Abstract
Multiple separate tumors developing in a single salivary gland is rare in previously untreated patients. Tumors that can be multicentric include Warthin tumor, oncocytoma, basal cell adenoma and acinic cell carcinoma. The incidence of multiple primary unilateral pleomorphic adenomas is extremely rare in patients with no prior history of trauma or surgery. We report two cases of primary multicentric pleomorphic adenoma and review the literature. We also subjected one of our cases to a molecular clonality test. The molecular results suggested that the separate nodules were clonally related, arguing against an independent origin.
Keywords: Pleomorphic adenoma, Multifocal, Parotid gland
Materials and Methods
The computerized files of Clinpath Laboratories were searched for cases diagnosed as primary parotid pleomorphic adenoma or mixed tumor over a 10-year period, 1995–2004. A total of 210 cases were identified. Cases with a solitary tumor nodule were excluded and patients who had prior treatment or trauma were excluded. There was one case of a multifocal primary pleomorphic adenoma. A second case was seen in consultation. In each case hematoxylin and eosin (H&E)-stained slides were reviewed to confirm the diagnosis. Clinical case records were reviewed. A paraffin block was available for clonality studies from the non-referred case.
Molecular Testing
An H&E stained slide and six unstained slides were prepared from the paraffin block. Targets were marked for microdissection on the H&E slide and were subsequently microdissected from the unstained slides using a beveled surgical blade and a stereoscopic microscope. DNA was extracted from the resulting tissue fragments after proteinase digestion, using a Qiagen column extraction kit (DNEasy Qiagen, Valencia, California). Polymerase chain reaction was performed using a standard protocol for 13 different short tandem repeat markers (Table 1). The short tandem repeat markers are known to co-localize with tumor suppressor genes at the locations given in Table 1. Analysis of the PCR product was performed using capillary electrophoresis (ABI, 3100, and Genescan software, applied by Systems Inc., Foster City, CA). The PCR products from normal tissue were analyzed first to identify loci that were heterozygous. All heterozygous loci were then examined in the tumor tissue for evidence of loss of heterozygosity. The ratio of the two peaks of heterozygous samples was compared between the tumor and the normal and ratios that were greater than 1.4 or less than 0.7 were considered to be evidence of loss of heterozygosity.
Table 1.
This table illustrates the molecular markers used, along with their cytogenetic locations
| Marker | Location | Nodule 1 | Nodule 2 | Result |
|---|---|---|---|---|
| D1s162 | 1p32.2 | No LOH | No LOH | Matched |
| D1s1183 | 1q25.3 | Non-informative | Non-informative | NA |
| D1s187 | 1p13.2 | LOH A | LOH A | Matched |
| D3s1516 | 3p25.3 | No LOH | No LOH | Matched |
| D3s1600 | 3p14.2 | No LOH | No LOH | Unmatched |
| D5s659 | 5q23.2 | Non-informative | Non-informative | NA |
| D10s1173 | 10q23.3 | No LOH | No LOH | Matched |
| D12s375 | 12q21.1 | LOH B | LOH B | Matched |
| D17s1161 | 17q21 | Non-informative | Non-informative | NA |
| D17s516 | 17p13.1 | Non-informative | Non-informative | NA |
| D17s768 | 17p13.1 | Non-informative | Non-informative | NA |
| D18s463 | 18q21.2 | No LOH | No LOH | Matched |
| D22s1150 | 22q12.2 | Non-informative | Non-informative | NA |
The results for nodule one and nodule two are given in the last two columns. LOH A indicates loss of heterozygosity with loss of the larger allele, while LOH B indicates loss of heterozygosity with loss of the smaller allele. Non-informative indicates that the patient was wild-type homozygous for the marker and No LOH indicates that the lesions had a normal allele pattern with no loss of heterozygosity
The overall patterns of loss of heterozygosity between the different nodules within the parotid were examined and compared.
Case 1
A 70-year-old female presented with a 5-month history of an increasing painless left preauricular swelling. There was no past history of surgery, or other trauma. Clinical examination revealed two nodules each in maximum approximately 1.0 cm in dimension in the parotid superficial lobe. Ultrasound examination showed two larger hypoechoic nodules 1.1 and 1.2 cm in dimension and two smaller nodules each 0.2 cm. A left superficial parotidectomy was carried out. Five years postoperatively there is no evidence of local recurrence.
Case 2
A 68-year-old female presented with a 10-year history of a painless left preauricular mass. There was no pain or past history of surgery or trauma. Ultrasound imaging revealed two hypoechoic nodules toward the superficial anterior edge in the region of the accessory part of the parotid gland, 0.6 and 0.9 cm in maximum dimension. Local excisions were carried out. The patient died in a motor vehicle accident 24 months after her operation. There was no local recurrence of disease.
Pathologic Findings
Gross Findings
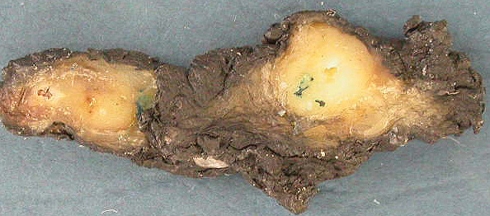
Each case contained two predominant encapsulated or well demarcated nodules with a grey-white cut surface. In case one they were 1.0 and 1.2 cm in maximum dimension (Fig. 1) while in case two they were 0.6 and 0.9 cm. The nodules were separated by intervening grossly normal parotid tissue by at least 0.8–1.0 cm.
Fig. 1.
Macroscopic photograph of the two nodules
Histologic Findings
The nodules in both cases contained a thin fibrous capsule. The tumor contained the typical stromal components of chondromyxoid and hyalinized tissue. The cellular areas exhibited characteristic epithelial and myoepithelial cells in solid, tubular and focally cystic areas (Fig. 2). In addition, at the surface of the capsule of the dominant nodule in each case were minute scattered nodules. These were also located sparsely between the dominant nodules seen only at the histologic level. These foci ranged from 0.02 to 0.2 cm, and some also had a fibrous capsule. The cytology of the lesions was bland with no increase in cellular atypia or pleomorphism. There were no appreciable mitoses and no necrosis.
Fig. 2.
Microscopic photograph of the two nodules (H&E×4)
Molecular Findings
Adequate quantity and quality DNA was obtained from the two nodules and from normal parotid in this case. All of the PCR primers successfully amplified PCR product. Of the 13 different markers, 6 were informative (heterozygous) in this patient and 7 were non-informative (homozygous). Lost of heterozygosity was identified in two loci and the LOH patterns were similar for the two nodules. These results can be seen in Table 1.
Discussion
Most tumors of major salivary gland are discovered clinically as a single mass involving only one gland. Involvement of bilateral major salivary glands synchronously is uncommon and multiple foci of tumor within the same gland is even more unusual [8, 9, 13]. Warthin tumor is the most common bilateral synchronous and multifocal unilateral tumor [9]. Of the other more commonly reported bilateral synchronous tumors are pleomorphic adenoma, acinic cell carcinoma, oncocytoma and basal cell adenoma [4, 9].While basal cell adenoma and oncocytoma may also be multifocal and unilateral [3, 9]. Nearly all of the unilateral multifocal salivary tumors occur in the parotid gland [3, 9].
Benign mixed tumors account for 55–65% of all parotid gland neoplasms. They are typically unicentric and uninodular. Pleomorphic adenomas can become multifocal, particularly when they recur after surgery or when there has been trauma. Small nodular protrusions (“pseudopodia”) extending from the tumor surface or tumor spillage and seeding at the time of surgery are presumably responsible for these tumor recurrences.
The incidence of a primary (untreated) multifocal parotid pleomorphic adenoma has been estimated at 0.14–0.6% [2, 6, 7, 15]. Enroth[6] showed an incidence of 0.6% but all of those cases had undergone prior fine needle aspiration. The incidence in the study presented here is estimated at was 0.5%. With the additional two cases presented here, there are at least 17 cases of primary unilateral multifocal pleomorphic adenoma in the literature [1–4, 6, 7, 10, 14–16]. Eleven of these cases are only briefly mentioned in the literature with no significant details (Table 2), with details only being available for the six remaining cases [1, 3, 4, 14]. These patients range in age from 40 to 87 years and all were female. The masses had been present clinically for between 1 month and 10 years. The number of nodules ranged from two per gland to “multiple”, but in the case described as “multiple” there were two dominant nodules. No recurrences were reported in the patients who had follow up ranged from 1 to 5 years.
Table 2.
Clinical details from cases of primary multifocal unilateral pleomorphic adenoma
| References | Age | Sex | Site | Number | Symptoms | Size (cm) | Follow up |
|---|---|---|---|---|---|---|---|
| Foote and Frazell [7] | N/A | N/A | Parotid | 2 | N/A | N/A | N/A |
| Foote and Frazell [7] | N/A | N/A | R Parotid | 2 | N/A | N/A | N/A |
| Enrotha [6] | N/A | N/A | Parotid | “multiple” | N/A | N/A | N/A |
| Carlsoo and Ekstrand [4] | 40 | F | R Parotid | 2 | Mass 10 years | 1.5 + 1.5 | N/A |
| Behnke [3] | 61 | F | R Parotid | 2 | Mass 2–3 years | 1.0 + 1.0 | 1 year no recurrence |
| Batsakis [2] | N/A | N/A | Parotid | N/A | N/A | N/A | N/A |
| Turnbulla and Franzell [15] | N/A | N/A | Parotid | N/A | N/A | N/A | N/A |
| Andre [1] | 56 | F | R Parotid | 2 | Mass 4 months | N/A | N/A |
| Tanimoto et al. [14] | 87 | F | L Parotid | 2 | Mass 1 month | 1.8 + 1.7 | 2 year no recurrence |
| Helmstaedter et al. [10] | 20 | N/A | Parotid | Multiple (1 major) | Mass | 1.5 | N/A |
| Yu et al [16] | N/A | N/A | Parotid | N/A | N/A | N/A | N/A |
| Present Case 1 | 70 | F | L Parotid | Multiple (2 major) | Mass 5 months | 1.0 + 1.2 | 5 year no recurrence |
| Present Case 2 | 68 | F | L Parotid | Multiple (2 major) | Mass 10 years | 0.6 + 0.9 | 2 year no recurrence |
N/A = Not available
a3 cases
The presence of multifocality can be explained in several different ways. The lesions could be arising independently by multifocal pathogenesis. Or, the tumor nodules could be parasitic nodules that have become detached from a main nodule. This could arise directly from the well-described pseudopodia or from undisclosed trauma.
In prior molecular studies of pleomorphic adenomas it has been shown that both epithelial and mesenchymal elements have a common cell of origin [5, 11, 12] and arise from a common origin. In one of the two cases reported in this paper, we were able to perform a molecular assessment of clonality. The two different nodules within this parotid gland were separately microdissected and a PCR was performed for a panel of genetic loci. When the pattern of allelic loss is similar in two different lesions, it suggests that they are clonally related. In our case the two different tumor nodules within this parotid gland showed almost identical allelic loss patterns. These results indicate that the two nodules most likely represent a multifocal pleomorphic adenoma rather than two synchronous primary tumors.
In summary, we present two cases of primary unilateral multifocal pleomorphic adenoma arising within the parotid gland. In both cases, the patients did not have any history of trauma or prior surgery. The similar molecular profile in the one case that could be tested suggested that the two lesions were clonally related, and did not arise independently. It would be important to test additional cases with two or more nodules of pleomorphic adenoma within the same parotid gland to further define the instances of independent versus clonally related multifocality.
References
- 1.Andre MP. Double tumeur mixte de la parotide. Ann Otolaryngol. 1954;71:474–5. [PubMed] [Google Scholar]
- 2.Batsakis JG. Tumors of the head and neck: clinical and pathological consideration, 2nd ed. Baltimore: Williams and Wilkins Co, 1979:25.
- 3.Behnke EE. Unilateral multiple benign mixed tumors of the parotid gland. Laryngoscope. 1982;92:1265–8. doi: 10.1288/00005537-198211000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Carlsoo B, Ekstrand T. Unilateral multiple mixed tumors of the parotid gland. J Laryngolotol. 1977;91:629–31. doi: 10.1017/s0022215100084152. [DOI] [PubMed] [Google Scholar]
- 5.Debiec-Rychter M, Valckenborough I, Broeck C, Hagemeijer A, Ven WJ, Kas K, Damme B, Voz ML. Histologic localization of PLAG1 (pleomorphic adenoma gene 1) in pleomorphic adenoma of the salivary gland: cytogenetic evidence of common origin of phenotypically diverse cells. Lab Invest. 2001;81:1289–97. doi: 10.1038/labinvest.3780342. [DOI] [PubMed] [Google Scholar]
- 6.Enroth C-M. Histological and clinical aspects of parotid tumors. Acta Otolaryngol (suppl) 1964;191:1–99. [PubMed] [Google Scholar]
- 7.Foote FW, Frazell El. Tumors of the major salivary glands. Cancer. 1953;6:1065–1133. doi: 10.1002/1097-0142(195311)6:6<1065::AID-CNCR2820060602>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Gates GA, Ritter FN, Mesara BW. Multiple primary mixed tumors of the salivary gland. A case report. Arch Otolaryngol. 1966;84:329–31. doi: 10.1001/archotol.1966.00760030331013. [DOI] [PubMed] [Google Scholar]
- 9.Gnepp DR, Schroeder W, Heffner D. Synchronous tumors arising in a single major salivary gland. Cancer. 1989;63:1219–24. doi: 10.1002/1097-0142(19890315)63:6<1219::AID-CNCR2820630631>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Helmstaedter V, Beutner D, Drebber U, Streppel M, Huttenbrink K. Multiple primary pleomorphic adenomas of the unilateral parotid gland. Laryngorhinootologie. 2007;86:448–50. doi: 10.1055/s-2006-945134. [DOI] [PubMed] [Google Scholar]
- 11.Lee P-S, Sabbath-Solitare M, Redondo TC, Ongoapin EH. Molecular evidence that the stromal and epithelial cells in pleomorphic adenomas of salivary gland arise from the same origin: clonal analysis using human androgen receptor gene (HUMARA) assay. Hum Pathol. 2000;31:498–503. doi: 10.1053/hp.2000.6716. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi S, Aihara T, Yoshinok K, Motomura K, Inaji S, Koyama H. Demonstration of monoclonal origin of human parotid gland pleomorphic adenoma. Cancer. 1996;71:431–5. doi: 10.1002/(SICI)1097-0142(19960201)77:3<431::AID-CNCR2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Seifert G, Donath K. Multiple tumors of the salivary glands—terminology and nomenclature. Oral Oncol Eur J Cancer. 1996;32B:3–7. doi: 10.1016/0964-1955(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 14.Tanimoto H, Kumoi K, Otsuki N, Hirayama Y. Multiple primary pleomorphic adenomas in a single parotid gland: report of a new case. Ear Nose Throat J. 2003;81:341–5. [PubMed] [Google Scholar]
- 15.Turnbull AD, Franzell EL. Multiple tumors of the major salivary glands. Am J Surg. 1969;118:787–9. doi: 10.1016/0002-9610(69)90230-X. [DOI] [PubMed] [Google Scholar]
- 16.Yu GY, Ma DQ, Zhang Y, Peng X, Cai Z, Gao Y, Chen Y. Multiple primary tumors of the parotid gland. Int J Oral Maxillofac Surg. 2004;33:531–4. doi: 10.1016/j.ijom.2004.03.010. [DOI] [PubMed] [Google Scholar]