Abstract
Diagnosis of basaloid squamous carcinoma (BSCC) currently relies mainly on histological criteria, with variable immunohistochemical results reported in small series. We explored the use of a battery of immunohistochemical stains to elucidate this diagnosis on 45 cases of BSCC. To further elucidate the immunohistochemical profile of BSCC, to explore potential genetic pathways of malignant transformation using proliferation markers, and to investigate a possible link with Human Papillomavirus (HPV). Forty-five cases of BSCC and 34 site-matched cases of squamous cell carcinoma (SCC) were obtained from the archives of the pathology department at our institution. Extensive literature review was undertaken utilizing Medline. Ber-EP4 is a useful diagnostic marker for BSCC, positive in 82% (37/45) of the cases and in 68% (23/34) of SCC. An alternative is the combination of cytokeratins CK14 and CK7, known to be negative, and CK1, known to be positive, which achieves an accuracy of 73% (33/45) in BSCC and 88% (30/34) in SCC. The two diagnostic approaches were in agreement in 66% of the cases; both methods were equally accurate in the divergent cases. Increased expression of the proliferation markers supports the concept that BSCC is a rapidly growing tumor. Results of p16 stains support an etiological link between BSCC and HPV; interestingly, HPV was present significantly more in BSCC (71% (32/45)), than in SCC (59% (20/34)) in this study (P = 0.02).
Keywords: Basaloid squamous carcinoma, Basosquamous carcinoma, Squamous cell carcinoma, Immunohistochemistry
Introduction
Basaloid squamous carcinoma (BSCC) is an uncommon variant of squamous cell carcinoma (SCC), that has predilection for the head and neck, though it has been described throughout the body [1–6]. The diagnosis of BSCC is currently based on histological criteria, including focal squamous differentiation, a basaloid pattern associated with frank SCC or carcinoma in situ [7]. Despite these criteria, the tumor can be histologically challenging. Poorly differentiated SCC, basal cell carcinoma, small-cell neuroendocrine carcinoma, and adenoid cystic carcinoma are mainly responsible for diagnostic dilemmas in the head and neck [8–10], while in other locations the differentials may vary [11]. Immunohistochemistry has been utilized in an attempt to clarify this difficulty, but the results have been variable and sometimes conflicting [3, 8, 10, 12–15], with the exception of Ber-EP4, a monoclonal epithelial membrane antigen (EMA), which has demonstrated some specificity for BSCC in a few other studies [8, 9, 16]. Coletta et al. also proposed a panel of three cytokeratin stains which were helpful in a series of five cases [17, 18]. In this study we sought to test these two previously described stains in a larger number of cases.
Many authors state BSCC is a more aggressive variant of SCC, citing more advanced stage at presentation, earlier metastasis, and recommending more definitive treatment of such patients [19–21]. This view has been questioned in recent articles which note these conclusions are based largely on limited numbers of case reports [22–24]. Several genetic mechanisms have been proposed for this assumed aggressive behavior, including cell-cycle regulators [25], increased tumor angiogenesis [26], and tumor suppressors and lack of apoptosis [9, 13, 14, 27], with variable results, and precise genetic mechanisms for more aggressive behavior remain elusive. In addition to examining proliferation markers c-kit, MIB-1 and p63, we sought a connection with human papilloma virus (HPV) using p16 staining. HPV-positive head and neck SCCs have a more basaloid morphology [28–30], compared to site-matched SCCs, but head and neck BSCC has not been definitively linked to HPV [14] as it has been in other anatomic locations [5].
Materials and Methods
Patients
After obtaining study approval from the Institutional Review Board (IRB) and Vermont Cancer Center (VCC), the archives of the Department of Pathology at Fletcher Allen Healthcare were searched for the diagnoses of “basosquamous carcinoma,” “basaloid squamous carcinoma,” or “squamous carcinoma with basaloid features,” and the original hematoxylin and eosin (H & E)-stained slides and pathology reports were reviewed according to the criteria of Wain et al. [7], for a total of 41 cases, with distribution as follows. Twenty-one were from head and neck sites (five from neck lymph nodes, four each from tongue base and tonsil, two from vallecula, one each from, pharynx, epiglottis, floor of mouth, nasal bridge and lateral tongue), eight from anorectal sites, eight from genitourinary sites, and eight from lung. Thirty-eight site-matched squamous cell carcinoma cases were located for comparison; however, upon review of the original H & E slides before any immunostaining had been performed, four of these squamous cell carcinoma cases were reclassified as BSCC since greater that 50% of the tumor was made up of basaloid cells with the typical growth patterns described above, and were included in the study group for data analysis [21], with distribution as follows. Eighteen were from head and neck sites (five from neck lymph nodes, five from tongue base, three from tonsil, two from larynx, and one each from floor of mouth, vallecula, epiglottis and pharynx), eight from anorectal sites, six from genitourinary sites, and two from lung.
Immunostaining
The formalin-fixed and paraffin-embedded tissue blocks from the 45 basaloid squamous and 34 squamous cell carcinoma were sliced into 5 μm sections and incubated overnight at 60°C for adherence to glass slides. Deparaffinization of tissue was achieved with xylene, and rehydration was performed in graded alcohol solutions into water, and subsequent peroxidase quenching in 3% hydrogen peroxide in methanol for 15 min at room temperature, followed by another water rinse. Antigen retrieval was performed utilizing the Dako Antigen Retrieval system (Dako, Carpinteria, CA), incubated 30 min at room temperature. Appropriate positive and negative control slides were prepared for each antibody, as per respective manufacturer’s instructions (Table 1).
Table 1.
Antibodies, sources, and dilutions
| Antibody | Full name & clone | Manufacturer | Concentration |
|---|---|---|---|
| Ber EP4 | Mouse IgG anti-Human Epithelial Antigen, clone Ber-EP4 | Dako | 0.625 mg/ml |
| c-kit (CD117) | Rabbit polyclonal anti-Human CD117 | Dako | 1.24 mg/ml |
| CK1a | Mouse IgG anti-cytokeratin 1, clone 34βB4 | Vector Laboratories | 7.6 μg/ml |
| CK7 | Mouse IgG anti-cytokeratin 7, clone OV-TL 12/30 | Dako | 5 μg/ml |
| CK14 | Mouse IgG anti-cytokeratin 14, clone LL002 | BioGenex | 5.8 μg/ml |
| MIB-1 (Ki-67) | Mouse IgG anti-Human Ki-67 Antigen, clone MIB-1 | Dako | 10.6 μg/ml |
| p16 | Mouse IgG anti-p16, clone JC8 | Lab Vision | 2 μg/ml |
| p63 | Mouse IgG anti-p63, clone 4A4 | Dako | 5.9 μg/ml |
aIncubated at 4°C overnight for antigen retrieval
Immunohistochemistry slides were interpreted by the diagnosing pathologist with regard to two parameters: 1) The percentage of tumor cells with positive staining (cases with <10% of tumor cells expressing stain were deemed “negative”) and 2) The intensity of stain expression, reported as either “strong” or “weak.” Any consistent pattern of positive staining, with regard to the tumor architecture, was also noted. The only exception to this reporting system is MIB-1, where we reported a percentage of positive stained cells. This is in keeping with prior publications which reported that the number of cells expressing MIB-1 is diagnostically important, whereas the intensity of this expression is not [31].
Statistical Analysis
The percentage of cells with positive stain in BSCC and SCC were compared using the Mann-Whitney test, and stain intensity was analyzed with Fisher’s Exact Test. Tree regression analyses were then used to delineate which set of tests could best discriminate between the two tumor types, and to provide cutoff values for the continuous variable (the percentage of positive cells).
Results
The original H & E-stained slides revealed 26 of the 45 BSCC (57.8%) were composed of basaloid nests with scant cytoplasm, prominent nuclear pleomorphism, palisading of the nuclei at the periphery of each nest, and central necrosis, all with a lobular growth pattern and small foci of squamous differentiation. The remaining 19 BSCC (42.2%) possessed more prominent squamous areas, some with minimal keratin, and predominant or prominent basaloid areas admixed.
Immunohistochemistry demonstrated the proliferation markers c-kit (CD117), MIB-1 (Ki-67), and p63 were expressed in a significantly larger percentage (P < 0.05) of BSCC tumor cells when compared with site-matched SCC tumors. Likewise Ber-EP4 and p16 were found to be present in significantly larger percentages of BSCC cells. Cytokeratins (CK) 1, 7, and 14 were expressed at significantly different levels in the two groups of tumors. CK1 and CK14 were both present in greater numbers in SCC, whereas CK7 was present in a greater amount in BSCC cells (Table 2).
Table 2.
Total cases positive, median, range, and P-value for each antibody
| Antibody | Cases Pos./total | Median % Pos. | Range % Pos. | P-value Mann–Whitney |
|---|---|---|---|---|
| c-kit (CD117) | 6/45a | 0a | 0–90a | 0.03 |
| 0/34b | 0b | 0–0b | ||
| MIB-1 (Ki-67) | 42/45a | 60a | 0–90a | 0.0009 |
| 32/34b | 40b | 0–80b | ||
| p63 | 43/45a | 100a | 0–100a | 0.002 |
| 34/34b,c | 80b | 10–100b | ||
| Ber-EP4 | 40/45a | 70a | 0–100a | <0.0001 |
| 20/34b | 10b | 0–100b | ||
| p16 | 32/45a | 90a | 0–100a | 0.02 |
| 20/34b | 45b | 0–100b | ||
| CK1 | 9/45a | 0a | 0–90a | 0.0004 |
| 19/34b | 10b | 0–100b | ||
| CK7 | 24/45a | 10a | 0–100a | 0.004 |
| 7/34b | 0b | 0–100b | ||
| CK14 | 24/45a | 10a | 0–100a | <0.0001 |
| 32/34b | 90b | 0–100b |
aBSCC
bSCC
cAll p63 positive staining in SCC was limited to peripheral, basal layer only
There were no significant differences for any of the stains with regard to intensity, except for the cytokeratins and Ber-EP4. CK1 and CK14 displayed significantly greater “strong” staining in SCC (P = 0.0005 and P = 0.01, respectively), and CK7 displayed “strong” staining significantly more often in BSCC (P = 0.01). Ber-EP4 was also strongly positive more often in BSCC (P = 0.0008).
Regression tree analysis determined the most specific marker of BSCC was Ber-EP4 positivity (P < 0.0001). If Ber-EP4 is used as a single marker, with any positive result considered as the diagnostic of BSCC, then the stain results correctly identified 89% of BSCC and 47% of SCC, for an overall accuracy of 71%. This high false-positive rate (identifying SCC as BSCC) can be partially rectified by establishing a cutoff value of 30% positivity. That is, if at least 30% of tumor cells must be positive to diagnose BSCC, the overall accuracy rose to 77%, correctly identifying 82% of BSCC and 68% of SCC. This is a novel finding within the literature, as other, smaller, studies did not share these results [32]. Although a higher proportion of BSCC cells were CK14 positive, there was a greater discrimination between BSCC and SCC cells if negative CK14 stain was used as the criterion for diagnosing BSCC. Additional BSCC cells were diagnosed if there were positive CK1 and negative CK7 results. This combination (negative CK14 or positive CK1 and negative CK7) correctly identified 73% of BSCC and 88% of SCC. The two methods agreed on 66% of the BSCC and SCC diagnoses, though further analysis to determine method was more likely to be accurate in these divergent cases found to be equivalent.
Discussion
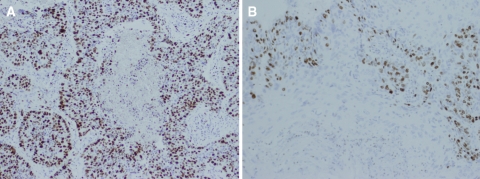
Our study represents the largest series of basaloid squamous carcinoma (BSCC) examined immunohistochemically, notably larger than prior studies concurrently comparing BSCC to SCC [3, 8–10, 13, 18, 26, 33]. While not universally accepted [22–24], the hypothesis that BSCC represents a more aggressive variant is supported by the significantly increased expression of three proliferation markers in this series. Of the three proliferation markers investigated, the increased expression of MIB-1 (Ki-67) diffusely throughout the tumor mass denotes a large percentage of cells (median = 60%) actively dividing in BSCC, because the target protein accumulates beginning in the G1 phase, and is not present in cells in G0. In contrast, MIB-1 expression was predominantly within the basal layers of SCC, representing lower total percentages (median = 40%) of tumor cells (Fig. 1). These trends were present in large absolute numbers of both BSCC and SCC groups (Table 2). While these findings are supportive of the hypothesis that BSCC is a rapidly growing and, perhaps, biologically more aggressive tumor, other proliferation markers may be of more practical use when faced with a diagnostic dilemma.
Fig. 1.
Diffused expression of MIB-1 (Ki-67) is seen in BSCC (a) compared with SCC (b), where it is largely restricted to the basal cell layer. Both images are shown at 10× magnification
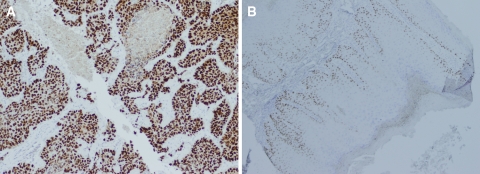
A broadly similar pattern emerged with p63 immunostaining. Within the BSCC, positive staining occurred diffusely throughout the tumor mass, and these cells represented large portions of the overall tumor (median = 100%). The SCC universally displayed some p63 overexpression, but this was always localized to a very obvious basal layer, even to a peripheral row of single cells (Fig. 2). The precise role of p63 in tumor development is an ongoing area of research, but it is known to be overexpressed in epithelial cancers of the head and neck [34], and is essential for the proliferative capacity of epithelial stem cells [35]. Whereas great numbers of cells overexpressing p63 dispersed throughout the tumor are again suggestive of a rapidly growing malignancy, this variation in the pattern of overexpression may be diagnostically helpful to the practicing pathologist. Thirty-four out of 34 total SCC (100%) displayed this prominent pattern of staining, suggesting variance from this configuration, or more diffusely positive staining, could represent features of BSCC or, possibly, other phenotypic SCC variants not addressed in this study.
Fig. 2.
Expression of p63 is widespread in BSCC (a, at 10× magnification), whereas it is exclusive to the basal cell layer in SCC (b, at 4× magnification)
The proliferation marker c-kit (CD117) was also positive significantly more often in BSCC than in SCC, though the absolute numbers are quite small (6/45 BSCC and 0/34 SCC). Thus, from a diagnostic standpoint, c-kit reactivity would seem to exclude the diagnosis of SCC, although the small absolute numbers make its use limited when differentiating BSCC from SCC. From a pathophysiologic viewpoint, the stem cell factor (SCF)/c-kit pathway seems to play a minimal role in the development of either tumor.
Numerous environmental and genetic factors likely play a role in the development of BSCC or SCC; some of these vary with the anatomic location of the malignancy. Tobacco exposure has long been associated with SCC of the lung and upper aerodigestive tract, and more recently, with BSCC of the oropharynx [23]. Human Papillomavirus (HPV) has been associated with SCC of the anogenital region as well as the head and neck [36–38]. More controversial is the relationship of HPV with the development of BSCC, with several small studies yielding conflicting results. Cabanillas et al., found no evidence of HPV DNA in nine cases of pharyngeal BSCC [14], whereas Rubin et al. [15] and Kleist et al., demonstrated significant associations between HPV and male genital, and oropharyngeal BSCC [39], respectively.
It seems likely that the conflicting results within the literature regarding the presence of HPV in BSCC can be attributed to the small sample size of the relevant studies. In this subset of 45 BSCC, p16 positivity (used as a surrogate marker for HPV, as the p16 cyclin-dependent kinase is epigenetically inactivated in non-HPV positive tumors [40]) was demonstrated in 32 cases (71.1%) of these positive cases, 14 were head and neck specimens, eight anorectal, eight urogenital, and two were from lung. The majority of these p16-positive specimens were from locations commonly associated with HPV infection (oropharynx, genitalia, anus and rectum), and although p16 is not commonly encountered in lung malignancies [41], it has been described in subsets of non-small cell lung cancers [42, 43]. These results illustrate HPV can be associated with BSCC, especially in anatomic locations known to be predisposed to HPV-related malignancy, as with other SCC varieties. While HPV-related oropharyngeal SCC is thought by some to have a favorable prognosis [38], further investigation will be required to determine whether this is also the case in HPV-related BSCC.
In addition to any prognostic differences between BSCC and SCC, there can also be significant diagnostic difficulty, particularly in correctly identifying BSCC. Whereas there are many BSCC that fulfill all of the histologic criteria set forth by Wain et al. [7] and will not present problems to veteran surgical pathologists, there are many others that will not be so easily characterized. With such a broad differential and corresponding differences in treatment, accurate diagnosis is both challenging and essential. Two immunohistochemical methods found to be helpful by other authors were employed in this study, to determine their utility in a larger sample. The first, proposed by Tellechea et al. [16], used Ber-EP4 as a marker of BSCC, and this has been documented in other small series to be efficacious as well [8, 9]. The second, proposed by Coletta et al. [17, 18], uses the combination of positive CK1 and positive CK14 with negative CK7. CK1 was reported positive only in the keratinizing component of the tumor, while CK14 was expected to be diffusely positive in the basaloid cells; negative CK7 staining was important to exclude adenoid cystic carcinoma or other salivary gland tumors.
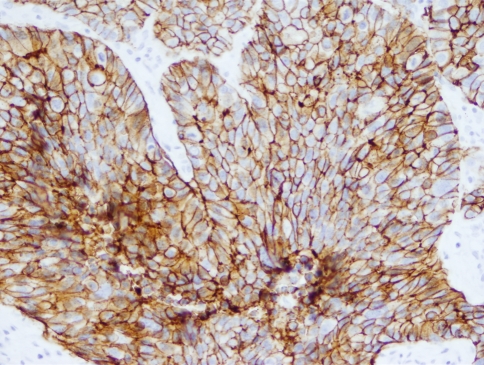
The use of Ber-EP4 in this sample confirmed prior reports of its increased activity in BSCC (Fig. 3). While there is at least one report of a BSCC series negative for Ber-EP4, it examined only six cases and thus could be explained by chance [32]. It is also important to note 20/34 SCC were at least focally positive, but the median positivity for BSCC was significantly higher (Table 2). As a single marker, Ber-EP4 can correctly identify BSCC in 71–77% of the cases, depending on parameters used. If any positive result was deemed sufficient to diagnose BSCC, it was correct in 71% of cases, whereas if a cutoff was established requiring at least 30% of cells positive before BSCC could be diagnosed, overall accuracy increased to 77%.
Fig. 3.
Ber-EP4 expression in BSCC. Note the specificity of the stain for the cell membranes, sparing the nuclei, seen here at 20× magnification
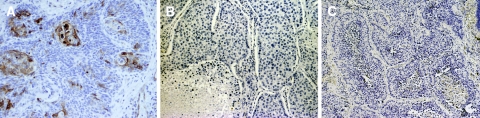
The panel of cytokeratins proved similarly useful in this series (Fig. 4). Of the three stains employed, CK14 was by far the most statistically important, with the other two results contributing little to the overall diagnostic accuracy from a statistical standpoint. Individually, CK1 had the highest overall accuracy (71% versus 67% for CK14 and 65% for CK7), as well as the highest sensitivity rate for BSCC at 80%. However, higher overall accuracy and sensitivity was attained if a cutoff of ≤60% was used with CK14 (76 and 82%, respectively). In their original article, Coletta et al., noted that CK14 was diffusely positive in both of their BSCC cases [17], whereas in our series CK14 was still useful if ≤60% of the tumor cells were positive. Several reasons for this discrepancy may exist. Differing dilutions of reagent may have been used, as the parameters were not published in the letter by those authors; also, there may have been variable amounts of basaloid and squamous components in the tumors used in our study compared to those two cases. It is noteworthy, though, that even focal positivity was diagnostically useful in this cytokeratin panel in including the diagnosis of BSCC, particularly since CK14 reactivity helps exclude neuroendocrine tumors and undifferentiated carcinomas [18, 44].
Fig. 4.
(a) CK1 expression in BSCC may be quite focal, occurring only in keratinizing areas, as seen in this 20× magnified image. Negative staining for CK7 (b) and CK14 (c), both at 10× magnification, were characteristic of BSCC in this study, although some BSCC did stain positively for CK14
CK1 and CK7 can be used to increase detection of BSCC but at the cost of misidentifying SCC—namely the overall accuracy does not improve. Likewise, a negative CK7 result did not dramatically influence the statistical analysis, but is certainly important clinicopathologically to rule out other tumors such as adenoid cystic carcinoma, adenocarcinoma, or adenosquamous carcinoma. When implementing this diagnostic battery, pathologists must remember to vigilantly examine the CK1-stained sections, as this expression can be quite focal and demonstration of the squamous component is one of the diagnostic criteria for BSCC. Although this combination of three cytokeratin stains may be more cumbersome and time-consuming to implement, it achieves similar or better accuracy (76–80%, depending on numerical cutoffs used) than Ber-EP4 alone in correctly identifying BSCC and SCC.
Acknowledgements
The authors have no financial conflicts of interest to disclose, and funding for the project came from departmental research funds and NIH grant PHS 22435-22.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
This data has been presented, in abstract form, at the 2008 United States and Canadian Academy of Pathology Annual Meeting, March 4, in Denver, Colorado.
References
- 1.Barnes L, Ferlito A, Altavilla G, MacMillan C, Rinaldo A, Doglioni C. Basaloid squamous cell carcinoma of the head and neck: clinicopathological features and differential diagnosis. Ann Otol Rhinol Laryngol. 1996;105:75–82. doi: 10.1177/000348949610500115. [DOI] [PubMed] [Google Scholar]
- 2.Cakir E, Demirag F, Ucoluk GO, Kaya S, Memis L. Basaloid squamous cell carcinoma of the lung: a rare tumour with a rare clinical presentation. Lung Cancer. 2007;57(1):109–11. doi: 10.1016/j.lungcan.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Koide N, Koike S, Watanabe H, Yazawa K, Adachi W, Amano J. Basaloid squamous carcinoma of the esophagus with analysis by in situ nick end labeling and PCNA immunostaining. Hepatogastroenterology. 1999;46:265–71. [PubMed] [Google Scholar]
- 4.Wong CS, Tsao MS, Sharma V, Chapman WB, Pintilie M, Cummings BJ. Prognostic role of p53 protein expression in epidermoid carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 1999;45:309–14. doi: 10.1016/s0360-3016(99)00188-1. [DOI] [PubMed] [Google Scholar]
- 5.Lam KY, Chan KW. Molecular pathology and clinicopathologic features of penile tumors: with special reference to analyses of p21 and p53 expression and unusual histologic features. Arch Pathol Lab Med. 1999;123:895–904. doi: 10.5858/1999-123-0895-MPACFO. [DOI] [PubMed] [Google Scholar]
- 6.Grayson W, Taylor LF, Cooper K. Adenoid cystic and adenoid basal carcinoma of the uterine cervix: a comparative morphologic, mucin, and immunohistochemical profile of two rare neoplasms of putative “reserve cell” origin. Arch Pathol Lab Med. 1999;123:448–58. doi: 10.1097/00000478-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol. 1986;17(11):1158–66. doi: 10.1016/S0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 8.Beer TW, Shepherd P, Theaker JM. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000;37:218–23. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones MS, Helm KF, Maloney ME. The immunohistochemical characteristics of the basosquamous cell carcinoma. Dermatol Surg. 1997;23:181–4. doi: 10.1016/S1076-0512(97)00105-2. [DOI] [PubMed] [Google Scholar]
- 10.Klijanienko J, El-Najjar A, Ponzio-Prion A, Marandas P, Micheau C, Caillaud JM. Basaloid squamous carcinoma of the head and neck: Immunohistochemical comparison with adenoid cystic carcinoma and squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1993;119:887–90. doi: 10.1001/archotol.1993.01880200093013. [DOI] [PubMed] [Google Scholar]
- 11.Hammar SP. Approach to the diagnosis of neuroendocrine lung neoplasms: variabilities and pitfalls. Semin Thorac Cardiovasc Surg. 2006;18(3):183–90. doi: 10.1053/j.semtcvs.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Shen Y, Liang Y, Wu X. Basaloid squamous cell carcinoma of the esophagus: an immunohistochemical study of 8 cases. Chin Med J. 2001;114(10):1084–8. [PubMed] [Google Scholar]
- 13.Sampaio-Goes FCG, Oliviera DT, Dorta RG, Nonogaki S, Landman G, Nishimoto IN, Kowalski LP. Expression of PCNA, p53, BAX, and BCL-X in oral poorly differentiated and basalooid squamous cell carcinoma: relationships with prognosis. Head Neck. 2005;27:982–9. doi: 10.1002/hed.20258. [DOI] [PubMed] [Google Scholar]
- 14.Cabanillas R, Rodrigo JP, Ferlito A, Rinaldo A, Fresno AF, Aguilar C, et al. Is there an epidemiological link between human papillomavirus DNA and basaloid squamous cell carcinoma of the pharynx? Oral Oncol. 2007;43(4):327-32. [DOI] [PubMed]
- 15.Rubin MA, Kleter B, Zhou M, Ayala G, Cubilla AL, Quint WG, Piroq EC. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159(4):1211–8. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellechea O, Reis JP, Domingues JC, Baptista AP. Monoclonal antibody Ber EP4 distinguishes basal-cell carcinoma from squamous-cell carcinoma of the skin. Am J Dermatopathol. 1993;15:452–5. doi: 10.1097/00000372-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Coletta RD, Almeida OP, Vargas PA. Cytokeratins 1, 7, and 14 immunoexpression are helpful in the diagnosis of basaloid squamous carcinoma. Histopathology. 2006;48:774. doi: 10.1111/j.1365-2559.2006.02345.x. [DOI] [PubMed] [Google Scholar]
- 18.Coletta RD, Cotrim P, Almeida OP, Alves VAF, Wakamatsu A, Vargas PA. Basaloid squamous carcinoma of the oral cavity: a histologic and immunohistochemical study. Oral Oncol. 2002;38:723–9. doi: 10.1016/S1368-8375(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 19.Banks ER, Frierson HF, Mills SE, George E, Zarbo RJ, Swanson PE. Basaloid squamous carcinoma of the head and neck. A clinicopathologic and immunohistochemical study of 40 cases. Am J Surg Pathol. 1992;16:939–46. doi: 10.1097/00000478-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Farmer ER, Helwig EB. Metastatic basal cell carcinoma: a clinicopathologic study of seventeen cases. Cancer. 1980;46:748–57. doi: 10.1002/1097-0142(19800815)46:4<748::AID-CNCR2820460419>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Winzenburg SM, Niehans GA, George E, Daly K, Adams GL. Basaloid squamous carcinoma: a clinical comparison of two histological types with poorly differentiated squamous cell carcinoma. Otolaryngol Head Neck Surg. 1998;119:471–5. doi: 10.1016/S0194-5998(98)70104-4. [DOI] [PubMed] [Google Scholar]
- 22.Zbaren P, Nuyens M, Stauffer E. Basaloid squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2004;12:116–21. doi: 10.1097/00020840-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Paulino AF, Singh B, Shah JP, Huvos AG. Basaloid squamous cell carcinoma of the head and neck. Laryngoscope. 2000;110:1479–82. doi: 10.1097/00005537-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Erisen LM, Coskun H, Ozuysal S, Basut O, Onart S, Hizalan I, Tezel I. Basaloid squamous cell carcinoma of the larynx: a report of four new cases. Laryngoscope. 2004;114:1179–83. doi: 10.1097/00005537-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Salerno G, Di Vizio D, Staibano S, Mottola G, Quaremba G, Mascolo M, Galli V, Rosa G, Insabato L. Prognostic value of p27Kip1 expression in basaloid squamous cell carcinoma of the larynx. BMC Cancer. 2006;6:146. doi: 10.1186/1471-2407-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marioni G, Gaio E, Giacomelli L, Marchese-Ragona R, Staffieri A, Marino F. Endoglin (CD105) expression in head and neck basaloid squamous cell carcinoma. Acta Otolaryngol. 2005;125:307–11. doi: 10.1080/00016480410023047. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Tojo MJ, Garcia Cano FJ, Infante Sanchez JC, Velazquez Sanchez E, Aguirre Urizar M. Immunoexpression of p53, Ki-67 and E-cadherin in basaloid squamous cell carcinoma of the larynx. Clin Transl Oncol. 2005;7(3):110–4. doi: 10.1007/BF02708743. [DOI] [PubMed] [Google Scholar]
- 28.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 29.Poetsch M, Lorenz G, Bankau A, Kleist B. Basaloid in contrast to nonbasaloid head and neck squamous cell carcinomas display aberrations especially in cell cycle control genes. Head Neck. 2003;25(11):904–10. doi: 10.1002/hed.10301. [DOI] [PubMed] [Google Scholar]
- 30.Wilczynski SP, Lin BT, Xie Y, Paz IB. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol. 1998;152:145–56. [PMC free article] [PubMed] [Google Scholar]
- 31.Davies L, Hardin NJ, Beatty BG. Can Ki-67 predict recurrence of N0 squamous cell carcinoma of the tongue? Ann Otol Rhinol Laryngol. 2006;115(1):12–7. doi: 10.1177/000348940611500102. [DOI] [PubMed] [Google Scholar]
- 32.Bracero F, Gamiz MJ, Soldado L, Conde JM, Redondo M, Gonzalez MA, Lopez Garrido J, Esteban F. Hypopharynx and larynx basaloid squamous carcinoma: our experience with 6 cases. Acta Otorrinolaringol Esp. 2001;52(3):229–36. doi: 10.1016/s0001-6519(01)78202-7. [DOI] [PubMed] [Google Scholar]
- 33.Akyol MU, Dursun A, Akyol G, Edalt N. Proliferating cell nuclear antigen immunoreactivity and the presence of p53 mutation in basaloid squamous cell carcinoma of the larynx. Oncology. 1998;55:382–3. doi: 10.1159/000011882. [DOI] [PubMed] [Google Scholar]
- 34.Matheny KE, Barbieri CE, Sniezek JC, Arteaga CL, Pietenpol JA. Inhibition of epidermal growth factor receptor signaling decreased p63 expression in head and neck squamous carcinoma cells. Laryngoscope. 2003;113(6):936–9. doi: 10.1097/00005537-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129(3):523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 36.Lu DW, El-Mofty SK, Wang HL. Expression of p16, Rb, and p53 proteins in squamous cell carcinomas of the anorectal region harboring human papillomavirus DNA. Mod Pathol. 2003;16(7):692–9. doi: 10.1097/01.MP.0000077417.08371.CE. [DOI] [PubMed] [Google Scholar]
- 37.Santos M, Landolfi S, Olivella A, Lloveras B, Klaustermeier J, Suarez H, Alos L, Puig-Tintore LM, Campo E, Ordi J. p16 overexpression identifies HPV-positive vulvar squamous cell carcinomas. Am J Surg Pathol. 2006;30(11):1347–56. doi: 10.1097/01.pas.0000213251.82940.bf. [DOI] [PubMed] [Google Scholar]
- 38.Tran N, Rose BR, O’Brein CJ. Role of human papillomavirus in the etiology of head and neck cancer. Head Neck. 2007;29(1):64–70. doi: 10.1002/hed.20460. [DOI] [PubMed] [Google Scholar]
- 39.Kleist B, Bankau A, Lorenz G, Jager B, Poetsch M. Different risk factors in basaloid and common squamous head and neck cancer. Laryngoscope. 2004;114(6):1063–8. doi: 10.1097/00005537-200406000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc Natl Acad Sci USA. 2006;103(38):14152–7. doi: 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguayo F, Castillo A, Koriyama C, Higashi M, Itoh T, Capetillo M, Shuyama K, Corvalan A, Eizuru Y, Akiba S. Human papillomavirus-16 is integrated in lung carcinomas: a study in Chile. Br J Cancer. 2007;97:85–91. doi: 10.1038/sj.bjc.6603848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ota N, Kawakami K, Okuda T, Takehara A, Hiranuma C, Oyama K, Ota Y, Oda M, Watanabe G. Prognostic significance of p16(INK4a) hypermethylation in non-small cell lung cancer is evident by quantitative DNA methylation analysis. Anticancer Res. 2006;26(5B):3729–32. [PubMed] [Google Scholar]
- 43.Yoo J, Jung JH, Lee MA, Seo KJ, Shim BY, Kim SH, Cho DG, Ahn MI, Kim CH, Cho KD, Kang SJ, Kim HK. Immunohistochemical analysis of non-small cell lung cancer: correlation with clinical parameters and prognosis. J Korean Med Sci. 2007;22(2):318–25. doi: 10.3346/jkms.2007.22.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sturm N, Lantuejoul S, Laverriere MH, et al. Thyroid transcription factor 1 and cytokeratins 1, 5, 10, 14 (34betaE12) expression in basaloid and large-cell neuroendocrine carcinomas of the lung. Hum Pathol. 2001;32:918–25. doi: 10.1053/hupa.2001.27110. [DOI] [PubMed] [Google Scholar]