Clinical Presentation
A 31-year-old female patient presented with a swollen eye (Fig. 1). Intraoral examination showed a necrotic ulcerated lesion extending from the midline involving the buccal sulcus over the right central and lateral incisors (teeth 7 and 8) (Fig. 2). The CT scan showed an erosive mass anterior to the ethmoid cells and an opacified maxillary sinus. The patient is HIV negative.
Fig. 1.
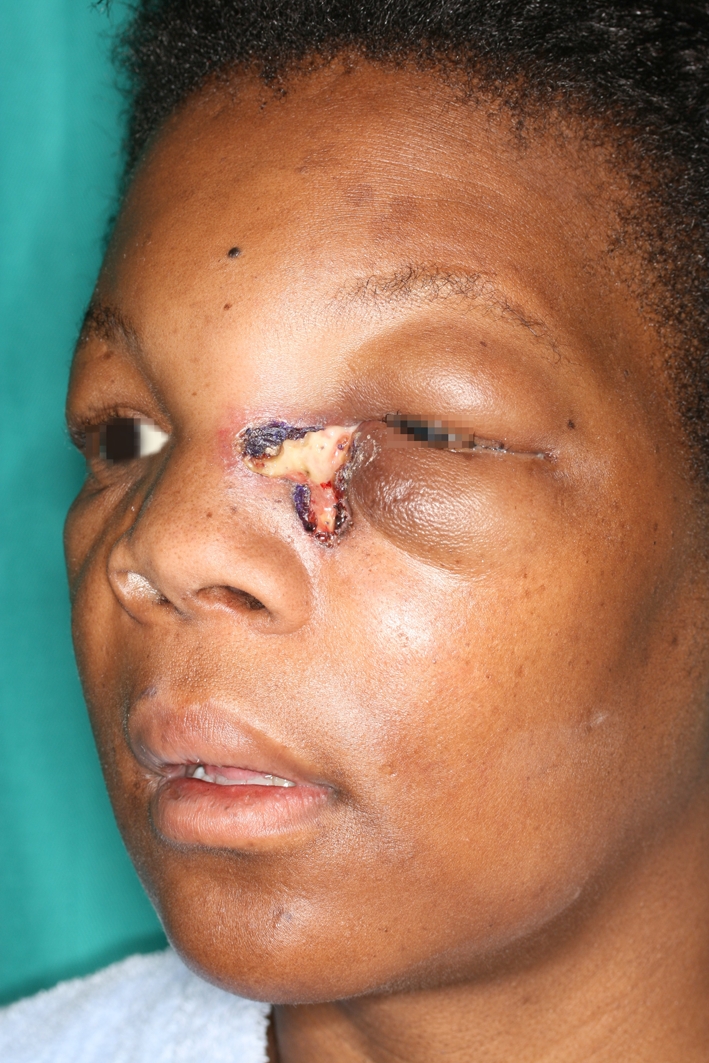
Extraorally, there was diffuse swelling extending from the glabella, involving the periorbital soft tissues and the nasolabial fold
Fig. 2.
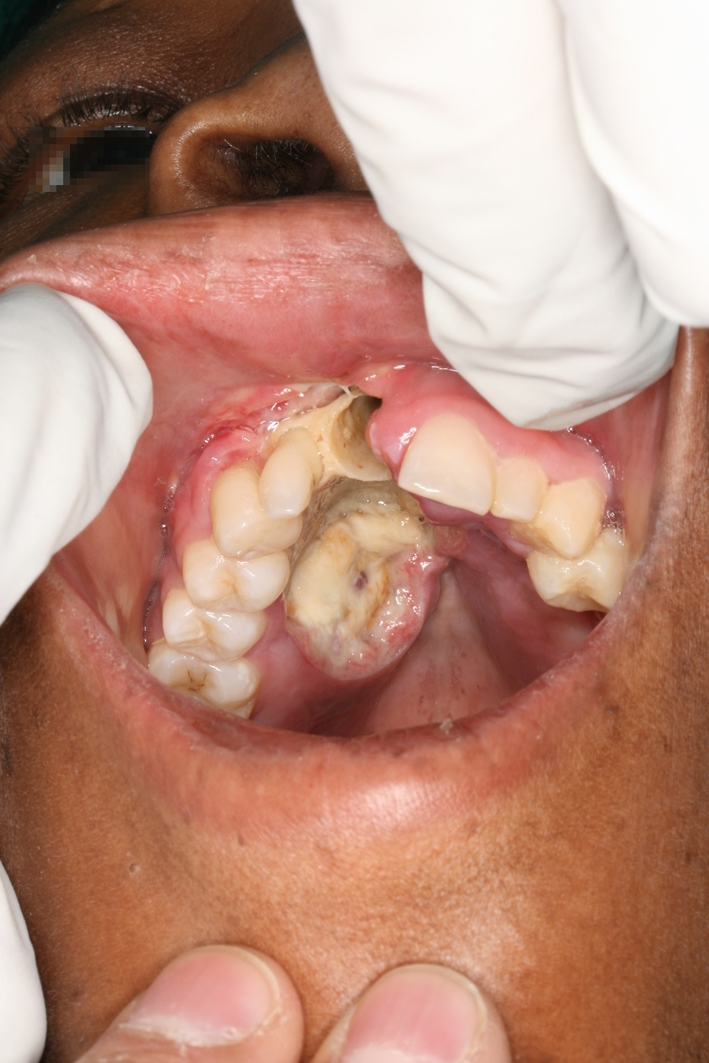
Intraorally, the necrotic mid-palatal ulcer had raised, rolled margins, and showed extension across the alveolar ridge to involve the buccal sulcus over the anterior teeth
Differential Diagnosis
Important clinical features from the clinical photos (Figs. 1 and 2) and history include:
an ulcerated intraoral tumor in the right anterior maxilla extending to the right buccal sulcus
an avascular, possibly necrotic socket of the right maxillary central incisor (tooth 8) with no evidence of alveolar bone destruction, features suggestive of ischemic change and exfoliation of the central incisor (tooth 8)
tumor tissue in the maxillary and ethmoidal sinuses
periorbital swelling around the left eye suggestive of tumor infiltration or cavernous sinus thrombosis
skin ulceration suggestive of ischemic changes
These features are suggestive of an infective or neoplastic process affecting a large area of the maxilla, crossing the midline, with bone and soft tissue involvement, and showing an affinity for blood vessels. The clinical differential diagnosis includes mid-facial necrotizing granulomatous diseases, deep fungal infections, primary intraoral neoplasms, maxillary sinus neoplasms and the so-called midline tumors.
Mid-facial necrotising granulomatous diseases include diseases such as Wegener’s granulomatosis, leprosy, sarcoidosis and tuberculosis. These conditions may present with skin lesions and oral ulceration but are seldom associated with ulcerative “tumors” or periorbital swelling.
Deep fungal infections such as zygomycosis, aspergillosis and histoplasmosis must be excluded. Zygomycosis remains a prime consideration as it has a known affinity for blood vessels resulting in thrombus formation and associated ischemic necrosis. Oral ulceration and periorbital swelling are common features. Patients frequently present with a short history of symptoms and are often uncontrolled diabetics. Intraoral tumor formation however is not a common presentation.
The clinical presentation of intraoral necrotic ulceration accompanied by paranasal sinus involvement and periorbital swelling are highly indicative of malignant disease. Of the wide spectrum of primary intraoral malignancies, squamous cell carcinoma or salivary gland carcinomas should be foremost considered. This patient is relatively young and furthermore the hard palate is a relatively uncommon site for a squamous cell carcinoma. The intraoral site is more compatible for a salivary gland malignancy. Tooth exfoliation associated with such malignancies is the result of direct bone destruction by tumor infiltration which is absent in the current case. Ischemic alveolar bone change without destruction is not a feature of salivary gland malignancy.
Maxillary sinus neoplasms include both primary sinus carcinomas and metastases. Metastases to the maxillary sinus are rare. Long standing primary sinus carcinomas, either squamous cell carcinomas or adenocarcinomas, can present with intraoral extension and ulceration. Bone destruction rather that ischemic necrosis is usually present in these cases. It is also rare for maxillary sinus tumors to cross the midline of the face.
There are a host of other “midline tumours.” Most soft tissue malignancies affecting the nasal area, such as rhabdomyosarcoma and esthesioneuroblastoma present as solitary tumors. They are seldom associated with ischemic bone necrosis. Lymphomas of the nasal area are usually T-cell lymphomas. The nasal type extranodal NK/T-cell lymphoma is compatible with the clinical presentation of the present case, including the presence of intraoral tumor tissue with necrosis, necrotic alveolar bone as well as changes in the maxillary and ethmoidal sinuses. Skin involvement with ischemic ulceration and periorbital infiltration, is also frequently seen in patients with the nasal type of NK/T-cell lymphoma. The latter appears to be the most likely diagnosis.
Diagnosis and Discussion
Extraoral examination showed a diffuse swelling extending from the glabella, involving the medial canthus of the eye, the eyelids, the peri- and infraorbital soft tissues and the nasolabial fold. Intraorally, the necrotic ulcerated lesion had raised, rolled margins which extended from the midline of the palate, across the alveolar ridge to involve the buccal sulcus over the right central and lateral incisors (teeth 7 and 8). The right central incisor (tooth 8) was previously extracted (Fig. 2). The ulcerated mass measured 4.5 × 3.0 × 1.5 cm3. The clinical differential diagnosis included a deep fungal infection and a squamous cell carcinoma.
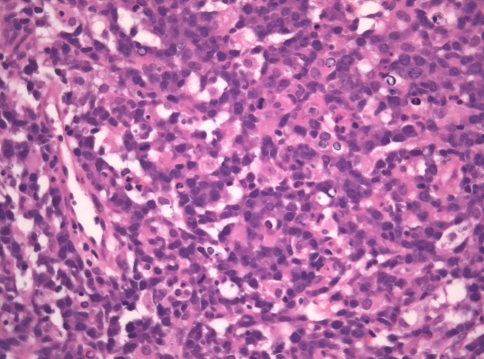
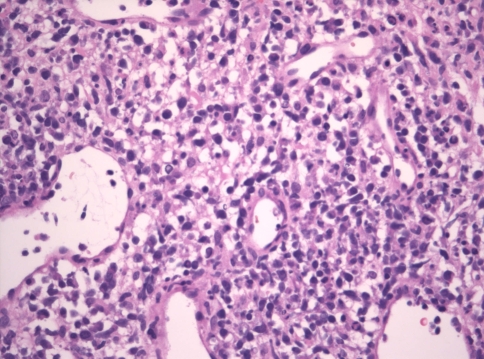
Microscopic examination of the soft and hard tissue incisional biopsies from the palate, middle meatus and periorbital regions revealed extensive ulceration with intense suppurative permeation of the overlying epithelium where present. Epithelial dysplasia was not observed. The deeper submucosa showed an infiltrative high-grade extranodal hematolymphoid malignancy. The neoplastic population consisted of intermediate to large pleomorphic lymphoid cells with hyperchromatic nuclei, increased nuclear to cytoplasmic ratios, marked apoptosis and increased mitotic activity, including atypical forms (Figs. 3 and 4). The tumor showed many arborizing vessels and an angiocentric distribution of the tumor cells was noted focally (Fig. 4). There were multifocal areas of tumor necrosis and a prominent mixed inflammatory background.
Fig. 3.
The tumor showed a predominance of medium to large cells, with irregularly shaped hyperchromatic nuclei, increased nuclear to cytoplasmic ratios, marked apoptosis and increased mitotic activity (hematoxylin–eosin stain, original magnification ×400)
Fig. 4.
High endothelial venules were increased and arborizing vessels were evident (hematoxylin–eosin stain, original magnification ×400)
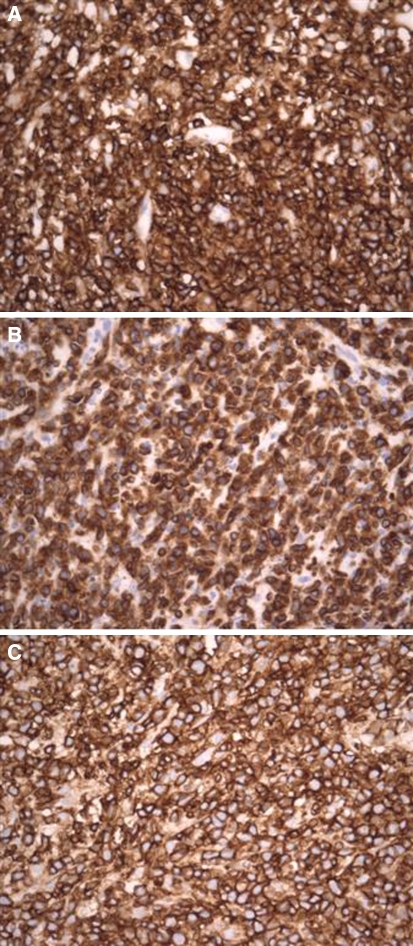
Special stains (Periodic acid-Schiff, Grocott’s and Ziehl Neelson) failed to demonstrate the presence of specific infective etiological agents, including fungal elements. The tumor had strong and diffuse immunoreactivity for CD45RB, CD3ε (cytoplasmic) and CD45 RO (T-cell UCHL1) (Fig. 5a–c) and CD56 (NCAM) and no immunoreactivity with CD20, CD79a, CD3 (surface), EBV-LMP1 and AE1/3 (Dako, Glostrup, Denmark). The EBV sequence (269 bp) was confirmed by DNA sequencing, following PCR amplification of DNA isolated from the formalin-fixed, paraffin-embedded tissue. Amplification for TCR-γ showed a monoclonal band, suggesting rearrangement of this gene. Based on the morphologic, immunophenotypic, genotypic and clinical characteristics, a diagnosis of extranodal NK/T-cell lymphoma, nasal type(WHO Classification) [1] was made.
Fig. 5.
Immunopositivity with CD45RB (a), CD3ε (b) and CD45RO (c) (Dako; Glostrup, Denmark) confirmed the T-cell lymphoma lineage of the neoplasm (original magnification ×400)
Extranodal NK/T-cell lymphoma, nasal type comprises about 1.4% of all non-Hodgkin’s lymphomas [2]. The frequency depends largely on the country of origin of the patients, with a frequency of 22.6% being reported in a review of cases of peripheral T-cell and natural killer (NK)/T-cell lymphomas from North America, Europe and Asia [3]. The WHO reports this tumor as accounting for about 50% of the peripheral T-cell lymphomas seen in Western countries [1].
Most T-cell lymphomas in this category occur in adults, are more common in males, and are more prevalent in Asia, Mexico and Central and South America. These lymphomas have also been described in immunosuppressed and post-transplant patients [1]. They show a predominantly extranodal presentation, with the sites of predilection being the nasal cavity, and paranasal sinuses with extension into the nasopharynx, orbit, oral cavity, palate, and oropharynx [1, 4]. Nasal lymphomas present as localized disease with symptoms such as nasal obstruction, discharge, or epistaxis due to the presence of a mass lesion, or with extensive midfacial destructive lesions (so-called lethal midline granuloma) [1, 5].
Whilst this disease is often localized to the upper aerodigestive tract at presentation, with bone marrow involvement being very uncommon, it may disseminate rapidly to skin, soft tissue, gastrointestinal tract, testis and cervical lymph nodes. Fever, hemophagocytosis and disseminated intravascular coagulation may ensue [5]. Those occurring outside the nasal cavity vary in presentation depending on the site of involvement. Skin involvement often results in ulcerated nodules, and intestinal lesions manifest as perforation. Patients often have high stage disease at presentation, with involvement of multiple extranodal sites. Cases with marrow and blood involvement often overlap with aggressive NK-cell leukemia [1, 3].
Not much is known about the etiology of extranodal NK/T-cell lymphoma. The nasal type is uniformly associated with Epstein-Barr virus (EBV) irrespective of the ethnic origin of the patients, signifying a probable pathogenic role of the virus. Those presenting in organs other than the nose also show a strong EBV association in Asian patients, but the strength of the association in White patients is less obvious [1].
Phenotypic markers expressed in extranodal NK/T-cell lymphoma include CD2, CD56 and CD3ε (cytoplasmic). Most cases are also positive for cytotoxic associated granules (granzyme B, TIA-1 and perforin), and negative for CD3 (surface), CD4, CD5, CD8, TCRβ, TCRδ, CD16 and CD57. Other commonly expressed antigens include CD43, CD45RO, HLA-DR, interleukin-2 receptor, Fas (CD95) and Fas ligand, with occasional cases being positive for CD7 and CD30.
Most extranodal NK/T-cell lymphomas have germline configurations of T-cell receptor (TCR) and immunoglobulin genes. A variety of cytogenetic aberrations have been reported, however no specific chromosomal translocations have thus far been identified [1, 5]. The most common cytogenetic abnormality is del(6)(q21q25) or i(6)(p10), however it is unclear whether this represents a primary or progression-associated event [1]. Virtually all cases of extranodal NK/T-cell lymphomas are positive for EBV, which is usually present in a clonal episomal form [1, 6]. It is important that the diagnosis of extranodal NK/T-cell lymphoma not be made in the absence of cytotoxic molecule expression or EBV positivity.
Nasal NK/T-cell lymphoma have a variable prognosis, with some patients responding well to therapy and others dying of disseminated disease despite aggressive therapy. Nasal-type NK/T-cell lymphoma occurring outside the nasal cavity is highly aggressive, with short survival times and poor response to therapy. Expression of multi-drug resistance genes may contribute to chemotherapy resistance in a majority of cases [1].
Acknowledgments
We thank Dr. Yusuf F Suleman (BDS) a registrar in the Division of Maxillofacial and Oral Surgery, University of the Witwatersrand, Johannesburg, South Africa, for his contribution of the clinical case. We also thank Dr. Salim Kaskar [BSc, BChD, MChD (Ortho)] and Ms Amina Kaskar for their help with formatting of the electronic images.
References
- 1.Chan JKC, Jaffe ES, Ralfkiaer E. Extranodal NK/T-cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO classification of tumours. Pathology & Genetics. Tumours of haematopoietic and lymphoid tissues. Lyon France: IARC Press; 2001. pp. 204–7. [Google Scholar]
- 2.The Non-Hodgkin’s Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909–18. [PubMed] [Google Scholar]
- 3.Rodriguez-Abreu D, Filho VB, Zucca E. Peripheral T-cell lymphomas, unspecified (or not otherwise specified): a review. Hematol Oncol. 2008;26:8–20. doi: 10.1002/hon.836. [DOI] [PubMed] [Google Scholar]
- 4.Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin’s lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype and treatment outcome in 113 patients. J Clin Oncol. 1998;16:70–7. doi: 10.1200/JCO.1998.16.1.70. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki R, Takeuchi K, Oshima K, Nakamura S. Extranodal NK/T-cell lymphoma: diagnosis and treatment cues. Hemtol Oncol. 2008;26:66–72. doi: 10.1002/hon.847. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi E, Asano N, Li C, et al. Nodal T/NK-cell lymphoma of nasal type: a clinicopathological study of 6 cases. Histopathology. 2008;52:585–96. doi: 10.1111/j.1365-2559.2008.02997.x. [DOI] [PubMed] [Google Scholar]