Abstract
Calcifying fibrous pseudotumor (CFT) is a rare distinct soft-tissue lesion characterized histologically by lymphoplasmacytic aggregates in a rich collagenized background with abundant psammomatous and dystrophic calcifications. Occurring most often in children and young adults, CFTs are clinically benign lesions that can form over a broad anatomic distribution, including in subcutaneous and deep soft tissues, as well as in serosal and visceral locations. The cause and mechanisms of pathogenesis of CFT are unknown. Simple excision with a margin of normal tissue is the treatment of choice. The risk for local recurrence is low. In this article, we describe a case of CFT in a 29-year-old woman with a 7-cm mass on the right upper gingiva and hard palate, discuss the differential diagnosis with other oral spindle cell lesions, such as, desmoid fibromatosis, nodular fasciitis, inflammatory myofibroblastic tumors, solitary fibrous tumor and also review the recent literature on this rare benign entity.
Keywords: Calcifying, Fibrous, Tumor, Pseudotumor, Oral
Introduction
Calcifying fibrous pseudotumor (CFT) is a rare lesion histologically characterized by abundant hyalinized collagen tissue with focal lymphoplasmacytic infiltrate and psammomatous and dystrophic calcifications [1–3]. This tumor was recognized first in peripheral axial soft tissues [1]. In the head-and-neck region, the most common location is the neck [2–4]. We report here the second known case of an intraoral CFT, review what is known about CFTs, and discuss the other oral clinicopathologic entities with which these tumors may be confused.
Case Report
Clinical History
An obese 29-year-old woman was referred to St. Joseph Medical Center by her primary-care physician for an enlarging, painless oral mass. The patient had no history of trauma or surgery to the oral cavity and no systemic symptoms. On physical examination, the mass was noted to be 6 × 4 × 2 cm, firm, nontender, and involving the right upper gingiva and hard palate.
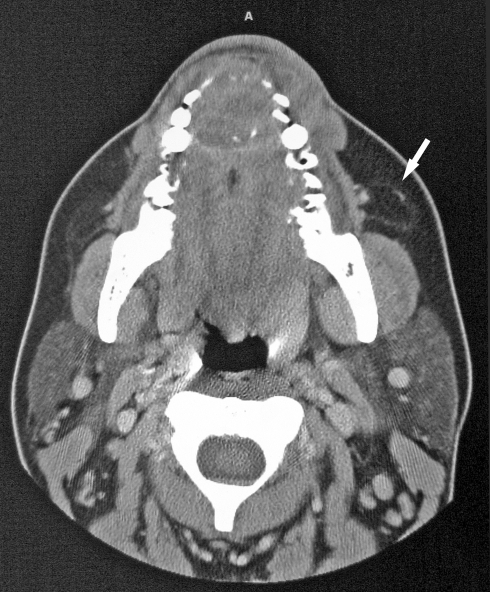
The patient underwent computed tomography (CT) of the face and sinuses, which showed a mass infiltrating the soft tissues overlying the anterior maxilla with direct extension into the anterior alveolar ridge, involving the central incisor and to a lesser degree the canines, particularly on the left side (Fig. 1). The mass did not involve the palate.
Fig. 1.
Computed tomographic scan of a CFT involving the soft tissue of the anterior alveolar ridge
The patient was taken to the operating room, and the tumor was excised. It was very difficult to resect from the surrounding structures. Through frozen-section analysis at the time of surgery, the tumor was diagnosed as a benign fibrous neoplasm.
The patient recovered uneventfully and was discharged from the hospital 1 day after surgery. The patient is free of disease 7 months after treatment.
Pathologic Findings
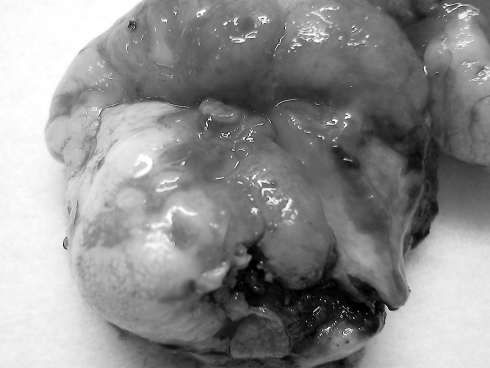
Grossly, the lesion was a solid, gray-white, lobulated mass measuring 7 × 5 × 2.5 cm with white, firm, gritty cut surfaces (Fig. 2). Microscopically, it was composed of a hyalinized fibrosclerotic tissue with calcifications that were psammomatous and dystrophic (Fig. 3). Within the tissue were inflammatory aggregates composed of lymphocytes and plasma cells (Fig. 4). Neither increased mitotic activity nor atypia were present. The lesion was uniformly hypocellular and well circumscribed but not encapsulated.
Fig. 2.
Gross appearance of the oral lesion
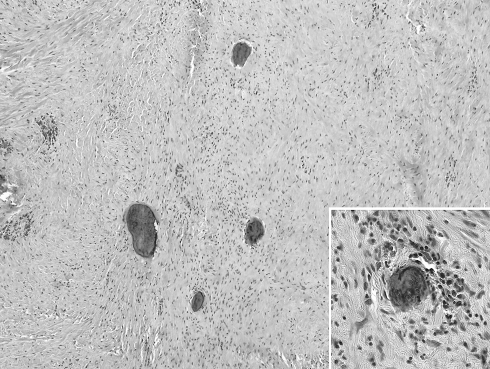
Fig. 3.
Dystrophic and psammomatous calcifications in a sclerotic background characteristic of CFT. Inset; high power view of a psammomatous body
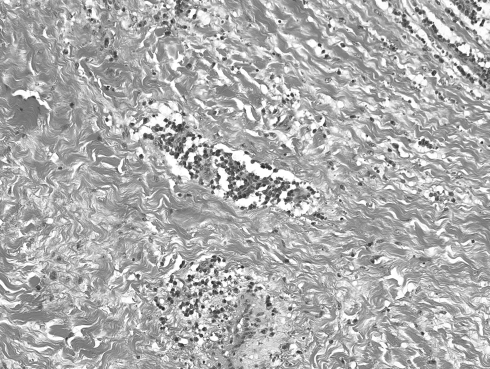
Fig. 4.
Single or aggregated plasma cells and lymphocytes scattered throughout the tumor
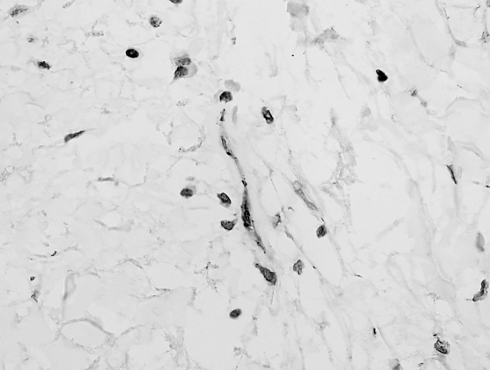
Immunostaining showed strong diffuse positivity for vimentin and focal nuclear for β-catenin, (Fig. 5) rare positive cells for smooth-muscle actin, and negativity for activin receptor-like kinase-1 (ALK-1), caldesmon, CD34, CD68, CD99, S-100 protein, and pancytokeratin.
Fig. 5.
Focal nuclear immunoreactivity for beta-catenin in the spindle cells
Discussion
CFT was originally described by Rosenthal and Abdul-Karim [1] in 1988; they called the lesion childhood fibrous tumor(s) with psammoma bodies. In 1993, Fetsch et al. [2] renamed the entity calcifying fibrous pseudotumor, emphasizing three important features: (1) the lesion is not limited to the pediatric population, (2) the calcifications are not always psammomatous, and (3) in the authors’ opinion, the tumor is the result of a reactive fibroinflammatory process. Nascimento et al. [3] have suggested that the designation pseudotumor been supplanted by tumor in recognition of the entity’s presumptive neoplastic characteristics. However, there is need of additional research to definitively answer this question.
A review of the largest series and recently published cases of CFT [2–10] yields the following information. Both sexes are equally affected, and although the age distribution ranges from 1 to 65 years, children and young adults are affected most often. Tumor diameters have ranged from 0.6 to 15 cm, and the tumors have been present from 2 months to 10 years at diagnosis. A wide range of anatomic locations, including the extremities, trunk, neck, mesenterium, mediastinum, and paratesticular area, has been observed. Recently, the tumor has been reported to involve the soft palate [5], gastrointestinal tract [6], lung, pleura, and myocardium [7–10]. Rarely, CFT may occur as multiple lesions. [8] Malignant transformation has not been reported. Simple excision with a margin of normal tissue has been curative. Recurrence has been noted in only four instances, and in these cases, the authors speculated that incomplete excision had occurred [2, 3]; however, follow-up data have been limited.
Immunohistochemical staining of CFT has been reported, but such analysis is not necessary for diagnosis [3]. The spindle cells express vimentin [3, 10, 11], α-smooth-muscle actin [3, 10], desmin [3], factor VIIIa [12], and CD34 [3]. Staining for cytokeratin CAM 5.2, epithelial membrane antigen, S-100 protein, ALK-1 neurofilaments, and CD31 have been negative [3, 10–12]. In the present case beta-catenin was focally expressed in the nuclei of the neoplastic cells. Nuclear positivity is not exclusive of desmoid fibromatosis, is also express in low-grade myofibroblastic sarcoma, solitary fibrous tumors, infantile fibrosarcoma, desmoplastic fibroblastomas and in gastrointestinal stromal tumors [13].
The cause and mechanisms of pathogenesis of CFT are unclear. It has been postulated that CFT may be a sclerosing end stage of inflammatory myofibroblastic tumor (IMT), based on the characteristics of two cases, one involving multiple lesions that had combined histologic features of both entities [14] and another in which CFT and IMT occurred synchronously [15]. However, Sigel et al. [16] and Nascimento et al. [3] also noted that ALK positivity and chromosome 2p22–24 abnormalities, which are often observed in IMT, are absent in CFT. Furthermore, there are distinct electron microscopic differences between these two entities [12] (Table 1). Trauma has not been postulated as cause of CFT, only in the case reported by Zamecnik et al. [17], there was a history of antecedent trauma.
Table 1.
Comparison of Inflammatory myofibroblastic tumor and calcifying fibrous pseudotumor
| Characteristics | IMF | CFT |
|---|---|---|
| Age of patients | Children | Adolescents |
| Young adults | Young adults | |
| Sex M:F ratio | 1:1.3 | 1:1 |
| Predominant sites | Lung | Soft tissues. |
| Intra-abdominal | Subserosal | |
| Duration of symptoms | Short | Long |
| Systemic symptoms | Sometimes | No |
| Laboratory abnormalities | Yes | No |
| Recurrences | Sometimes | Rarely |
| Metastasis | Rarely | Never |
| Histology | ||
| Architecture | Multi-patterned | Uniform |
| Myofibroblasts | Yes | Yes |
| Hyalinization | Yes | Yes |
| Cell appearance | Florid and bland | Bland |
| Stromal calcifications | Occasional | Always |
| Inflammatory infiltrate | Polymorphous | Lymphoplasmacytic |
| E.M appearance of fibroblasts | Activated | Immature |
| Immunohistochemistry | ||
| ALK-1 expression | Common | Rarely and focal |
| Factor VIII | Intense | Focal |
| SMA expression | Strong diffuse | Occasional and focal |
| Chromosome rearrangements | Yes (2p22–24) | Unknown |
The histologic features of CFTs are reasonably distinct and usually allow easy recognition from other reactive or benign neoplastic lesions that one might consider in the differential diagnosis. The most common are IMT, [18] (Table 1), desmoid fibromatosis, [19] nodular fasciitis, [20] and solitary fibrous tumor [21] (Table 2). Other oral lesions that should be distinguished from CFT are: desmoplastic fibroblastoma [22], true fibroma [23], giant cell fibroma, [24] irritation fibroma [25] and amyloidoma [26] (Table 2).
Table 2.
Differential diagnosis of calcifying fibrous pseudotumor
| Calcifying fibrous pseudotumor | Table 1 |
| Inflammatory myofibroblastic tumor | Table 1 |
| Desmoid fibromatosis | Prominent myofibroblastic elements arranged in long fascicles Invasive character Rare lymphoid infiltrates Infrequent calcifications Express beta-catenin |
| Nodular fasciitis | Abundant myofibroblastic component with mitotic activity Myxoid matrix “tissue culture” appearance Hyalinized stroma in long-standing Rare or absent calcifications |
| Solitary fibrous tumor | Patternless arrangement of spindle cells. Hypo and hypercellular areas within a collagenous background Rare calcifications Express CD34, Bcl-2 |
| Desmoplastic fibroblastoma | Stellate fibroblasts. Myxocollagenous stroma No calcifications |
| True oral fibroma | Sharp margins, well demarcated from adjacent tissue. Difference in character of the collagen fibers between the lesion and surrounding tissue Calcifications not a feature |
| Giant cell fibroma | Large stellate cells and multinucleated giant cells embedded in a loosely collagenous stroma Rare calcifications |
| Irritation fibroma | Most common fibrous oral lesion Excessive collagen fibers production. Rare calcifications |
| Amyloidoma | Homogenous waxy quality of amyloid. Giant cell reaction Congo-red positivity |
To the best of our knowledge, there are no published reports of the cytogenetic characterization of CFT. Hoffman et al. [4] attempted fluorescent in situ hybridization in archival paraffin-embedded tissue to detect trisomy 7 and trisomy 8 because these karyotypes have been reported in other benign fibrous tumors [27], but the signal intensity was too low for the study to be completed. Further fluorescent in situ hybridization studies were not attempted. One tumor was reported to be diploid after flow cytometry [11]. Future cytogenetic studies would be helpful for ascertaining whether CFT is better classified as a neoplasm or as a reactive process.
Awareness of oral CFT and its distinctive morphologic features is important in averting the diagnostic pitfalls caused by its gross and microscopic similarities to other oral spindle cell tumors.
References
- 1.Rosenthal NS, Abdul-Karim FW. Childhood fibrous tumor with psammoma bodies. Arch Pathol Lab Med. 1988;112:798–800. [PubMed] [Google Scholar]
- 2.Fetsch JF, Montgomery EA, Meis JM. Calcifying fibrous pseudotumor. Am J Surg Pathol. 1993;17:502–8. doi: 10.1097/00000478-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Nascimento AF, Ruiz R, Hornick JL, et al. Calcifying fibrous pseudotumor: clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol. 2002;10:189–96. doi: 10.1177/106689690201000304. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman H, Beaver ME, Maillard AAJ. Calcifying fibrous pseudotumor of the neck. Arch Pathol Lab Med. 2000;124:435–7. doi: 10.5858/2000-124-0435-CFPOTN. [DOI] [PubMed] [Google Scholar]
- 5.Mardi K, Sharma J. Calcifying fibrous pseudotumor of the soft palate—a case report. Indian J Pathol Microbiol. 2006;49:394–5. [PubMed] [Google Scholar]
- 6.Emanuel P, Qin L, Harpaz N. Calcifying fibrous tumor of small intestine. Ann Diagn Pathol. 2008;12:138–41. doi: 10.1016/j.anndiagpath.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Soyer T, Ciftci AO, Gucer S, et al. Calcifying fibrous pseudotumor of lung: a previously unreported entity. J Pediatr Surg. 2004;39:1729–30. doi: 10.1016/j.jpedsurg.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Shibata K, Yuki D, Sakata K. Multiple calcifying fibrous pseudotumors disseminated in pleura. Ann Thorac Surg. 2008;85:e3–5. doi: 10.1016/j.athoracsur.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 9.Kirby PA, Sato Y, Tannous R, et al. Calcifying fibrous pseudotumor of the myocardium. Pediatr Dev Pathol. 2006;9:384–7. doi: 10.2350/06-01-0022.1. [DOI] [PubMed] [Google Scholar]
- 10.Dumont P, Muret A, Skrobala D, et al. Calcifying fibrous pseudotumor of the mediastinum. Ann Thorac Surg. 1997;63:543–4. doi: 10.1016/S0003-4975(96)01022-3. [DOI] [PubMed] [Google Scholar]
- 11.Fukunaga M, Kikuchi Y, Endo Y, et al. Calcifying fibrous pseudotumor. Pathol Int. 1997;47:60–3. doi: 10.1111/j.1440-1827.1997.tb04435.x. [DOI] [PubMed] [Google Scholar]
- 12.Hill KA, Gonzalez-Crussi F, Chou PM. Calcifying fibrous pseudotumor versus inflammatory myofibroblastic tumor: a histological and immunohistochemical comparison. Mod Pathol. 2001;14:784–90. doi: 10.1038/modpathol.3880390. [DOI] [PubMed] [Google Scholar]
- 13.Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cell lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509–14. doi: 10.1111/j.1365-2559.2007.02794.x. [DOI] [PubMed] [Google Scholar]
- 14.Dorpe J, Ectors N, Geboes K, et al. Is calcifying fibrous pseudotumor a late sclerosing stage of inflammatory myofibroblastic tumor? Am J Surg Pathol. 1999;23:329–35. doi: 10.1097/00000478-199903000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Pomplun S, Goldstraw P, Davies SE, et al. Calcifying fibrous pseudotumour within an inflammatory pseudotumor: evidence of progression from one lesion to the other? Histopathology. 2000;37:380–2. doi: 10.1046/j.1365-2559.2000.00997-1.x. [DOI] [PubMed] [Google Scholar]
- 16.Sigel JE, Smith TA, Reith JD, et al. Immunohistochemical analysis of anaplastic lymphoma kinase expression in deep soft tissue calcifying fibrous pseudotumor: evidence of a late sclerotic stage of inflammatory myofibroblastic tumor? Ann Diagn Pathol. 2001;5:10–4. doi: 10.1053/adpa.2001.21474. [DOI] [PubMed] [Google Scholar]
- 17.Zamecnik M, Dorociak F, Vesely I. Calcifying fibrous pseudotumor after trauma. Pathol Int. 1997;47:812. doi: 10.1111/j.1440-1827.1997.tb04464.x. [DOI] [PubMed] [Google Scholar]
- 18.Johann AC, Caldeira PC, Abdo EN, et al. Inflammatory myofibroblastic tumor of the alveolar mucosa of the mandible. Minerva Stomatol. 2008;57:59–63. [PubMed] [Google Scholar]
- 19.Gonzalez-Garcia R, Escorial-Hernandez V, Munoz-Guerra MF, et al. Fibromatosis of the oral tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:e31–4. doi: 10.1016/j.tripleo.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 20.Dayan D, Nasrallah V, Vered M. Clinico-pathologic correlation of myofibroblastic tumors of the oral cavity: 1. Nodular fasciitis. J Oral Pathol Med. 2005;34:426–35. doi: 10.1111/j.1600-0714.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 21.Fusconi M, Ciofalo A, Greco A, et al. Solitary fibrous tumor of the oral cavity: case report and pathologic considerations. J Oral Maxillofac Surg. 2008;66:530–4. doi: 10.1016/j.joms.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Shimoyama T, Horie N, Ide F. Collagenous fibroma (desmoplastic fibroblastoma): a new case originating in the palate. Dentomaxillofac Radiol. 2005;34:117–9. doi: 10.1259/dmfr/22428083. [DOI] [PubMed] [Google Scholar]
- 23.Christopoulos P, Skalovounou A, Patrikiou A. True fibroma of the oral mucosa: a case report. Int J Oral Maxillofac Surg. 1994;23:98–9. doi: 10.1016/S0901-5027(05)80601-0. [DOI] [PubMed] [Google Scholar]
- 24.Takeda Y, Kaneko R, Suzuki A, et al. Giant cell fibroma of the oral mucosa. Report of a case with ultrastructural study. Acta Pathol Jpn. 1986;36:1571–6. doi: 10.1111/j.1440-1827.1986.tb02828.x. [DOI] [PubMed] [Google Scholar]
- 25.Toida M, Murakami T, Kato K, et al. Irritation fibroma of the oral mucosa: a clinicopathological study of 129 lesions in 124 cases. Oral Med Pathol. 2001;6:91–4. doi: 10.3353/omp.6.91. [DOI] [Google Scholar]
- 26.Basak PY, Ergin S, Sezer MT, et al. Amyloidosis of the tongue with kappa light chain disease. Australas J Dermatol. 2001;42:55–7. doi: 10.1046/j.1440-0960.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson HL, Hawkin EP, Cooley LD. Infant cardiac fibroma with clonal t(1;9)(q32-;q22) and review of benign fibrous tissue cytogenetics. Cancer Genet Cytogenet. 1996;87:34–7. doi: 10.1016/0165-4608(95)00264-2. [DOI] [PubMed] [Google Scholar]