considering each of the different cellular types in this tumor.
The aim of this study was to analyze the CK immunoprofile of 80 cases of salivary gland MEC, considering the expression of each cellular type in comparison to the immunoprofile of normal salivary gland components.
Material and Methods
A total of 80 cases of MEC from major and minor salivary glands were retrieved from the files of the AC Camargo Cancer Hospital, São Paulo, Brazil, the same database included in previous studies [3, 4]. Clinical data were obtained from the patients records. This study was carried out with approval of the Research Ethics Committee, Piracicaba Dental School, State University of Campinas.
All cases were histologically reviewed using hematoxylin and eosin (HE), periodic acid-Schiff and mucicarmine stainings to confirm the diagnosis of MEC. Tumors were graded according to the histological criteria suggested by the World Health Organization and the Armed Forces Institute of Pathology as low, intermediate, and high-grade lesions [6, 7]. Each tumor was also assessed according to the cellular types components—squamous, intermediate, mucous, clear, columnar, and oncocytic; and the presence of adjacent normal salivary glands and each of its components—mucous and serous acini, intercalated, striated and excretory ducts, and myoepithelial cells.
For immunohistochemical reactions, 3-μm sections mounted on glass silanized histological slides were used. Primary antibodies were selected based on the MEC’s CK immunoexpression described in the literature [10, 14, 21–24] and included: anti-cytokeratin 6 (clone LHK6B, Novocastra Laboratories, dilution 1:200), anti-cytokeratin 7 (clone OV-TL 12/30, Dako, dilution 1:400), anti-cytokeratin 8 (clone 35H11, Dako, dilution 1:200), anti-cytokeratin 13 (clone KS-1A3, Novocastra Laboratories, dilution 1:400), anti-cytokeratin 14 (clone LL002, Novocastra Laboratories, dilution 1:200), anti-cytokeratin 18 (clone DC10, Dako, dilution 1:400) and anti-cytokeratin 19 (clone RCK108, Dako, dilution 1:400). Microwave antigen retrieval using citrate buffer, overnight incubation with the primary monoclonal antibodies and conjugated secondary antibodies, followed by the use of diaminobenzidine as the chromogen in all cases. Slides were counterstained with hematoxylin, mounted and analyzed by two authors (RSA, FRP). Negative and positive controls were used in all reactions to all antibodies.
Expression of CK was considered negative (0–5% of positive cells) or positive (>5% of positive cells), based on the average percentage of positive cells in 10 high-power fields of each specimen under light microscopy. Expression was independently assessed in each cellular type and each adjacent normal salivary gland components.
Results
From the 80 cases included in this study, 40 were from major salivary glands, mainly parotid (38 cases), and 40 from minor salivary glands, mainly palate (21 cases). Forty-one patients were male and the median age at diagnosis was 46 years (range 6–96), with a peak of incidence between 5th and 7th decades of life.
The tumors were classified according to their histological grade of malignancy in high-grade (36 cases, 45%), low-grade (35 cases, 44%), and intermediate-grade tumors (9 cases, 11%). Cellular types encountered included squamous (78 cases, 98%), intermediate (57 cases, 71%), mucous (43 cases, 54%), clear (24 cases, 30%), columnar (23 cases, 29%), and oncocytic cells (2 cases, 3%) (Fig. 1). Some tumors presented all cell types while in others one cellular type was the predominant, variable in accordance to the histological grade of malignancy. High-grade and intermediate-grade tumors presented a prevalence of squamous and intermediated cells, whereas low-grade tumors presented a wide distribution of the three main cellular types. Among the 80 cases of MEC, 40 presented adjacent normal salivary glands on the surgical margins, mainly from parotid and palate (21 and 9 cases, respectively).
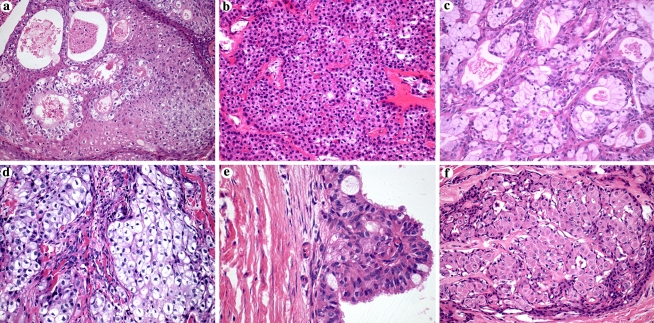
Fig. 1.
Cellular types found in salivary gland mucoepidermoid carcinomas (HE, original magnification, 200×). (a) Squamous cells; (b) intermediate cells; (c) mucous cells; (d) clear cells; (e) columnar cells (original magnification, 400×); (f) oncocytic cells
In general, most of the MEC cases presented some positivity for all CK studied, especially CK7, the main CK expressed in all cellular types (Fig. 2). CK expression varied according to the cellular type and, consequently, histological grade of malignancy. Squamous cells were mainly immunopositive for CK 6, 7, 8, 14, 18, and 19; intermediate and mucous cells for CK7; clear cells for CK 6, 7, and 8; and columnar cells for CK 6, 7, 8, and 18. Oncocytic cells, observed in only two cases, were immunopositive for CK 6, 7, 8, 18, and 19 in one of the cases (Table 1 and Fig. 3). CK13 was less frequently expressed in all cases, usually being found in a weak staining pattern.
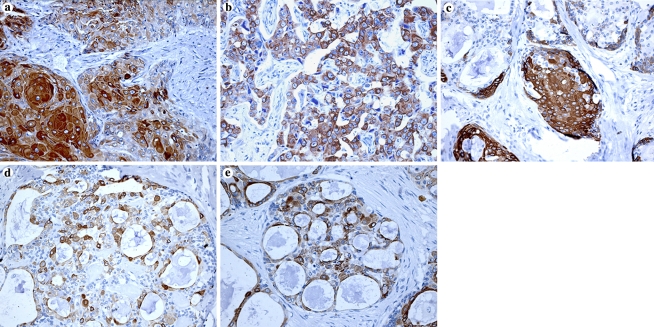
Fig. 2.
Immunoexpression of cytokeratin 7 in each cellular type found in mucoepidermoid carcinomas (immunoperoxidase, original magnification, 400×). (a) Squamous cells; (b) intermediate cells; (c) mucous cells; (d) clear cells; (e) columnar cells; (f) oncocytic cells
Table 1.
Cytokeratins (CK) immunohistochemical expression according to the cellular types in 80 cases of salivary gland mucoepidermoid carcinomas (MEC)
| CK | Mucoepidermoid carcinoma cellular types—n (%) | |||||
|---|---|---|---|---|---|---|
| Squamous (78 cases) | Intermediate (57 cases) | Mucous (43 cases) | Clear (24 cases) | Columnar (23 cases) | Oncocytic (2 cases) | |
| Simple epithelial CK | ||||||
| CK7 | 65 (83%) | 35 (61%) | 20 (47%) | 14 (58%) | 17 (74%) | 1 (50%) |
| CK8 | 58 (74%) | 27 (47%) | 4 (9%) | 14 (58%) | 13 (57%) | 1 (50%) |
| CK18 | 44 (56%) | 20 (35%) | 6 (14%) | 4 (17%) | 12 (57%) | 1 (50%) |
| CK19 | 42 (54%) | 13 (23%) | 4 (9%) | 7 (29%) | 7 (30%) | 1 (50%) |
| Stratified epithelial CK | ||||||
| CK6 | 54 (69%) | 23 (40%) | 3 (7%) | 15 (63%) | 12 (52%) | 1 (50%) |
| CK13 | 26 (33%) | 5 (9%) | 1 (2%) | 7 (29%) | 4 (17%) | 0 (0%) |
| CK14 | 43 (55%) | 10 (18%) | 0 (0%) | 3 (13%) | 1 (4%) | 0 (0%) |
In parenthesis the number of cases that showed the respective cells
n = number of positive cases; (%) = percentage of positive cases
Fig. 3.
Immunoexpression of the remaining cytokeratins expressed in squamous cells found in mucoepidermoid carcinomas (immunoperoxidase, original magnification, 200×). (a) CK6; (b) CK8; (c) CK14; (d) CK18; (e) CK19
The CK immunoprofile in MEC in the different histological grade of malignancy was variable reproducing the encountered cellular types, but in general all CK were positive. Low-grade and high-grade tumors immunoexpressed mainly CK 6, 7, 8, 14, 18, and 19 and intermediate-grade tumors CK 6, 7, 8, and 14. CK 13 was the least commonly CK expressed in all histological grades of malignancy (Table 2).
Table 2.
Cytokeratins (CK) immunohistochemical expression according to the histological grade of malignancy in 80 cases of salivary gland mucoepidermoid carcinomas (MEC)
| CK | Histological grading os malignancy—n (%) | ||
|---|---|---|---|
| Low-grade (35 cases) | Intermdiate-grade (9 cases) | High-grade (36 cases) | |
| Simple epithelial CK | |||
| CK7 | 35 (100) | 6 (68) | 30 (83) |
| CK8 | 30 (86) | 8 (89) | 28 (78) |
| CK18 | 23 (66) | 4 (44) | 23 (64) |
| CK19 | 23 (66) | 4 (44) | 20 (56) |
| Stratified epithelial CK | |||
| CK6 | 23 (66) | 8 (89) | 26 (72) |
| CK13 | 15 (43) | 3 (33) | 12 (33) |
| CK14 | 23 (66) | 5 (56) | 21 (58) |
n = number of positive cases; (%) = percentage of positive cases
Normal salivary glands found adjacent to tumors also showed immunoexpression according to each individual structure. Mucous and serous acini were immunopositive for CK 7, 8, and 18, both presenting CK18 as the CK most frequently expressed. In serous acini CK 6 and 19 were eventually expressed. Intercalated ducts were mainly positive for CK 6, 7, 18, and 19 and striated ducts for CK 6, 7, 8, 13, 18, and 19. In excretory ducts luminal cells were mainly immunopositive for CK 6, 7, 8, 18, and 19, and basal cells for CK14. Myoepithelial cells were especially immunopositive for CK 14 (Table 3).
Table 3.
Cytokeratins (CK) immunohistochemical expression in 40 normal salivary glands adjacent to mucoepidermoid carcinomas (MEC)
| CKs | Normal salivary glands—n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Mucous acini (19 cases) | Serous acini (28 cases) | Intercalated ducts (28 cases) | Striated ducts (38 cases) | Excretory ducts (31 cases) | Myoepithelial cells (40 cases) | ||
| Luminal cells | Basal cells | ||||||
| Simple epithelial CK | |||||||
| CK7 | 5 (26%) | 8 (29%) | 25 (89%) | 35 (92%) | 30 (97%) | 11 (36%) | 4 (10%) |
| CK8 | 2 (11%) | 7 (25%) | 12 (43%) | 36 (95%) | 29 (94%) | 10 (32%) | 2 (5%) |
| CK18 | 7 (37%) | 17 (61%) | 21 (75%) | 36 (95%) | 22 (71%) | 7 (23%) | 5 (13%) |
| CK19 | 0 (0%) | 2 (7%) | 19 (68%) | 35 (92%) | 27 (87%) | 12 (39%) | 0 (0%) |
| Stratified epithelial CK | |||||||
| CK6 | 0 (0%) | 2 (7%) | 19 (68%) | 30 (79%) | 21 (68%) | 14 (45%) | 1 (3%) |
| CK13 | 0 (0%) | 0 (0%) | 7 (25%) | 21 (55%) | 11 (36%) | 1 (3%) | 0 (0%) |
| CK14 | 0 (0%) | 1 (4%) | 5 (18%) | 15 (40%) | 5 (16%) | 25 (81%) | 13 (33%) |
n = number of positive cases; (%) = percentage of positive cases
In general, MEC presented immunopositivity for CK, 6, 7, 8, 14, 18, and 19, independently of the cellular type, morphological pattern of organization, and histological grade of malignancy; whereas normal salivary glands presented immunopositivity for all CK, independently of the salivary gland component (Table 4).
Table 4.
Summary of Cytokeratins (CK) immunoexpression in salivary gland mucoepidermoid carcinomas and normal salivary glands
| Component | Immunoexpression profile | ||
|---|---|---|---|
| Most frequent expressiona | Least frequent/absent expression | ||
| Mucoepidermoid Carcinomab | CK 6, 7, 8, 14, 18, and 19 | CK13 | |
| Normal salivary glands | |||
| Acinar cellsc | CK 18 | CK 6, 7, 8, 13, 14, and 19 | |
| Intercalated ducts | CK 6, 7, 18, and 19 | CK 8, 13, and 14 | |
| Striated ducts | CK 6, 7, 8, 13, 18, and 19 | CK14 | |
| Excretory ducts | CK 6, 7, 8, 14, 18, and 19 | CK13 | |
| Myoepithelial cellsc | CK 14 | CK 6, 7, 8, 13, 18, and 19 | |
aConsidering CK expression in at least half of the cases; b Overall tumor expression regardless of the cellular type; c Considering the single CK expressed in most of the cases
Discussion
MEC nomenclature includes reference to only two cellular types, although they are not unique in these tumors. In fact, MEC is characterized by a variety of cell types and growth patterns, which also include intermediate, clear, columnar, and oncocytic cells, arranged in solid nests and cystic or ductal structures [1, 5, 6, 11, 25, 26]. This cellular heterogeneity contributes to the difficulties in understanding its histogenesis. In our study, squamous cells were observed in almost all cases, followed by intermediate and mucous cells, confirming the predominance of these three cellular types in MEC. Clear cells, although less commonly found, are usually arranged in lobular or solid nests that can eventually be the predominant architectural finding, giving rise to the so-called clear cell variant of MEC [1, 25]. Columnar cells were observed in about one-third of the cases, usually lining cystic and ductal structures, and we could also identify two cases with nests of oncocytic cells, an uncommon finding in MEC [1, 26].
Salivary gland tumors histogenesis is based on the ductal-acinar unity, resulting in three models of development: origin from luminal cells, from non-luminal cells, and from a mixed population of acinar and/or ductal cells with basal and/or myoepithelial cells [5]. Due to the wide cellular diversity observed in MEC, its histogenesis remains controversial. Most authors believe that MEC origin is from pluripotential reserve cells from excretory ducts [3, 9, 10, 22, 27]. Exclusion of an origin similar to pleomorphic adenoma and adenoid cystic carcinoma from intercalated ducts is based on the absence of myoepithelial differentiation in MEC [9, 10]. Moreover, a recent study suggested a striated duct differentiation in MEC based on an immunohistochemical profile [23]. The CK are the major intermediate filaments group and have been used for diagnostic and histogenetic purposes in salivary gland tumors, including MEC [10, 14, 21–24].
CK7 was the main CK expressed in all cellular types of MEC. It is the first CK expressed during salivary gland development [28, 29], being retained in adult salivary glands [18–20, 28]. It also represents an important immunomarker on different salivary gland tumors [30]. On the other hand, CK13 was the CK least commonly expressed in all cellular types of MEC. It is a CK not expressed in developing salivary glands, but it may be found in some luminal cells of excretory ducts in fully developed normal salivary glands and in striated ducts as observed in this study [10, 31]. Nevertheless, CK13 has been previously described as an immunomarker of MEC [10, 14, 21, 24].
Squamous cells from MEC frequently express CK14 [10, 14, 21, 23, 32], a finding that was confirmed by our results. This CK has been also described as an immunomarker of myoepithelial and basal cells from excretory ducts [10, 14, 19, 23, 31], which was confirmed in normal salivary glands from our study. Nevertheless, Martins et al. [29] was not able to identify CK14 immunoexpression in myoepithelial cells of fetal salivary glands and concluded that this CK indicates basal membrane anchorage of these cells. It is important to point towards CK14 expression being frequent only in squamous cells, particularly in high-grade MEC, as this also happens in squamous cell carcinomas [24]. CK13 expression in squamous cells was already described by some authors, but it may vary from absence to intense positivity [10, 14, 21, 32]. Our results also identified some positivity to CK13 in squamous cells, but it was not frequent.
Intermediate cells were especially immunopositive for CK 7 similarly to the literature [10, 14, 21, 23]. CK 7 is described in acinar cells of developing salivary glands [29] and acinar and ductal cells of normal salivary glands in adults [10, 18, 19, 28, 29]. CK 6 and 8 were expressed in less than 50% of the intermediate cells but were identified in more than 50% percent of the squamous, clear, and columnar cells. These findings may be correlated with the possibility of intermediate cells being in a more advanced stage of cytodifferentiation of reserve cells from salivary duct unit but less differentiated than these other cell types, and afterwards the source of the other cell types in MEC [1, 11].
Overall, mucous cells presented less immunoexpression of all CK, what is probably explained by the displacement of the intermediate filaments to the periphery by mucous secretory granules. They were especially positive for CK 7, a finding also verified by other authors [10, 14, 21, 23, 32] and that reinforces CK 7 as the main immunomarker of salivary gland MEC. Contrarily to other studies [10, 14, 21, 23], CK 8 immunoexpression in mucous cells was not frequent in our results.
Clear cells expressed mainly CK 6, 7, and 8 in our study, a CK profile similar to intermediate cells. CK 6, which has been usually expressed in hyperproliferative squamous epithelia [20], was the most common CK identified in clear cells. A CK profile of clear cells including CK 7, 8, 14, 17, 18, and 19 was also identified [23, 32]. Clear cells are characterized microscopically by failure to stain with hematoxylin and eosin due to fixation artifacts, sparsity of organelles, or presence of mucin, glycogen, lipids or ribosomes [32] and are described by some authors in MEC as closely related to squamous and intermediate cells [11, 25, 32–34]. Intermediate cells are considered the progenitor cells in MEC, due to its ability of differentiation towards the other cells types, including clear cells [1], and probably justifying the similar CK profile between these both cell types. Terada et al. [25] reported a case of MEC composed of clear cells, presenting a gradual transition into intermediate cells, reinforcing this finding.
Columnar cells resemble the cells found in the major secretory ducts of salivary glands [1] and were mainly immunopositive for CK 6, 7, 8, and 18 in our study, a pattern similar to intermediate cells, except for prominent CK 18 expression. Loyola et al. [10] and Foschini et al. [23] also identified CK 13, 17, and 19 in columnar cells, and CK 13 and CK 19 were expressed in 17% and 30% of our cases with columnar cells, respectively. It is worthwhile to call attention that we found frequent CK 6 expression in columnar cells from MEC, a feature not previously described in this tumor.
Oncocytic cells, characterized on light microscopy by abundant granular eosinophilic cytoplasm as a result of increased amount of mitochondria, are rarely found in MEC [1, 26]. Oncocytic cells were found in two of our 80 MEC, with the cells expressing CK 6, 7, 8, 18, and 19 in only one of the cases. This contrast in an extremely rare MEC cellular type did not allow any further conclusion. Intermediate cells of MEC are also rich in mitochondrias [23], which reinforces the concept of intermediate cells being the first step of differentiation of the remaining cellular types found in salivary MEC.
Considering the normal salivary glands in this study, we can state that acinar and myoepithelial cells presented uncommonly expression of all CK, except for CK 18 and 14, respectively. Ductal cells presented a wide range expression of all CK. Excretory ducts presented expression of CK 6, 7, 8, 14, 18, and 19, which can be differentiated from intercalated ducts that uncommonly presented expression of CK 8 and 14, and from striated ducts that usually did not showed expression of CK 14 but showed expression of CK 13.
In conclusion, MEC presents variable immunohistochemical profile depending on the cellular type and, consequently on the histological grade of malignancy, since the presence and proportion of each cellular type is variable according to the latter. CK7 represents the main CK expressed in all cell types of MEC, being the only CK highly expressed in intermediate and mucous cells. On the other hand, CK13 represents the CK less expressed in all cell types of MEC, especially in intermediate and mucous cells, the former considered the first step of cytodifferentiation from the reserve cells. Normal salivary glands also present a variable immunohistochemical profile depending on the component, but in general, ductal structures expressed most of the CK studied with subtle differences that do not allow us to strictly state the origin of MEC. However, cytokeratin profile in MEC showed similarity to the immunoexpression on the excretory duct unit of normal salivary glands.
Acknowledgments
This research was supported by CAPES, FAPESP and CNPq, Brazil. The authors thank Ana Cristina do Amaral Godoy for the immunohistochemical assistance.
References
- 1.Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol. 2006;13:293–307. doi: 10.1097/01.pap.0000213058.74509.d3. [DOI] [PubMed] [Google Scholar]
- 2.Ito FA, Ito K, Vargas PA, et al. Salivary gland tumors in a brazilian population: a retrospective study of 496 cases. Int J Oral Maxillofac Surg. 2005;34:533–6. doi: 10.1016/j.ijom.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Pires FR, Alves FA, Almeida OP, et al. Carcinoma mucoepidermóide de cabeça e pescoço: estudo clínico-patológico de 173 casos. Rev Bras Otorrinolaringol (Engl Ed) 2002;68:679–84. [Google Scholar]
- 4.Pires FR, Almeida OP, Araujo VC, et al. Prognostic factors in head and neck mucoepidermoid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:174–80. doi: 10.1001/archotol.130.2.174. [DOI] [PubMed] [Google Scholar]
- 5.Dardick I. Color Atlas/text of salivary gland pathology. New York: Igaku-Shoin Medical Publishers; 1996. [Google Scholar]
- 6.Ellis GL, Auclair PL. Tumors of the salivary glands—Atlas of tumor pathology, 3rd Series, Fascicle 17. Washington: Armed Forces Institute of Pathology; 1996. [Google Scholar]
- 7.Barnes L, Eveson JW, Reichart P, Sidransky D. World health organization classification of tumours. Pathology & genetics—Head and neck tumors. Lyon: IARC Press; 2005. [Google Scholar]
- 8.Dardick I, Daya D, Hardie J, et al. Mucoepidermoid carcinoma: ultrastructural and histogenetic aspects. J Oral Pathol. 1984;13:342–58. doi: 10.1111/j.1600-0714.1984.tb01433.x. [DOI] [PubMed] [Google Scholar]
- 9.Dardick I, Gliniecki MR, Heathcote JG, et al. Comparative histogenesis and morphogenesis of mucoepidermoid carcinoma and pleomorphic adenoma. An ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1990;417:405–17. doi: 10.1007/BF01606029. [DOI] [PubMed] [Google Scholar]
- 10.Loyola AM, Sousa SO, Araujo NS, et al. Study of minor salivary gland mucoepidermoid carcinoma differentiation based on immunohistochemical expression of cytokeratins, vimentin and muscle-specific actin. Oral Oncol. 1998;34:112–8. doi: 10.1016/S1368-8375(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhry AP, Cutler LS, Liefer C, et al. Ultrastructural study of the histogenesis of salivary gland mucoepidermoid carcinoma. J Oral Pathol Med. 1989;18:400–9. doi: 10.1111/j.1600-0714.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 12.Hassanin MB, Ghosh L, Das AK, et al. Immunohistochemical and fluorescent microscopic study of histogenesis of salivary mucoepidermoid carcinoma. J Oral Pathol Med. 1989;18:291–8. doi: 10.1111/j.1600-0714.1989.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 13.Regezi JA, Zarbo RJ, Batsakis JG. Immunoprofile of mucoepidermoid carcinomas of minor salivary glands. Oral Surg Oral Med Oral Pathol. 1991;71:189–92. doi: 10.1016/0030-4220(91)90466-P. [DOI] [PubMed] [Google Scholar]
- 14.Silveira EJ, Veras Barros SS, Amorim RF, et al. Cytokeratin profile in mucoepidermoid carcinoma is not related to its histological grading of malignancy. Exp Mol Pathol. 2006;81:72–6. doi: 10.1016/j.yexmp.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 16.Coulombe PA, Omary MB. “Hard” and “soft” principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110–22. doi: 10.1016/S0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- 17.Kirfel J, Magin TM, Reichelt J. Keratins: a structural scaffold with emerging functions. Cell Mol Life Sci. 2003;60:56–71. doi: 10.1007/s000180300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawaf MH, Ouhayoun JP, Forest N. Cytokeratin profiles in oral epithelia: a review and a new classification. J Biol Buccale. 1991;19:187–98. [PubMed] [Google Scholar]
- 19.Berkovitz BK, Barret AW. Cytokeratin intermediate filaments in oral and odontogenic epithelia. Bull Group Int Rech Sci Stomatol Odontol. 1998;40:4–23. [PubMed] [Google Scholar]
- 20.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–39. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 21.Araujo VC, Sousa SO, Carvalho YR, et al. Application of immunohistochemistry to the diagnosis of salivary gland tumors. Appl Immunohistochem Mol Morphol. 2000;8:195–202. doi: 10.1097/00022744-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Pires FR, Chen SY, Perez DEC, et al. Cytokeratin expression in central mucoepidermoid carcinoma and glandular odontogenic cyst. Oral Oncol. 2004;40:545–51. doi: 10.1016/j.oraloncology.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Foschini MP, Marucci G, Eusebi V. Low-grade mucoepidermoid carcinoma of salivary glands: characteristic immunohistochemical profile and evidence of striated duct differentiation. Virchows Arch. 2002;440:536–42. doi: 10.1007/s00428-001-0585-6. [DOI] [PubMed] [Google Scholar]
- 24.Sobral AP, Loducca SV, Kowalski LP, et al. Immunohistochemical distinction of high-grade mucoepidermoid carcinoma and epidermoid carcinoma of the parotid region. Oral Oncol. 2002;38:437–40. doi: 10.1016/S1368-8375(01)00089-6. [DOI] [PubMed] [Google Scholar]
- 25.Terada T, Ikeuchi S, Inomoto C, et al. Mucoepidermoid carcinoma of the palate composed exclusively of clear cells (clear cell variant) Virchows Arch. 2004;445:541–3. doi: 10.1007/s00428-004-1086-1. [DOI] [PubMed] [Google Scholar]
- 26.Brannon RB, Willard CC. Oncocytic mucoepidermoid carcinoma of parotid gland origin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:727–33. doi: 10.1016/S1079-2104(03)00377-9. [DOI] [PubMed] [Google Scholar]
- 27.Eversole LR. Histogenic classification of salivary tumors. Arch Pathol. 1971;92:433–43. [PubMed] [Google Scholar]
- 28.Gustafsson H, Kjorell U, Eriksson A, et al. Distribution of intermediate filament proteins in developing and adult salivary glands in man. Anat Embryol (Berl) 1988;178:243–51. doi: 10.1007/BF00318227. [DOI] [PubMed] [Google Scholar]
- 29.Martins MD, Cavalcanti de Araujo V, Raitz R, et al. Expression of cytoskeletal proteins in developing human minor salivary glands. Eur J Oral Sci. 2002;110:316–21. doi: 10.1034/j.1600-0722.2002.21360.x. [DOI] [PubMed] [Google Scholar]
- 30.Meer S, Altini M. CK7+/CK20—immunoexpression profile is typical of salivary gland neoplasia. Histopathology. 2007;51:26–31. doi: 10.1111/j.1365-2559.2007.02728.x. [DOI] [PubMed] [Google Scholar]
- 31.Araujo VC, Sousa SO. Expression of different keratins in salivary gland tumours. Eur J Cancer B Oral Oncol. 1996;32B:14–8. doi: 10.1016/0964-1955(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 32.Prado RF, Lima CF, Pontes HA, et al. Calcifications in a clear cell mucoepidermoid carcinoma: a case report with histological and immunohistochemical findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e-40–4. doi: 10.1016/j.tripleo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Ellis GL. Clear cell neoplasm in salivary glands: clearly a diagnostic challenge. Ann Diagn Pathol. 1998;2:61–78. doi: 10.1016/S1092-9134(98)80035-X. [DOI] [PubMed] [Google Scholar]
- 34.Chen SY. Ultrastructure of mucoepidermoid carcinoma of minor salivary glands. Oral Surg Oral Med Oral Pathol. 1979;47:247–55. doi: 10.1016/0030-4220(79)90149-X. [DOI] [PubMed] [Google Scholar]