Abstract
Head and neck paragangliomas are rare neuroendocrine tumors derived from neural crest cells of parasympathetic ganglia or the widely dispersed neuroendocrine cells of the head and neck region. Paragangliomas of the sinonasal tract and nasopharynx are rare. The clinicopathologic features of this unique example of a hereditary, nasopharyngeal paraganglioma, and selective entities that are included in its differential diagnosis are presented.
Keywords: Paraganglioma, Sinonasal paraganglioma, Head and neck pathology, Clinicopathologic correlation
Clinical Presentation
A 53 years old man presented to the University of Alabama at Birmingham Division of Otolaryngology-Head and Neck Surgery following referral from his primary care physician for the evaluation of nasal airway obstruction, intermittent epistaxis, breathing difficulties, headaches, and repeated sinus infections. His symptoms were slow in onset occurring over an approximate three-year period. Review of his past medical history revealed the presence of mild hypertension and a history of Penicillin allergy. He had rhinoplasty 20 years earlier and a nasal biopsy was also performed during childhood but was non-diagnostic. Review of his social history revealed tobacco and drug use 20 years ago and only occasional alcohol consumption.
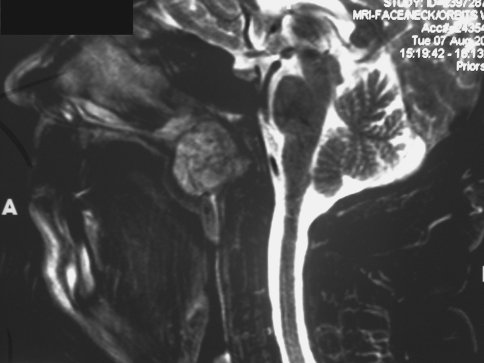
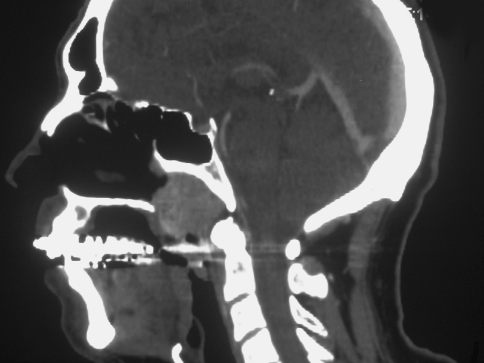
Imaging studies revealed a relatively well-defined but radiolucent lesion, measuring approximately 3 cm in greatest dimension, situated within the nasopharynx just inferior to the sphenoid sinus. The lesion did not extend to the clivus and did not appear to cause destruction of the sinonasal bones. The mass had a salt and pepper pattern on sagittal MRI imaging without contrast (Fig. 1) and was hypointense on post-contrast coronal CT imaging (Fig. 2).
Fig. 1.
Sagittal MRI without contrast, showing a relatively well-demarcated lesion in the nasopharynx, exhibiting salt and pepper architecture
Fig. 2.
Sagittal post -gadolinium T1-weighted CT image of the neck (soft tissue) demonstrating that the nasopharyngeal mass is hypointense
Differential Diagnosis
Numerous common and rare entities that involve the sinonasal and nasopharyngeal region were entertained in the differential diagnosis of the current tumor. A combination of detailed clinical, radiographic, and serological work-up, coupled with a thorough histological examination (requiring the application of a battery of immunohistochemical stains) was essential to establish the correct diagnosis. The differential diagnosis included juvenile angiofibroma, esthesioneuroblastoma, glomangiopericytoma/sinonasal hemangiopericytoma, pleomorphic adenoma, meningioma, and chordoma among others. Additionally, sinonasal paraganglioma needed to be distinguished from solitary plasmacytoma as tremendous overlap are known to be seen in both the clinical and radiographic features of these entities.
Diagnosis
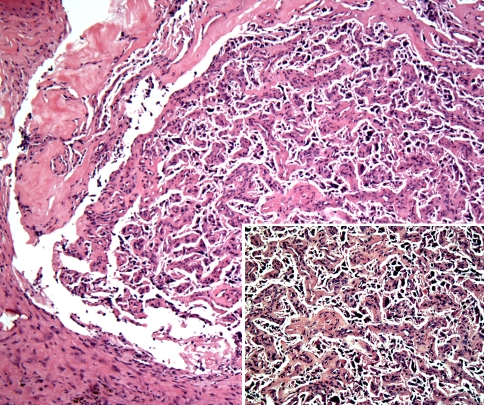
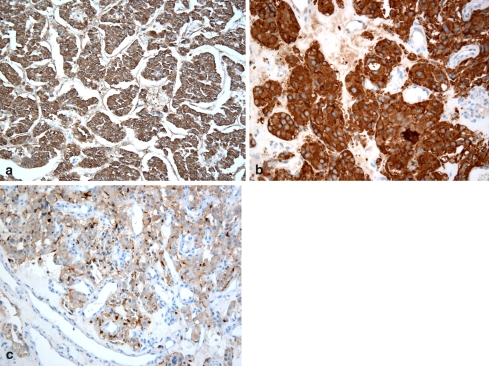
The lesion was biopsied and histologic examination revealed the presence of nests and clusters of a mostly monotonous population of cells (zellballen), separated by variably sized sinusoidal type vessels. Cellular pleomorphism and hyperchromatism was seen but no atypical mitotic figures were appreciated (Fig. 3). The inner round to oval tumor cells reacted positively with neuroendocrine markers (synaptophysin and chromogranin) while staining with anti-S-100 protein highlighted the sustentacular cells at the periphery of the clusters (Fig. 4). The features were diagnostic of paraganglioma. Subsequently, the patient underwent full laboratory work- up which revealed mild hyperlipidemia, mildly elevated Hct (50.1 g/dl), normal urinary vanillylmandelic acid levels (VMA = 7.4 mg/24 h), a normal plasma epinephrine level (31 pg/ml) but noticeably elevated levels of plasma norepinephrine (657 pg/ml) and plasma dopamine levels (935 pg/ml). Similarly, a fractionated normetanephrine level was elevated (215 pg/ml) while the fractionated metanephrine level measurement was unremarkable (44 pg/ml).
Fig. 3.
Histomorphological examination of the nasopharyngeal mass demonstrated the so-called “zellballen” pattern with tumor cells arranged in nests and in an organoid pattern. The cells are separate by a sinusoidal type vasculature. (Hematoxylin and eosin stain, original magnification ×20) Insert: The tumor cells exhibited pleomorphism but no mitotic figures were appreciated. (Hematoxylin and eosin stain, original magnification ×40)
Fig. 4.
The tumor cells reacted positively with synaptophysin (a) and chromogranin (b). Staining with anti S-100 protein (c) delineated the sustentacular cells (dark brown) peripherally
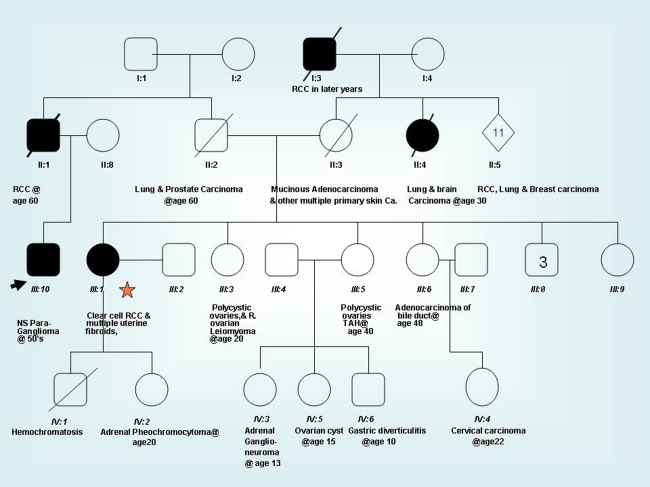
Upon questioning the patient regarding family history, he reported multiple unusual tumors in family members, but was unsure of the exact specifics. As the patient’s relatives were managed and followed elsewhere, their medical records were retrieved from across the southern United States, after obtaining proper consent. One of the patient’s first cousins was found to have renal cell carcinoma, clear cell type, and her daughter was diagnosed with pheochromocytoma. Review of the family’s genetic profile demonstrated various other neoplasms (Fig. 5).
Fig. 5.
The family pedigree tree of the patient (arrow) with nasopharyngeal hereditary paraganglioma. Note the patient’s cousin (star) and her child (designated as IV: 2) were diagnosed with renal cell carcinoma and adrenal phaeochromocytoma, respectively. Multiple other benign and malignant tumors existed in this family
Genetic sequence analysis performed on the cousin’s peripheral blood demonstrated heterozygosity for a missense variant in the SDHB gene. Specifically, there was a heterozygous nucleotide change of G to A at the second nucleotide codon 230 [Axon 7- sat] in nucleotide 689 (c689G > A).This missense variant which encodes R230DH had not been seen previously described in the literature nor seen in the reference laboratory where the genetic analysis was conducted, except in a single example in a patient with a pheochromocytoma diagnosed several years earlier. Genetic analysis favored classifying the present case as a class III variant by American College of Medical Genetics criteria [1].
Subsequent Course
Our patient received a general body CT to search for other lesions. He was subsequently presented with various treatment options, collectively comprising embolization and surgery, with or without adjuvant radiation therapy. He elected to continue his treatment at another institution. Measurements of his catecholamine levels at that time showed persistently elevated urinary norepinephrine and dopamine levels (114 μg/h and 487 μg/24 h respectively) as well as elevated plasma norepinephrine (657 pg/ml, normal range = 80–598) and plasma dopamine levels (935 pg/ml, normal range = 3–46).
A general body CT performed at an outside institution demonstrated the presence of a heterogeneous pericaval mass between the duodenum and the right kidney measuring 2 × 1.5 cm; consistent with paraganglioma. Two additional abdominal masses that showed contrast enhancement on axial and coronal T-1 weighted images were discovered, one located in the carotid sheath, measuring 3 × 3.5 cm and the other measuring 1 × 1.5 cm, identified just medial to the stylohyoid process. All three tumors were excised and the diagnosis of paraganglioma was confirmed in the pericaval and carotid sheath tumors while the third tumor was diagnosed as “glomus vagale”.
Ultrastructural examination was also performed on the periaortic mass demonstrating polygonal cells with ample cytoplasm containing small dense core granules that exhibited pleomorphism. A well-developed smooth endoplasmic reticulum, small lipid droplets and a fair number of mitochondria were also identified confirming the diagnosis of paraganglioma.
The patient subsequently underwent an excision of the remainder of his nasopharyngeal mass. Histomorphological examination further confirmed the original diagnosis. At the same time, he also underwent genetic testing and found to carry the same SDHB mutation identified in his cousin. All laboratory analysis performed systematically in the subsequent few months showed a gradual but marked decline in catecholamine levels to normal confirming that the tumors were functional, although it cannot be determine with 100% certainty if all the paragangliomas were functional. The levels remained at normal values when measured again 3 months and 9 months later, respectively). (Normal fractionated normetanephrine and metanephrine values ranged from 18 to 112 pg/ml and 21–61 pg/ml, respectively).
The patient is currently doing well with only mild hypertension. Attempts to perform genetic testing on the remainder of his family are planned but compliance and low socioeconomic status remain negative factors in completion of these studies.
Discussion
Paragangliomas are neuroendocrine tumors derived from neural crest cells. They may be found in adrenal or extra-adrenal location; arising from sympathetic and parasympathetic ganglia, respectively. Paraganglioma of the head and neck (H&N) are derived from parasympathetic ganglia or the widely dispersed neuroendocrine cells of the head and neck region [2]. H&N paragangliomas comprise 0.012% of all human tumors, 0.6% of all H&N neoplasms and approximately 3% of all paragangliomas [3]. These tumors often recapitulate the cells of paraganglia (both the principal and sustentacular cells). They most commonly present as asymptomatic, space-occupying lesions in middle age women [4].
Approximately, 10–50% of H&N paragangliomas are familial [5] and up to 40% of familial tumors, as well as up to 10% of sporadic tumors are bilateral and detected at an earlier age than unilateral cases [6]. While the vast majority of cases reported are benign, malignancy is more frequently encountered with sporadic rather than the hereditary tumors [7].
Review of the literature suggests that up to 27.4% of all cases of pheochromocytoma and paraganglioma are associated with gene mutations [8]. Four types of familial paraganglioma (PGL) syndromes have been identified with mutations in the SDH (succinate dehydrogenase) gene. The SDH gene encodes subunits of a heterotetrameric SDH complex that in turn is associated with hereditary paragangliomas [9]. This type of mutation has been identified in all patients with multifocal H&N paragangliomas. PGL type 1 is associated with germline mutations in the succinate dehydrogenase complex subunit D gene (SDHD), PGL type 3 (associated with mutations in the succinate dehydrogenase complex subunit C gene (SDHC) and PGL type 4 is associated with mutations in the SDHB gene as seen in the present case. Germline heterozygous mutations in SDHA (PGL type 2) are yet to be clarified.
Evidence support that SDHD mutations (PGL type 1) are associated with multifocal H&N paragangliomas while SDHB mutations (PGL type 4) are commonly linked to extra-adrenal disease and malignancy [10]. The present case had a well documented familial history of SDHB mutations but the benign nature was confirmed in its lack of biologic progression since excision.
Functional paragangliomas, including those in the H&N region, may synthesize a wide range of neuroendocrine products (e.g., serotonin, leu-enkephalin, gastrin, substance P, and others). In our case, the noticeable elevation in catecholamine levels and their subsequent decline following the excision of his tumors further confirmed their functional nature.
The four subtypes of paraganglioma recognized in the H&N are, in order of frequency; carotid body, jugulotympanic, vagal, and laryngeal with the remainder (much less frequently encountered) in the orbit, sinonasal region, nasopharynx, trachea, and thyroid gland areas. When compared to the rest, sinonasal neoplasms are considered rare [4, 11, 12] with no familial cases reported to date in the English language literature. A multifocal paraganglioma involving the nose, paranasal sinuses, and nasopharynx [4, 13] and another functional nasal paraganglioma causing Cushing’s syndrome have been reported [14] but to the best of our knowledge, no hereditary cases have been reported in the sinonasal region prior to the current case.
The majority of H&N paragangliomas are benign and, therefore, managed by surgery alone without radiation therapy. Angiographic arterial embolization is predominantly used to debulk in multicentric tumors. Adjunct chemotherapy is infrequently applied due to inconsistent responses.
Histologically, paragangliomas demonstrate a biphenotypic appearance comprising peripherally located sustentacular cells surrounding the round, paler staining clear, chief cells. The latter cells are organized into whorled, well-delineated nests of cells, referred to as “zellballen”. The sustentacular cells are best highlighted with anti-S-100 protein while the inner chief cells stain positively with neuroendocrine markers (synaptophysin, NSE, and chromogranin). Cellular pleomorphism may be identified and mitotic activity is not uncommon. A rich sinusoidal type vascular network supports the nests of cells. These later features are responsible for the characteristic imaging utilized to diagnose the lesion preoperatively. Computed tomography (CT) can best identify the extent of bony erosion while MRI highlights the arterial flow patterns, via post-contrast rapid sequential scanning. Paraganglioma often appears as a hypointense well defined mass with areas of signal (flow) voids on T1-weighted images while showing the so-called “salt and pepper appearance “on T2-weighted images. Furthermore, MRI may assist in locating multiple foci of disease and delineates intracranial from extracranial involvement.
Morphologic criteria alone do not distinguish benign from malignant tumors. Neither the presence of atypical histological features nor aggressive radiographic appearances (showing an infiltrative pattern) are accepted as criteria for malignancy. Rather, local or distant metastasis is required [7]. Data from the “National Cancer Data Base” suggest a 60%, 5-year survival rate based on 59 reported cases in which regional metastases was discovered. The identification of distant metastases [9], necrosis, mitotic activity, and vascular invasion in the primary tumor as well as the identification of fewer sustentacular cells histologically are collectively considered as factors that may negatively influence the prognosis [15].
Thorough clinicopathologic and radiographic evaluation is required to distinguish sinonasal paraganglioma from many other entities where features often overlap [2, 16, 17]. Paraganglioma may grow as a polypoid mass, simulating an inflammatory sinonasal polyp. Similarly, signs and symptoms may overlap with many other expansile sinonasal tumors that produce congestion, rhinorrhea, epistaxis, and nasal obstruction. The presence of typical histomorphologic features depicting the “zellballen pattern” that stain positively with neuroendocrine markers, coupled with classic salt and pepper radiographic appearance on MRI may help to initially establish the diagnosis.
Lobular capillary hemangioma and cavernous hemangioma of the sinonasal and pharyngeal region are included in the differential diagnoses of nasopharyngeal paraganglioma. They are most frequently encountered in the anterior nasal septum and in the tip of the turbinates as an obstructive, painless, polypoid nasal mass that is associated with epistaxis. Nasopharyngeal angiofibroma is another important tumor to include in the differential diagnosis. It is a rare benign neoplasm that commonly appears as a pedunculated or a polypoid mass with a locally destructive behavior. It primarily involves young adolescent boys. The signs and symptoms described are non-specific and include nasal obstruction, epistaxis, nasal discharge, and facial deformity. However, routine radiographic imaging is considered characteristic, demonstrating bowing of the posterior wall of maxillary antrum with distortion and posterior displacement of the walls of the pterygoid plates (Holman–Miller sign). An angiogram typically demonstrates large numbers of tortuous vessels within the lesion. Histologically, the tumor is characterized by variable sized staghorn type vasculature, supported by a moderately cellular spindle to stellate shaped fibroblasts. These cells characteristically stain positively for testosterone receptors and vimentin by immunohistochemistry but are non-reactive for cytokeratin.
Sinonasal hemangiopericytoma (glomangiopericytoma) is another uncommon, intermediate grade, vascular tumor that should be also included in the differential diagnosis of nasopharyngeal paraganglioma. It most commonly presents as a unilateral obstructive nasal mass, accompanied by chronic sinusitis-like features, headaches, congestion, and epistaxis. Conventional radiographic images and CT scans classically show an opacified polypoid nasal mass; however, 20% of cases reveal relatively unremarkable imaging. Histologically, sinonasal glomangiopericytoma may demonstrate variable patterns. Features include an unencapsulated, cellular proliferation of spindle to oval cells, arranged in short fascicles, whorls, or a storiform pattern often producing a distinct perivascular palisaded arrangement. Variable number of blood vessels, many of which assume the so called “staghorn appearance” exhibiting perivascular hyalinization are seen. Mitosis may be found but necrosis is not observed. The tumor cells stain positively with factor XIII related antigen, vimentin, smooth muscle actin, and muscle specific actin. Additionally, they react negatively with bcl-2 and CD99, which is considered to be the most important and reliable markers that help distinguish this neoplasm from solitary fibrous tumor in that region.
Olfactory neuroblastoma is another uncommon malignant neoplasm to be considered in the differential diagnosis of the current case. Similar to other tumors described, signs and symptoms are more or less non-specific, including nasal obstruction, epistaxis, headaches, anosmia, and visual changes. The tumor displays classic “dumbbell-shaped” lesion extending across the cribriform plate when occurring in the usual upper nasal cavity, however, ectopic tumors may be seen at a lower level where it is associated with sinus opacification and calcification. This is for the most part responsible for the speckled radiographic pattern and thus may be confused with other vascular lesions in the H&N region including paraganglioma. With MRI studies, the lesion shows enhancement on post contrast T1-weighted images.
Pleomorphic adenoma may also rarely occur in the sinonasal and pharyngeal region and thus may be added to the differential list. It may produce variable signs and symptoms including unilateral nasal obstruction, epistaxis, nasal swelling, epiphora, and mucopurulent rhinorrhea. CT and MRI imaging typically demonstrate a homogenous, well-defined soft tissue mass, lacking infiltration into the surrounding structures. Histologically, the tumor is similar in presentation to those seen in the salivary glands, demonstrating epithelial cords, nests, and ducts supported by a variably cellular stroma that typically display myxoid chondroid changes among other features.
Other neoplasms in the differential diagnosis include chordoma, a low to intermediate grade tumor, predominantly seen at the base of the skull, including the clivus and nasopharyngeal regions. It often produces various non-specific symptoms and signs including diplopia, nasal discharge, pain, and epistaxis. The tumor typically demonstrates an expansile destructive mass best evaluated by MRI, showing a high T2 signal and enhancement on T1-post-gadolinium. Histologic examination characteristically shows nests, cords or sheets of large S-100 protein and cytokeratin positive epithelioid cells exhibiting vesicular nuclei and vacuolated cells, referred to as physaliferous cells.
Sinonasal meningioma is a rare benign neoplasm, commonly seen in an intracranial location but ectopic tumors may be also found in the H&N including the sinonasal area, where they may be derived from heterotopic meningeal tissue. The tumor may also appear polypoid and produce non-specific signs and symptoms such as epistaxis, pain, headaches, and others. They also have prevalence for left sided involvement. Sinonasal tumors grow slowly and may be present for several years prior to establishing the diagnosis. Meningioma is characterized by variable histological presentations, which may include a lobular architecture, whorled rearrangement of tumor cells and concentric psammomatous type calcifications. Intranuclear cytoplasmic inclusions are often visualized. The cells characteristically stain positively by immunohistochemistry for vimentin and EMA. CT and MRI imaging predominantly help to identify any intracranial tumor component.
Lastly, alveolar soft part sarcoma is a rare tumor of unknown etiology. It predominately involves the extremities, with approximately one-third of tumors are identified in the H&N region. Here it shows prevalence for orbital and tongue occurrence while sinonasal and pharyngeal tumors are considered extremely rare. Alveolar soft part sarcoma appears hypervascular on angiography and on contrast enhanced CT. The tumor also demonstrates high signal intensity on T-1 and T-2 weighted imaging. Histologically, the tumor is characterized by an organoid, alveolar or nested pattern of growth with thin but distinct endothelial lined fibrovascular septa. The tumor cells are large and characterized by abundant granular eosinophilic cytoplasm. The demonstration of PAS positive intracytoplasmic crystals at the center of the nests is often extremely helpful especially when accompanied by a distinct dyscohesion of tumor cells, which may be attributed to possible necrotic changes within the tumor cells.
Primary nasopharyngeal paraganglioma must be also distinguished from other primary and metastatic tumors of the sinonasal tract such as solitary plasmacytoma, neurofibromas, schwannoma, solitary fibrous tumor, benign and malignant skeletal and smooth muscle tumors among others.
In conclusion, sinonasal paragangliomas are rare when compared to the rest of head and neck paragangliomas and sinonasal tumors in general with only 30 cases reported in the English literature. Additionally, while it is not unusual to identify functional tumors in the head and neck region, to the best of our knowledge, no functional tumors with familial background have been reported in that location to date and the current case describes a possible example of hereditary functional paraganglioma of the sinonasal tract. Because the patient had his multiple paragangliomas removed within a short time frame, it is impossible to determine with 100% certainty however, if the sinonasal tumor was functional.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Nasser Said-Al-Naief, Phone: +1-205-9341488, FAX: +1-205-975-5200, Email: nasser@uab.edu.
Junu Ojha, FAX: +1-313-4946672, Email: ojhaj@udmercy.edu.
References
- 1.Astuti F, Latif A, Dallol PL, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial phaeochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes L, Eveson JW, Reichart P, Sidransky D. Head and neck tumors. In: Gnepp DRE, editor. World health organization classification of tumors pathology & genetics. Lyon, France: IARC Press; 2005. pp. 361–70. [Google Scholar]
- 3.Suarez C, Rodrigo JP, Ferlito A, et al. Tumours of familial origin in the head and neck. Oral Oncol. 2006;42(10):965–78. doi: 10.1016/j.oraloncology.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Lack EE, Cubilla AL, Woodruff JM. Paragangliomas of the head and neck region: a pathologic study of tumors from 71 patients. Hum Pathol. 1979;10:191–218. doi: 10.1016/S0046-8177(79)80008-8. [DOI] [PubMed] [Google Scholar]
- 5.Pellitteri PK, Ferlito A, Bradley PJ, et al. Management of sarcomas of the head and neck in adults. Oral Oncol. 2003;39(1):2–12. doi: 10.1016/S1368-8375(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 6.Bikhazi PH, Roeder E, Attaie A, Lalwani AK. Familial paragangliomas: the emerging impact of molecular genetics on evaluation and management. Am J Otol. 1999;20(5):639–43. [PubMed] [Google Scholar]
- 7.Grufferman S, Gillman MW, Pasternak LR, et al. Familial carotid body tumors: case report and epidemiologic review. Cancer. 1980;46(9):2116–22. doi: 10.1002/1097-0142(19801101)46:9<2116::AID-CNCR2820460934>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Amar L, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;34:8812–8. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 9.Drucker A, Houlden R. A case of familial paraganglioma syndrome type 4 caused by a mutation in the SDHB gene. Nat Clin Pract Endocrinol Metab. 2006;2:702–6. doi: 10.1038/ncpendmet0342. [DOI] [PubMed] [Google Scholar]
- 10.Benn DE, Gimenez-Roqueplo AP, Reilly JR, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91(3):827–36. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- 11.Welkoborsky HJ, Gosepath J, Jacob R, et al. Biologic characteristics of paragangliomas of the nasal cavity and paranasal sinuses. Am J Rhinol. 2000;14(6):419–26. doi: 10.2500/105065800779954284. [DOI] [PubMed] [Google Scholar]
- 12.Ketabchi S, Massi D, Santoro R, Franchi A. Paraganglioma of the nasal cavity: a case report. Eur Arch Otorhinolaryngol. 2003;260(6):336–40. doi: 10.1007/s00405-002-0569-4. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn JA, Aronoff BL. Nasal and nasopharyngeal paraganglioma. J Surg Oncol. 1989;40(1):38–45. doi: 10.1002/jso.2930400110. [DOI] [PubMed] [Google Scholar]
- 14.Apple D, Kreines K. Cushing’s syndrome due to ectopic ACTH production by a nasal paraganglioma. Am J Med Sci. 1982;283(1):32–5. doi: 10.1097/00000441-198201000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Achilles E, Padberg BC, Holl K, Kloppel G, Schroder S. Immunocytochemistry of paragangliomas—value of staining for S-100 protein and glial fibrillary acidic protein in diagnosis and prognosis. Histopathology. 1991;18:453–8. doi: 10.1111/j.1365-2559.1991.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 16.Thompson L. Head and neck pathology. A volume in the foundations in diagnostic pathology. Elsevier, Inc.; 2006. p. 124–154.
- 17.Wenig B. Atlas of head and neck pathology. Elsevier, Inc.; 2008. p. 63–153.