Introduction
Parathyroid adenoma is part of a spectrum of parathyroid proliferative disorder that includes parathyroid hyperplasia, parathyroid adenoma, and parathyroid carcinoma. Patients typically present with evidence of primary hyperparathyroidism with elevated serum calcium levels and elevated serum parathyroid hormone levels. Hyperparathyroidism is divided into primary, secondary, and tertiary hyperparathyroidism. Most parathyroid hyperplasia is the result of secondary hyperparathyroidism due to renal disease. Tertiary hyperparathyroidism is the autonomous secretion of parathyroid hormone in the setting of long-standing renal disease resulting in hypercalcemia. Eighty to 85 percent of primary hyperparathyroidism is caused by parathyroid adenoma followed by primary parathyroid hyperplasia (15%) and parathyroid carcinoma (5%).
Patients with primary hyperparathyroidism may present with clinical evidence of elevated serum calcium levels which include non-specific symptoms such as fatigue, pain and weakness as well as polydipsia, polyuria, and nephrolithiasis. Gastrointestinal symptoms include constipation, anorexia, nausea, and vomiting. Extreme hypercalcemia can lead to cardiac arrhythmias, coma and death. These days most patients with hypercalcemia are discovered incidentally on routine work-up for other reasons. Elevated levels of serum calcium are noted on laboratory screening tests. Evaluation consists of radiographic studies and subsequent surgical exploration.
Normal parathyroid glands are too small to be detected on imaging (usually 5 × 3 × 1 mm,), but parathyroid disease typically results in enlargement of the glands allowing for visualization. Sonography and 99mTc-sestamibi scintigraphy are the primary imaging modalities utilized for the visualization of diseased glands. Prolonged and avid uptake of sestamibi is seen on 2 h delayed images within adenomas (Fig. 1). Single photon emission computed tomography (SPECT) increases the sensitivity of localizing enlarged parathyroid glands by scintigraphy over that of planar imaging by allowing identification of an enlarged parathyroid in three dimensions. Scintigraphy is approximately 90% sensitive for localizing a parathyroid adenoma, and can easily demonstrate glands greater than 500 mg. Combined SPECT/CT may even increase lesion localization, but preliminary data are not available to confirm this. Ultrasound imaging demonstrates parathyroid adenomas as typically homogeneously hypoechoic lesions compared with the adjacent thyroid, and they can easily be detected when they are larger than 1 cm. Cystic parathyroid adenomas are rare, and the cystic areas appear as regions of decreased echogenicity within the gland (Fig. 2a and b). Doppler imaging typically demonstrates a characteristic extrathyroidal feeding vessel entering the parathyroid gland at one of the poles.
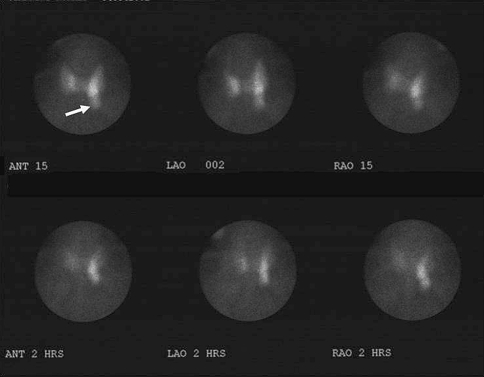
Fig. 1.
Fifty-seven year old man with mild hypercalcemia. 99mTc sestamibi scan demonstrates normal uptake by the thyroid gland with a focal area of pronounced uptake on the left (arrow). Over 2 h the thyroid uptake decreased to better reveal the prominent focal area of increased activity in the region of the lower left lobe of the thyroid consistent with parathyroid adenoma
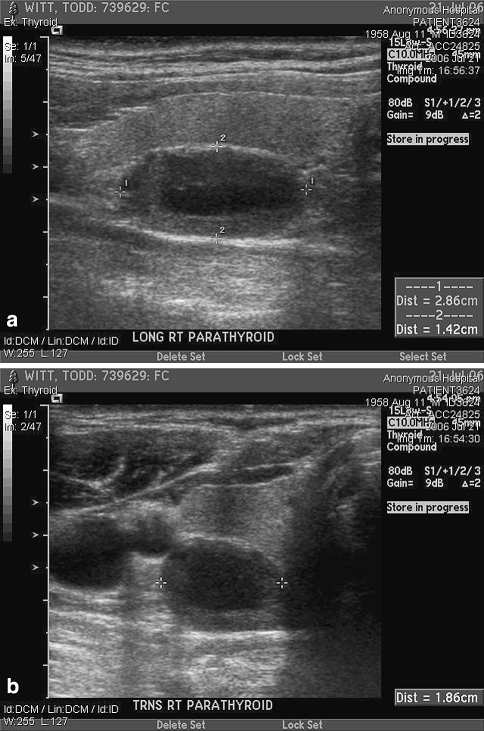
Fig. 2.
Sonographic image in the longitudinal (a) and transverse (b) plane demonstrates a hypoechoic parathyroid adenoma measuring 2.86 × 1.86 × 1.42 cm. Located centrally within the adenoma is an area of decreased echogenicity consistent with cystic degeneration
Contrast enhanced CT and MRI are less commonly used for preoperative localization, but may be of benefit in the setting of failed parathyroidectomy for the localization of ectopic glands. Adenomas demonstrate intense enhancement on thin collimation CT, and variable signal intensity on MRI. However, they are typically intermediate to low signal on T1-weighted MR images, and hyperintense on T2-weighted images. Due to the high T2 signal intensity, the addition of contrast for MR scanning does not significantly increase detection.
Parathyroid hyperplasia typically involves all four glands, i.e. all four glands are enlarged. Occasionally parathyroid hyperplasia may be uneven and one or two glands are more prominent and enlarged than the other glands causing confusion radiographically and on clinical examination. If one gland is significantly larger than the other glands it may be interpreted as parathyroid adenoma rather than hyperplasia and following resection of the presumed “adenoma” the hyperparathyroidism does not resolve.
On resection of parathyroid adenoma the surgeon typically is able to easily identify the single enlarged gland. In the past, sampling of the other glands was required to rule out parathyroid hyperplasia. These days however, with the accuracy of radiographic imaging and intraoperative parathyroid hormone monitoring, the surgeon does not necessarily need to sample tissue from the remaining glands. On gross examination the gland is obviously enlarged and is usually solid. However, as in the case presented here, parathyroid adenoma may rarely be cystic. (Figs. 3a and b and 4). The weight of parathyroid adenomas varies but in general, the mean weight for parathyroid adenoma is approximately 1 g (the weight of a normal parathyroid gland is typically less than 50 mg). Cut surface is typically smooth, soft, and reddish brown in color, distinguished from the yellow-brown color of normal parathyroid tissue. Microscopically, one classically sees a discrete mass separated from a rim of uninvolved parathyroid parenchyma by a thin fibrous capsule. The uninvolved parathyroid tissue should show the fat component of the normal parathyroid and is classically atrophic and compressed. Often this is not the case, however, and the rim of uninvolved parathyroid tissue cannot be identified. Not infrequently the thin fibrous capsule is disrupted and so attenuated that it cannot be identified. Also, the tumor itself may be multinodular and irregular. Microscopically, one expects to see a proliferation of a single cell type which most commonly is the chief cell (Fig. 4, inset) but often times oxyphilic cells are the dominant cell type. Occasionally a mixture of both types of cells can be seen, raising the question of parathyroid hyperplasia. These “out of the ordinary” gross and microscopic features of parathyroid adenoma are challenges that the pathologist may encounter on a regular basis with parathyroid adenoma and each case should be judged independently as to the likelihood of it being a true adenoma or part of parathyroid hyperplasia. Obviously clinical and radiographic correlation will also be helpful in making this determination. Growth patterns vary and may range from solid to pseudo-glandular, follicular, and acinar; occasionally cystic degeneration can be seen (Fig. 5a). There may be eosinophilic secretions within the follicular structures reminiscent of the colloid-filled follicles that can be seen in thyroid tissue (Fig. 5b). Some nuclear pleomorphism can be seen but it is usually focal and seen in clusters. Mitotic figures are inconspicuous (<1/10 hpf) and atypical mitotic figures should not be seen in typical parathyroid adenomas. Delicate fibrovascular bands may be present but dense fibrous bands should not be seen and if present should raise red flags for the possibility of parathyroid carcinoma. Hemorrhage, hemosiderin, inflammatory cells, and fibrosis may be seen as part of the degenerative changes, especially if tumors are large.
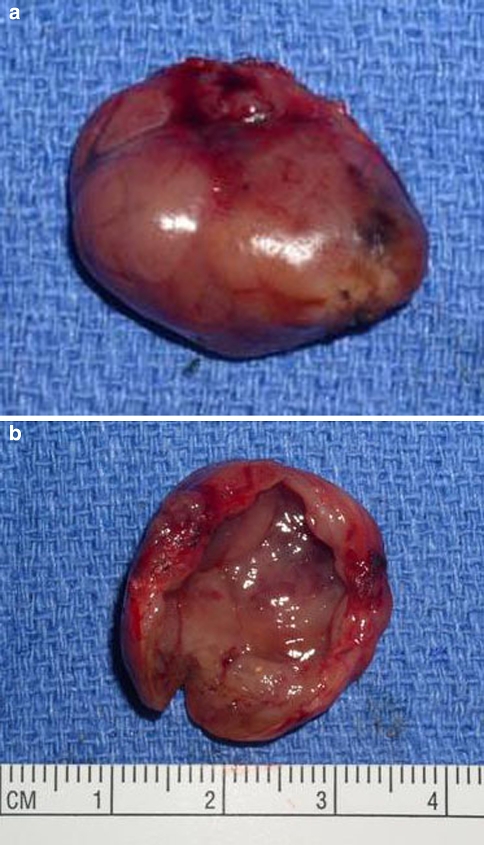
Fig. 3.
Gross specimen of a cystic parathyroid adenoma. (a) Exterior view shows a smooth surface with minimal hemorrhage and no significant adhesions. The specimen “shelled out” easily. (b) The mass is opened to demonstrate a smooth cyst wall lining
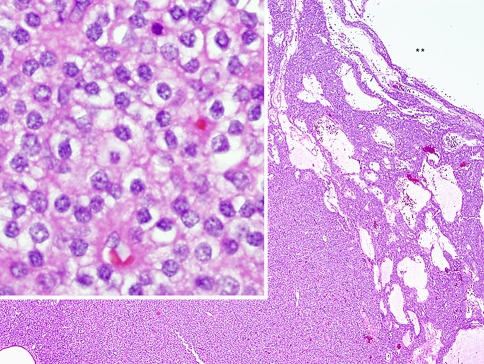
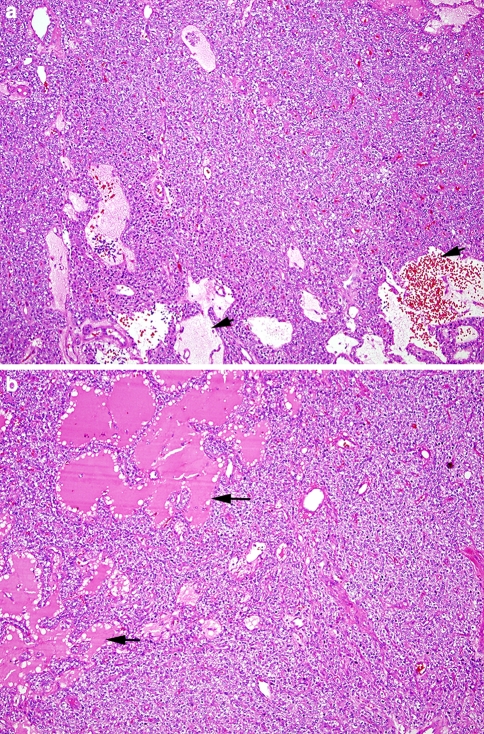
Fig. 4.
The low-power portion of this H&E stained section shows the hypercellular parathyroid adenoma with a cystic lumen indicated by the ** corresponding to the smooth cyst wall in Fig. 3b. High power examination (inset) depicts the proliferative parathyroid tissue to be composed of a population of chief cells. This parathyroid proliferation lacks the normal fat component
Fig. 5.
(a) H&E stained section shows a solid growth pattern interspersed with areas of cystic change (arrows). The cystic spaces are filled with a watery “proteinaceous” fluid as well as red blood cells. (b) A bright, eosinophilic material fills the “follicle-like” spaces (arrows) with peripheral scalloping reminiscent of the colloid-filled follicles seen in thyroid tissue
Immunohistochemical studies are, in general, not necessary for making the diagnosis of parathyroid adenoma. Normal, hyperplasic and neoplastic parathyroid tissue is reactive with antibodies to chromogranin and parathyroid hormone. Staining for parathyroid hormone however is difficult to perform and interpret accurately due to marked diffusion artifact. The one use where immunohistochemical staining may become useful is in differentiating atypical parathyroid adenoma from parathyroid carcinoma. There is a constellation of features one looks for in making the diagnosis of parathyroid carcinoma, including adherence to thyroid tissue, vascular and capsular invasion, extension into soft tissue structures, trabecular growth pattern, dense fibrotic bands, thick fibrous tumor capsule, tumor necrosis, tumor cell spindling, prominent macronucleoli, increased mitotic activity and atypical mitotic figures. There are times when a parathyroid neoplasm will have some histologic features of carcinoma but the full spectrum of features necessary to make the diagnosis is not present. Recent studies have suggested that a panel of immuohistochemical markers including bcl-1, Ki67, p27, and others may help to further distinguish benign vs. malignant parathyroid neoplasm. The most promising reports indicate loss of nuclear expression of parafibromin [a protein encoded by the tumor suppressor gene HRPT2] may indicate a higher likelihood that a parathyroid neoplasm is malignant. Further studies are needed to fully validate these proposals.
Finally, patients with MEN syndromes, most commonly MEN 1, frequently will have parathyroid proliferative disorder as part of their syndrome. Nearly 90% of patients with MEN 1 have parathyroid hyperplasia. Parathyroid adenoma and carcinoma can also be seen as part of these syndromes. While the vast majority of patients with parathyroid proliferative disorder present with sporadic disease, the possibility of an MEN syndrome should always be kept in mind when evaluating these patients.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Bibliography
- Smith JR, Oates ME. Radiolonuclide imaging of parathyroid glands: patterns, pearls, and pitfalls. Radiographics. 2004;24:1101–15. doi: 10.1148/rg.244035718. [DOI] [PubMed] [Google Scholar]
- Clark PB, Perrier ND, Morton KA. Detection of an intrathymic parathyroid adenoma using single-photon emission CT 99mTc sestamibi scintigraphy and CT. AJR Am J Roentgenol. 2005;184(Suppl 3):S16–8. doi: 10.2214/ajr.184.3_supplement.01840s16. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Tublin ME, Ogilvie JB. Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR Am J Roentgenol. 2007;188:1706–15. doi: 10.2214/AJR.06.0938. [DOI] [PubMed] [Google Scholar]
- Thompson LDR. Benign neoplasms of the parathyroid gland. In: Thompson LDR, editor. Endocrine pathology, foundations in diagnostic pathology. Philadelphia: Churchill Livingstone/Elsevier; 2006. pp. 157–164. [Google Scholar]
- Sharma J, Milas M, Berber E, et al. Value of intraoperative parathyroid hormone monitoring. Ann Surg Oncol. 2008;15(2):493–8. doi: 10.1245/s10434-007-9683-2. [DOI] [PubMed] [Google Scholar]
- Stojadinovic A, Hoos A, Nissan A, et al. Parathyroid neoplasms: clinical, histopathological and tissue microarray-based molecular analysis. Hum Pathol. 2003;34(1):54–64. doi: 10.1053/hupa.2003.55. [DOI] [PubMed] [Google Scholar]
- Erickson LA, Jin L, Wollan P, et al. Parathyroid hyperplasia, adenomas and carcinomas. Differential expression of p27 protein. Am J Surg Pathol. 1999;23(3):288–95. doi: 10.1097/00000478-199903000-00007. [DOI] [PubMed] [Google Scholar]
- Gill AJ, Clarkson A, Gimm O, et al. Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism-jaw tumor syndrome-related adenomas from sporadic parathyroid adenomas and hyperplasias. Am J Surg Pathol. 2006;30(9):1140–9. doi: 10.1097/01.pas.0000209827.39477.4f. [DOI] [PubMed] [Google Scholar]
- Mittendorf EA, McHenry CR. Parathyroid carcinoma. J Surg Oncol. 2005;89(3):136–42. doi: 10.1002/jso.20182. [DOI] [PubMed] [Google Scholar]
- Tan MH, Morrison C, Wang P, et al. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin Cancer Res. 2004;10(19):6629–37. doi: 10.1158/1078-0432.CCR-04-0493. [DOI] [PubMed] [Google Scholar]