Clinical Presentation
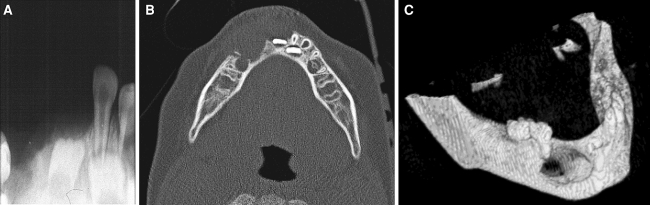
A 1.5-year-old boy got a blow to his mandibular anterior teeth. Less than a month later, there was a 2 × 2 cm2 bluish-brownish soft mass in the vestibule extending between the deciduous canines. Central incisors were mobile. Radiographically, there was a midline lytic lesion with periosteal elevation. The lesion was curetted and the right central incisor extracted. During the next year there were three episodes of recurrence, for which the child was re-treated by curettage. The third recurrence appeared clinically as a secreting fistula and soft tissue swelling adjacent to the right mandibular first deciduous molar. The swelling did not respond to antibiotic treatment. The periapical X-ray, axial CT scan, and 3-D mandibular reconstruction images from this recurrence are illustrated in Fig. 1a, b, c, respectively.
Fig. 1.
(a) Periapical X-ray from the third recurrence, (b) axial view, and (c) 3D-CT scan reconstruction
Differential Diagnosis
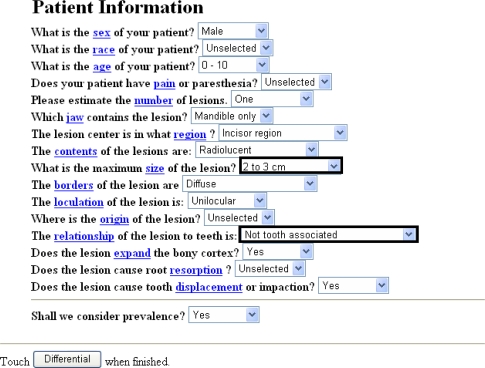
The tool for initiating the analysis of the differential diagnosis of this case was a computerized program of a 16-multiple choice questionnaire on the patient clinical data available at www.orad.org (Fig. 2). Four different datasets were produced by alternating the item “what is the maximum size of the lesion?” to either “2–3 cm” or “<2 cm” combined with two choices of the item of “the relationship of the lesion to teeth is: either “Not tooth associated” or “Missing tooth associated”. This program also yielded the relative frequency of the differential diagnoses for each set of data. A total of 12 entities were submitted by the program as possible different diagnoses for all the four supplied data combinations, out of which seven were more likely and five less likely differential diagnoses. Table 1 summarizes the relative frequency of the entities included in the differential diagnoses according to each set of data and Table 2 summarizes these entities in terms of compatibility with the known clinical data and radiological findings of the present case. Accordingly, our leading entities were Langerhans cell disease and central giant cell granuloma (CGCG), followed by keratocystic odontogenic tumor.
Fig. 2.
The form of the computerized program at www.orad.org provided with the patient’s data. Items emphasized by a bold frame were changed to alternative options
Table 1.
Differential diagnoses for the four data sets as submitted by the computerized program and their relative frequency (%)
| Differential diagnosis | Dataset Ia (%) | Dataset IIb (%) | Dataset IIIc (%) | Dataset IVd (%) |
|---|---|---|---|---|
| Central giant cell granuloma | 36 | 31 | 41 | 37 |
| Keratocystic odontogenic tumor | 26 | 44 | 20 | 35 |
| Langerhans cell disease (LCD) | 10 | 2 | 11 | 2 |
| Osteosarcoma | 5 | 5 | 4 | 5 |
| Burkitt’s lymphoma | 4 | 7 | 4 | 9 |
| Adenomatoid odontogenic tumor | 3 | 6 | 1 | 3 |
| Odontogenic fibroma | 3 | 1 | 4 | 1 |
| Arteriovenous malformation* | 3 | – | – | 2 |
| Central sqamous cell carcinoma* | 1 | – | – | – |
| Hemophilic pseudotumor* | – | 1 | 2 | 3 |
| Malignant fibrous hystiocytoma* | – | – | 1 | – |
| Metastatic tumor* | – | – | – | 1 |
aLesion size: 2–3 cm; relationship of lesion to teeth: “not tooth associated”
bLesion size: 2–3 cm; relationship of lesion to teeth: “missing tooth associated”
cLesion size: <2 cm; relationship of lesion to teeth: “not tooth associated”
dLesion size: <2 cm; relationship of lesion to teeth: “missing tooth associated”
* Entities that are the least likely to be compatible with the present case and therefore will not be further elaborated
Table 2.
Entities included in the differential diagnosis (DD)/compatibility of features with the clinical data or radiological findings of the case
| DD | Age | Gender | Jaw location | Clinical presentation | Radiographic appearance | General consideration | Probability for correct diagnosis |
|---|---|---|---|---|---|---|---|
| Adenomatoid odontogenic tumor | 3–82 years; +65% in 2nd decade/+− | M:F = 1:2/− | Maxilla > mandible/− | Slow growing, asymptomatic, associated with unerupted teeth/− | Well defined unilocular radiolucency/− | Excision usually curative; rare recurrence/− | Unlikely |
| Odontogenic fibroma | Mean 40 years; range 11–66 years/− | M:F = 1:2.8/− | Mandible:maxilla = 6.5 :1/+ | Asymptomatic, jaw expansion; loosening of teeth/+ | Well defined unilocular radiolucency/− | Excision—the treatment of choice; rare recurrence/− | Unlikely |
| Keratocystic odontogenic tumor | Peak 20–30 years; range 1st–9th decade/+ | M > F/+ | Mandible (60%–80%), posterior region/+− | Slow growing, minimal cortical expansion/− | Unilocular or multilocular radiolucency/+− | Curettage increases chances of recurrence/+ | Less likely |
| Langerhans cell disease | 50% of cases <10 years/+ | Definite male predilection/+ | More common in posterior mandible/− | “floating teeth” & mucosal involvement, as a proliferative gingival mass; common tenderness and pain/+− | Punched out or ill-defined unilocular radiolucency/−+ | Recurrence common/+ | Likely |
| Central giant cell granuloma | Peak 10–20 years, range 2–80 years/+ | Males (1st decade), females (after 1st decade)/+ | Occasionally crosses midline/+ | Usually asymptomatic; cortical perforation and extension into soft tissue when aggressive/+ | Unilocular or multilocular radiolucency; non-corticated margins/+ | Clinical details mostly in conformity with this lesion, except for the fistula | Most likely |
| Osteogenic sarcoma | Mean 33 years/− | Male predilection/+ | No definite jaw predilection | Swelling; loosening of teeth, pain, paresthesia/+− | Varies: radiolucency, mixed sclerotic and radiolucent or completely sclerotic/−+ | Rapid clinical course; curettage possibly lead to exacerbation/+− | Unlikely |
| Burkitt’s lymphoma | Peak 7 years/− | M:F = 2–4:1/+ | Maxilla:mandible = 2:1/− | Extensive alveolar bone destruction; rapidly growing; pain and paresthesia with/without systemic symptoms/− | Radiolucency with poorly defined margins/+− | Very rapid clinical course; curettage unlikely to achieve control | Unlikely |
+: compatible; +−: partly compatible; −: incompatible
Several aspects were addressed for further supporting the selected most probable differential diagnoses. With regard to the nature of the lesion, a benign rather than a malignant lesion was favored, as no symptoms were reported and the child was in general good health. A malignant lesion in a child would be expected to be associated with deterioration in the patient health status. In addition, as the lesion was conservatively treated several times it seemed to go well with a benign but locally aggressive lesion. The repeated curettage procedures were aimed to avoid extensive destruction of the jawbone and tooth structures of the child.
In respect to the etiology of the lesion, trauma, in general, may have led to teeth fracture, mucosal or skin damage and none of them were mentioned in the clinical details. Trauma is unlikely to be the causative agent in cases of true neoplastic lesions (e.g., keratocystic odontogenic tumor or Langerhans cell disease), but hemangioma and CGCG may be caused by trauma. An infectious agent (e.g., bacterial or fungal) may be associated with pain or tenderness of the teeth, which were not mentioned and, therefore, infection is most likely not the primary etiologic cause in this case. Regarding the origin of the lesion, peripheral or central (jawbones), we assumed that an aggressive infectious process, such as actinomycosis would have caused a “wooden” hard indurated area of fibrosis which would be bluish-brownish in color, thus infection, again, did not seem to be compatible with this lesion. Central hemangioma, Langerhans cell disease or CGCG may all originate from the bone and end up having bluish–brown soft tissue swellings. In addition, the periosteal elevation reported in the original manifestation of the lesion, would most likely support a central origin.
In relation to the submitted X-rays, we identified tooth germs of the left central and lateral incisors but not those of the right incisors. We assumed that the latter may have failed to develop due to the presence of an odontogenic tumor (e.g., keratocystic odontogenic tumor). Another possibility is that the missing tooth germs were lost during the repeated curettage procedures of the aggressive lesion (Langerhans cell disease or CGCG). On the 3D-CT scan, the right first deciduous molar appeared to be periodontally involved and this might be the explanation for the presence of the fistula. It was not clear why the fistula did not respond well to antibiotics, unless it represented a secondary infection (osteomyelitis?) of the primary neoplasm.
In summary, our final entities to enter the differential diagnosis were CGCG and Langerhans cell histiocytosis, probably associated with secondary infection (osteomyelitis).
Diagnosis and Discussion
“Atypical” Aggressive Central Giant Cell Granuloma
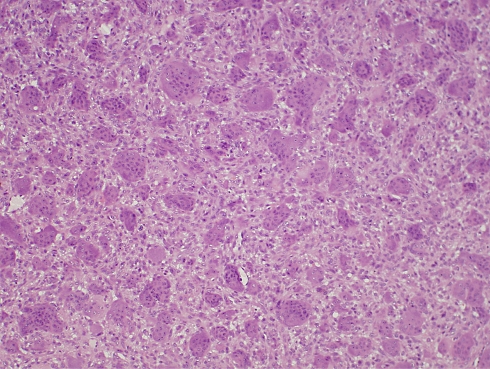
The tissue from the original lesion was submitted to histopathological evaluation and was diagnosed as CGCG (Fig. 3). Repetitive microscopic evaluation of the recurrent lesions re-confirmed this diagnosis.
Fig. 3.
Histopathologic diagnosis is consistent with central giant cell granuloma
The present case has been unfolding during a period of 5 years and is still ongoing.
During this period of time the patient experienced 10 episodes of recurrence in the mandible. As these episodes progressed, the lesions showed a tendency to develop in the body of the mandible and ascending ramus, distal to the site of initial occurrence in the midline of the mandible. In addition, concurrent with the 8th recurrence in the mandible, a focus of CGCG developed in the anterior maxilla. So far, only one episode of recurrence was reported in regard to the latter.
The most common causes for persistent/recurrent and multiple CGCG include hyperparathyroidism, cherubism, and Noonan syndrome and Noonan-like/Multiple giant cell lesion syndrome.
Primary hyperparathyroidism with uncontrolled production of the parathyroid hormone (PTH) is mainly associated with parathyroid adenoma (80–90%), less with parathyroid hyperplasia (10–15%) and only rarely with parathyroid carcinoma (~0.5%) [1]. In general, hyperparathyroidism is rare in pediatric patients, as is the case with our patient. Furthermore, repeated laboratory tests revealed that the PTH, Ca+2, and phosphorus levels were within normal limits, ruling out a diagnosis of hyperparathyroidism, at least in its overt state.
Cherubism is classically manifested as bi-lateral lesions in the posterior jawbones [2]. This long-time known observation has been recently explained on the basis of a spatio–temporal association between cherubism and the failure of development of the second and third permanent mandibular molars [3]. The histopathological findings in cherubism are essentially similar but not entirely identical to those seen in CGCG, the main difference lies in the presence of perivascular eosinophilic cuff-like deposits in the former. Neither clinical nor histopathological findings in this case supported a diagnosis of cherubism.
Noonan syndrome, originally described by Cohen and Gorlin [4], includes clinical features such as short stature, low intelligence or developmental delay, giant cell lesions of bones, joints and/or soft tissues, etc., and is associated with a mutation in the PTPN11 gene. As the patient in the present case did not carry a mutation in the PTPN11 gene, this diagnosis was also ruled out.
The treatment approach in the present case was originally based on surgical procedures constrained to curettage and extraction of involved deciduous teeth and tooth germs of permanent teeth in order to keep the functional and esthetic damage to a minimum—a critical necessity in such a young patient. For the same purpose, the surgical strategy was occasionally consolidated with pharmacological agents including calcitonin, interferon α-2a (INFα-2a) and corticosteroids.
The patient was on calcitonin treatment (injections) for as long as 12 months, but eventually developed recurrent lesions. One explanation for the absence of response to calcitonin may be the fact that the lesional cells were found to be constantly negative for calcitonin receptors as shown on several occasions by means of immunohistochemistry.
As CGCG is assumed, by some investigators, to be a vascular tumor [5], within 72 h after performing curettage for one of the recurrences, the patient started daily subcutaneous injections with INFα-2a. Retrospectively, we assessed angiogenesis within the lesional tissue by examining the frequency of newly formed blood vessels, using antibodies to vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). As the tumor contained only a few small stained blood vessels, indicating a relatively minimal process of angiogenesis, the effectiveness of an anti-angiogenic agent in this case do not seem to be of a great value, unless it has additional mechanisms of action, for example on the lesional cells [6]. Unfortunately, after a 5-month period of treatment with INFα-2a, a new recurrence developed. In addition, this treatment was ceased due to emergence of side effects.
In addition, the patient was treated with intralesional injections of corticosteroids [2]. This was supported by immunoreactivity of the lesional cells (both mononuclear and multinuclear giant) for corticosteroid receptors [7]. Injections were given over a period of 15 months, during which there were two episodes of recurrence in the mandible and a new focus in the maxilla. Based on the limited experience from the literature that combining steroids and calcitonin may have a synergistic effect [8], the patient was given one course of intralesional corticosteroids combined with calcitonin nasal spray, however, this did not prevent, yet, another recurrence.
Cases of CGCG with several recurrences and concomitant ipsilateral/contra-lateral multiple lesions in the absence of a systemic condition have been reported [9–11], however, they were not as complex and non-responsive as the present case. In order to emphasize the exceptional biologic behavior of our case, we chose to use the terms “atypical” and “aggressive” for the submitted diagnosis.
In regard to the possible biological mechanisms that could explain, at least in part, the evolvement of such a persistent lesion of CGCG in the absence of a systemic condition, we suggest that post-traumatic inflammatory reaction could serve as a trigger to a disturbance in the control of the osteoblast–osteoclast activities essential for the normal tooth development and eruption that naturally take place in this young patient. This suggestion is mainly based on the temporal proximity between the trauma to the mandible and the appearance of the lesion at the site of the trauma [12, 13]. Alternatively, it could be proposed that this is a case of occult hyperparathyroidism that would eventually develop into an overt state [14, 15]; or else, there is the possibility that this case might represent an abnormally amplified responsiveness of the local osteoclasts involved in tooth eruption, to normal levels of PTH. The progression of the lesion along the mandible to a posterior location compared to the point of origin in the midline and the expansion of the lesion to the ipsilateral jawbone, may be explained in terms of tumor spill and hematogeneous spread, respectively [9, 14].
This challenging case “consumed” all the armamentarium of the commonly used pharmacological agents for aggressive CGCG lesions. For exceptional cases such as the present one, new pharmacologic strategies should be considered. It is important to emphasize that the administration of a pharmacological agent should no longer be done empirically, but rather be supported by immunohistochemical evidence for the presence of the target cell/epitope, against which it is supposed to act.
Acknowledgments
The presenter would like to remark the Departments of Oral and Maxillofacial Surgery and Pediatric Hemato-Oncology, at the Chaim Sheba Medical Center, Tel Hashomer, Israel, for the dedicated treatment and follow-up of the patient.
References
- 1.Salen PN. Hyperparathyroidism. http://www.emedicine.com/emerg/TOPIC265.HTM.
- 2.Lange J, Akker P, Berg H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:603–15. doi: 10.1016/j.tripleo.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Hyckel P, Berndt A, Schleier P, Clement JH, Beensen V, Peters H, et al. Cherubism—new hypothesis on pathogenesis and therapeutic consequences. J Craniomaxillofac Surg. 2005;33:61–8. doi: 10.1016/j.jcms.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MM, Gorlin RJ. Noonan-like/multiple giant cell lesion syndrome. Am J Med Genet. 1991;40:159–66. doi: 10.1002/ajmg.1320400208. [DOI] [PubMed] [Google Scholar]
- 5.Kaban LB, Mulliken JB, Ezekowitz RA, Ebb D, Smith PS, Folkman J. Antiangiogenentic therapy of a recurrent giant cell tumor of the mandible with interferon alpha-2a. Pediatrics. 1999;103(6 Pt 1):1145–9. doi: 10.1542/peds.103.6.1145. [DOI] [PubMed] [Google Scholar]
- 6.Vered M, Buchner A, Dayan D. Giant cell granuloma of the jawbones—a proliferative vascular lesion? Immunohistochemical study with vascular endothelial growth factor and basic fibroblast growth factor. J Oral Pathol Med. 2006;35:613–9. doi: 10.1111/j.1600-0714.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 7.Vered M, Buchner A, Dayan D. Immunohistochemical expression of glucocorticoid and calcitonin receptors as a tool for selecting therapeutic approach in central giant cell granuloma of the jawbones. Int J Oral Maxillofac Surg. 2006;35:756–60. doi: 10.1016/j.ijom.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Pinto LP, Cherubinim K, Salum FG, Yurgel LS, Fugueirdo MA. Highly aggressive brown tumor in the jaw associated with tertiary hyperparathyroidism. Pediatr Dent. 2006;28:543–6. [PubMed] [Google Scholar]
- 9.Miloro M, Quinn PD. Synchronous central giant cell lesions of the jaws: report of a case and review of the literature. J Oral Maxillofac Surg. 1995;53:1350–5. doi: 10.1016/0278-2391(95)90600-2. [DOI] [PubMed] [Google Scholar]
- 10.Lange J, Akker P. Clinical and radiological features of central giant-cell lesions of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:464–70. doi: 10.1016/j.tripleo.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Curtis NJ, Walker DM. A case of aggressive multiple metachronous central giant cell granulomas of the jaws: differential diagnosis and management options. Int J Oral Maxillofac Surg. 2005;34:806–8. doi: 10.1016/j.ijom.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Haque AU, Moatasim A. Giant cell tumor of bone: a neoplasm or a reactive condition? Int J Clin Exp Pathol. 2008 (in press). [PMC free article] [PubMed]
- 13.Unal M, Karabacak T, Vayisoglu Y, Bagis HE, Pata YS, Akbas Y. Central giant cell reparative granuloma of the mandible caused by a molar tooth extraction: special reference to the maneuver of drilling the surgical field. Int J Pediatr Otorhinolaryngol. 2006;70:745–8. doi: 10.1016/j.ijporl.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Smith PG, Marrogi AJ, Delfino JJ. Multifocal central giant cell lesions of the maxillofacial skeleton: a case report. J Oral Maxillofac Surg. 1990;48:300–5. doi: 10.1016/0278-2391(90)90398-L. [DOI] [PubMed] [Google Scholar]
- 15.Hariss M. Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg. 1993;31:89–94. doi: 10.1016/0266-4356(93)90168-V. [DOI] [PubMed] [Google Scholar]