Abstract
Low-grade papillary adenocarcinoma (LGPA) represents a relatively rare histological variant of polymorphous low-grade adenocarcinoma (PLGA). There has been a debate as to whether LGPA is associated with greater aggressive potential compared to PLGA; this is further obfuscated by the fact that diagnostic criteria for LGPA have not been well-defined. We believe that this is the first report of a patient with LGPA who developed metastases to the femur and scalp. We review the published evidence for classifying LGPA as distinct from PLGA. The weight of published data does support the idea that LGPA is oncologically distinct from LGPA. However, as uniform diagnostic criteria are lacking, we suggest a cut-off value of 10% or greater papillary formation as being necessary to separate LGPA from PLGA.
Keywords: Low-grade papillary adenocarcinoma, Polymorphous low-grade adenocarcinoma, Oral salivary gland tumor
Introduction
Polymorphous low-grade adenocarcinoma (PLGA) is a malignancy arising predominantly from minor salivary glands. First appearing in the second edition of the WHO Classification of Salivary Tumours in 1991 [1], it was initially described in 1983 by Batsakis et al. and Freedman et al [2, 3]. As such, PLGA is a well-defined neoplasm characterized by architectural diversity, cytological uniformity, and indolent clinical behavior. Low-grade papillary adenocarcinoma (LGPA) was first described by Allen et al. in 1974 with a follow up publication by Mills et al. in 1984 [4, 5]. While it has been described as a histologic variant of PLGA, controversy exists in the literature regarding whether LGPA should be classified as an entity distinct from PLGA based on a more aggressive oncologic potential.
PLGA unified several histologic classifications, including LGPA, terminal ductal carcinoma (TDA), and lobular carcinoma [6, 7]. Prior to its recognition, it was commonly diagnosed as adenoid cystic carcinoma, while over the last two decades there has been substantial data published on the features of PLGA differentiating the two [8]. It is the second most common primary minor salivary gland malignancy after mucoepidermoid carcinoma, comprising 9–26.4% of all salivary malignancies [9–11]. It commonly arises in the palate (49–77.8%), followed by either the buccal mucosa or upper lip (7.4–13.4%), and can also involve the floor of the mouth, lower lip, alveolar ridge, and tongue [7, 9, 12, 13]. Additionally, PLGA can arise in the lung [14], parotid gland [15], submandibular gland [16], and maxilla [17], and two case reports describe it transforming into higher grade neoplasms [18, 19].
Despite the controversy regarding LGPA, only five groups have compared the biologic potential of PLGA and LGPA, arriving at conflicting conclusions [6–8, 20, 21]. Our purpose in this manuscript is to present a case report of a LGPA and to review the published evidence for classifying LGPA as distinct from PLGA; we believe this is the first case of LGPA with metastases to the femur and scalp.
Case Report
Clinical Course
In 1985, a 51-year-old man with a history of alcohol and tobacco use, presented with a mass of the right hard palate. The biopsy revealed LGPA. He underwent a right maxillectomy and probably also received adjuvant radiation therapy to the primary site at that time, although the medical records from this period are unavailable. In 1987, he developed right cervical metastases and a radical neck dissection was performed. In 1998, a chest X-ray revealed a right upper nodular mass, which was confirmed as metastatic disease by endoscopic brushings. In late 2006, metastatic disease was also found in the right femur, which was treated by radiation therapy at another institution. A resection of the right proximal femur with placement of an endoprothesis was performed at this institution. In July 2007, he presented with a subcutaneous mass of the occipital and vertex of the scalp, present for at least 3 months. This was initially treated with incision and drainage, and antibiotics. At the time, follow-up chest CT revealed evidence of progressive pulmonary metastatic disease. The left posterior scalp mass was 3.5 cm in diameter and freely mobile. It was resected and closed primarily with a split thickness skin graft.
Pathology
1985
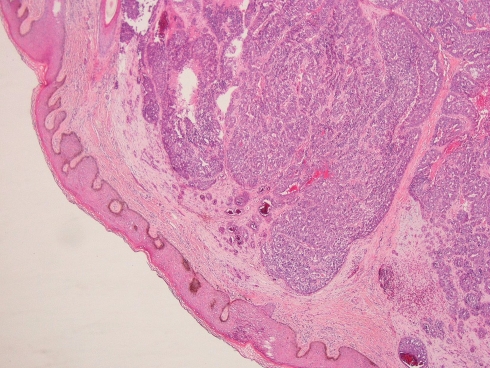
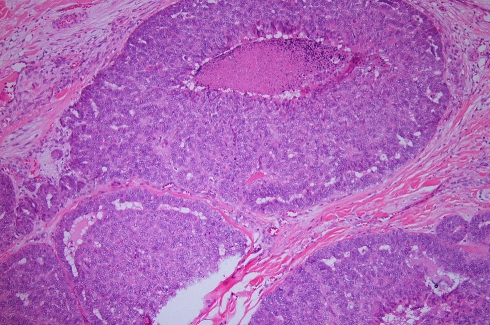
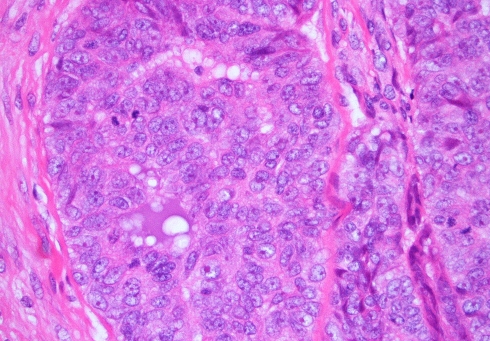
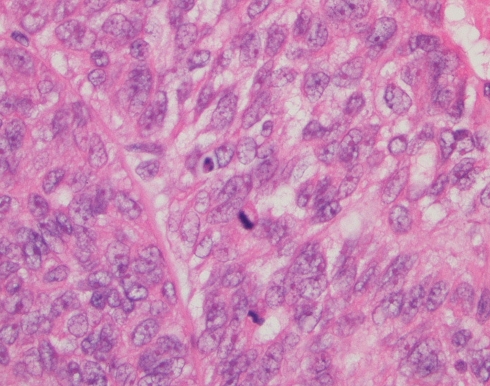
The en-bloc primary resection was composed of the right maxilla, hard palate, lateral wall and floor of the nasal cavity. The tumor contained gland-forming tumor islands, a papillary component, and a minor tubular component (Fig. 1). Solid areas and cribriform glandular architecture predominated; the cribriform areas had a filigree appearance. The papillary component represented about 10% of the tumor (Fig. 2). Indian-filing was present in about 5–10% (Fig. 3). Cytologically, the tumor demonstrated oval nuclei with moderate pleomorphism, prominent single nucleoli and fine chromatin. Only occasional mitotic figures were seen. The tumor infiltrated bone; focal perineural invasion and necrosis were also seen.
Fig. 1.
Low power view of the primary palatal tumor, forming tumor islands within the submucosa
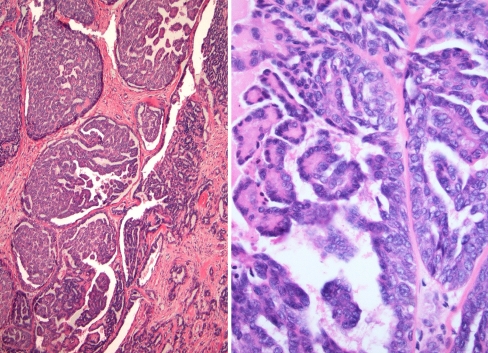
Fig. 2.
Papillary formation is seen. The tumor cells have bland vesicular nuclei
Fig. 3.
Low power view demonstrating streaming tubules and cords of tumor cells
1987
The cervical metastatic disease revealed a prominent solid pattern with sharply punched out cribriforming areas.
2006
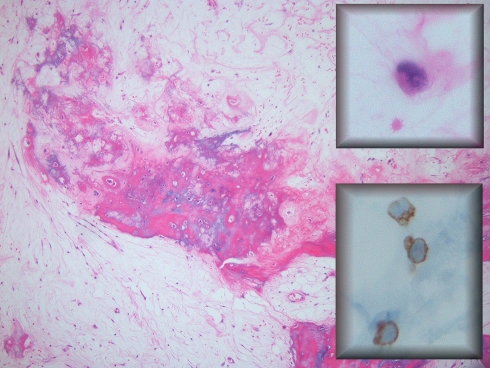
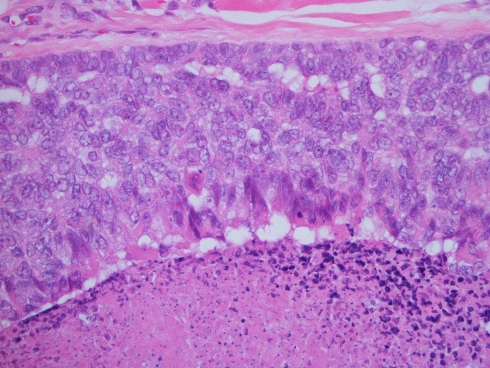
The resected femur and femoral head demonstrated focal intramedullary myxoid tissue with dystrophic calcification consistent with post-radiation osteitis in the subtrochanteric area of femoral shaft. No obvious carcinoma was seen on hematoxylin and eosin stained slides, however, scattered epithelial cells could be appreciated. Immunohistochemical staining for AE13 and CAM 5.2 confirmed the presence of scattered residual tumor cells (Fig. 4). These findings are consistent with near-complete response of metastatic carcinoma to external radiation therapy.
Fig. 4.
The resected femur in 2006, after radiotherapy. Osteitis and fracture callous is seen. Occasional single residual tumor cells are present with intranuclear holes, indicative of radiation effect (upper inset). These cells expressed AE1/3 (lower inset), confirming their nature as residual metastatic carcinoma
2007
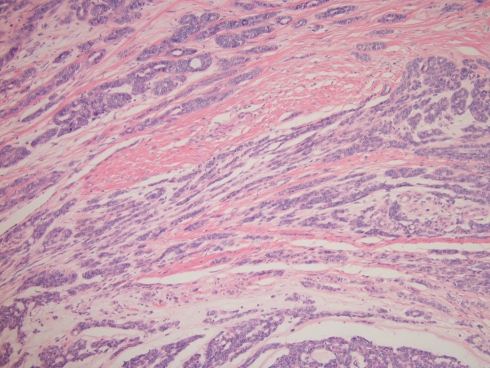
The metastatic scalp carcinoma revealed solid tumor nests (Figs. 5 and 6) and areas of filigree cribriform architecture. No Indian-filing or tubular formations were seen. The papillary component comprised less than 5% of the tumor. Mitotic activity was brisk (Fig. 7) and necrosis was present (Fig. 8). Cytologically, the nuclear chromatin was coarse, and the nucleoli were large and pleomorphic, representing progression in cytological grade as compared to the original neoplasm in 1985. Overall, about 90% of the tumor was solid with filigree cribriform architecture, and 25% necrotic, further representing progression in grade as compared to 1985.
Fig. 5.
Metastasis to scalp in 2007. A solid tumor pattern predominates
Fig. 6.
Filigree-type cribriforming—this pattern differs from the sharply punched out cribriform spaces seen in adenoid cystic carcinoma
Fig. 7.
This latest recurrence demonstrates larger tumor cells with greater pleomorphism, coarser chromatin, multiple nucleoli, and a brisk mitotic rate
Fig. 8.
Tumor necrosis is abundant (bottom of image)
Discussion
Since it was first coined in 1984 [22], PLGA has become characterized by its architectural diversity, cytological uniformity, and indolent clinical behavior. Cases have been documented in patients of almost all ages, ranging from 12–94 years, with the mean age commonly in the sixth decade; some studies show no gender predilection, while others demonstrate a 2:1 female-to-male ratio [7, 9, 12, 23]. However, controversy exists regarding the significance of histologic subtypes. Are PLGAs with solid and papillary components part of the spectrum of PLGA, or a distinct clinicopathologic entity?
General Diagnostic and Prognostic Issues
PLGAs are occasionally confused with pleomorphic adenomas and or adenoid cystic carcinomas (ACC). However, a pleomorphic adenoma can be easily distinguished from PLGA by its lack of infiltration. The need to distinguish PLGA from ACC arises more often, as there are overlapping histologic features (i.e., cribriform, tubular, and soild patterns), while the prognoses of these two tumors greatly differ. Clinically, both tumors are characterized by slow growth and multiple local recurrences. However, distant metastases (DM) are rare for PLGA and frequent for ACC. In fact, for ACC, the DM rate is greater than the regional metastases rate [24]. Not surprisingly, DM decreases patient survival for ACC. Sung et al. [24] found that the 5- and 10-year disease specific survival rates were 88% and 72% for patients with ACC without DM, respectively, and 76% and 48% for those with ACC and DM. By comparison, the disease specific survival for patients with PLGA was 97.6%, with an average follow-up of 9.6 years [12]. Therefore accurate prognostication is contingent upon an accurate diagnosis.
Light microscopy can distinguish PLGA from ACC. PLGA tumor cells are epithelioid with a moderate amount of eosinophilic cytoplasm and the nuclei are vesicular with fine chromatin. A specific characteristic feature of PLGA is the “Indian-file” pattern of invasion, which usually includes targetoid perineural invasion. Another specific feature is an overall blue-grey tumor hue that can be appreciated at low-power on hematoxylin and eosin stained sections. This is due to a blue-grey tumor matrix plus the vesicular nuclear chromatin. In contrast, ACC is usually composed of basaloid cells with little cytoplasm, and more angular, pleomorphic nuclei. Mitotic activity may also be increased in ACC, but is usually negligible in PLGA [12]. Architecturally, ACC is composed of three possible patterns: tubular, cribriform, and solid, while the diagnosis of ACC can usually be excluded when additional patterns (e.g., papillary, glandular, or Indian-file infiltration) are observed. Although the cribriform pattern is common to both ACC and PGLA, distinctions can still be observed. Cribriform areas for ACC are typically rigid, whereas both rigid and reticular, or “lacy” type, cribriforming may be observed in PLGA [25] (see Table 1).
Table 1.
Distinction between PLGA, LGPA, and ACC
| PLGA | LGPA | ACC | |
|---|---|---|---|
| Low-power architecture | |||
| Cribriforming | Rigid or reticular | Rigid or reticular | Rigid |
| Perineural invasiona | Targetoid, Indian-file pattern | Extensive | Extensive |
| Infiltration | Yes | Yes | Yes |
| Indian-file invasion | Yes | Yes | No |
| Blue-grey tumor matrix | Yes | Yes | No |
| Whorling tumor fasicles | Yes | Yes | No |
| Papillary structures | Yesb | Yesb | No |
| Solid component | Yes | Yes | Yesc |
| High power cytologic features | |||
| Cell type | Epithelioid tumor cells with abundant cytoplasm Myoepithelial cells may be present |
Epithelioid tumor cells with abundant cytoplasm Myoepithelial cells may be present |
Basaloid tumor cells with high nuclear/cytoplasmic ratio, little cytoplasm Myoepithelial cells may be present |
| Nuclei | Vesicular nuclei with fine chromatin | Vesicular nuclei with fine chromatin | Dark basaloid nuclei. High-grade ACC may have coarse chromatind |
| Mitotic activity | Occassional | Occassional | Varies with grade |
Legend
aCommon in all tumors
bThe cut-off distribution with respect to papillary structures justifying the classification of LGPA has not been adequately defined
cSolid architecture in ACC ≥30% corresponds to high-grade classification. HG ACC is invariably associated with other high-grade features such as necrosis and nuclear pleomorphism. By contrast, PLGA/LGPA are generally low-grade tumors. The presence of solid areas in PLGA/LGPA is not necessarily associated with high-grade cytology. In the present report, we do see a progression to high-grade cytologic features
dThe nuclear grade of ACC can vary, whereas PLGA and LGPA are generally low-grade tumors. The presence of a solid salivary tumor with high-grade cytology favors the diagnosis of high-grade ACC
As mentioned previously, one study of PLGA reported 97.6% disease specific survival with an average follow-up of 9.6 years [12]. Clearly, PLGA is an indolent neoplasm; some tumors have been present for up to 30 years prior to diagnosis [26]. Local recurrence rates range from 10.4–32.5%, regional metastases rates 0–15%, and DM rates 0–7.5% [7, 12, 13]. Not surprisingly, one group [25] has recommended long-term follow-up for at least 20 years. In general, clinicopathologic features such as growth pattern differences, mitotic rate, tumor necrosis, patient age, and tumor location are not prognostically significant [7].
Polymorphous Low-Grade Adenocarcinoma vs. Low-Grade Papillary Adenocarcinoma
There has been controversy regarding whether PLGA with extensive papillary morphology should be classified separately as low-grade papillary adenocarcinoma (LGPA) given its possible greater aggressive potential. Slootweg et al. [6] reviewed the distinctions between seven PLGAs (also referred to as terminal duct adenocarcinoma) and eight LGPAs. The authors stated that whorling tumor fasicles are characteristic for PLGA and absent in LGPA, while the presence of any papillary structures would necessitate the classification of LGPA. Slootweg et al. [6] describe LGPA as comprised of tumor “ducts lined by single- or multi-layered epithelium lying in a muco-hyaline stroma, with some ducts dilating to form microcysts,” some of which are “filled with papillary outgrowths”. Table 1 summarizes the histological features that distinguish ACC, PLGA, and LGPA.
Slootweg also compared the clinical outcomes between PLGA and LGPA. Local recurrences developed in 3/7 PLGAs and 1/8 LGPAs [6]. Regional metastases developed in 2/8 LGPAs, but none of the seven PLGAs [6]. Mitchell et al. [21] compared 75 PLGA cases with 15 LGPA cases reported in the literature and found that LGPAs more frequently developed local recurrences (46.6%) and cervical node metastasis (40%) as compared to PLGAs (19% and 6.6%, respectively). Lastly, Perez-Ordonez [20] reported regional metastases in 2 of 4 LGPA cases as compared to 3/13 PLGA. These reports support the contention that LGPA is more aggressive than PLGA.
However, this issue is far from resolved; 6 years later, Slootweg published a greater experience with 22 cases of PLGA, finding that papillary structures within typical PLGAs were not predictive of metastases, concluding that papillary structures form part of the spectrum of PLGA [27]. Similarly, Colmenero et al. [8] also did not find that papillary architecture was predictive of outcome. Table 2 compiles and summarizes the rates of local recurrence, regional and distant metastases, and disease-related mortality for PLGA and LGPA [7, 8, 12, 20, 21, 28–31]. We see that LGPA has significantly greater rates of local recurrence (45%) and regional metastases (35%) as compared to PLGA (23% and 10%, respectively). Thus, the distinction between these two tumors is warranted. However, one limitation of comparing outcomes is the lack of uniform diagnostic criteria for classifying LGPA. Evans and Luna [7] used the definition of “more than focal” papillary differentiation, which was not further defined. As uniform diagnostic criteria are lacking, we suggest that a cut-off of 10% or greater papillary formation should be criteria for distinguishing LGPA from PGLA. However, we would like to note that this percentage is an arbitrary choice with little data to support it.
Table 2.
Comparison between cases reported as PLGA and LGPA
| PLGA | LGPA | Pa | |
|---|---|---|---|
| Number of patients | 289 | 40 | |
| Local recurrence | 46 (15.9%) | 18 (45%) | 0.0001 |
| Cervical node metastasis | 12 (4.1%) | 14 (35%) | 0.0001 |
| Distant metastasis | 7 (2.4%) | 2 (5%) | 0.083 |
| Death from tumor | 1 (0.3%) | 2 (5%) | NS |
Treatment
For PLGAs the standard of care is surgical resection. Adjuvant radiation therapy (RT) is not a standard recommendation. Historically, RT was recommended for PLGA with inadequate margins, perineural or perivascular spread, and/or lymph node involvement [32, 33]. However, analyzing 40 patients with PLGA, Evans [7] found no significant differences in the rates of regional metastases, DM, and patient survival according to the type of initial therapy or margin status. In other words, RT had no impact on regional metastases, DM, or survival.
Distant Metastases for PLGA and LGPA
With respect to the development of distant metastases, we found reports on eight patients with PLGA and two with LGPA [6] who developed DM, most commonly to the lungs, including the pleura [31], as well as the vertebrae [6], ilium [7], orbit [30], and skin [7, 30] (see Table 3). However, we suspect that the rates and sites of distant metastases may be underestimated as cases of PGLA with distant metastases probably go unreported. One previous case describes PLGA metastases to the ilium, and two previous cases describe metastases to the skin. Evans and Luna [7] described one patient with metastasis in the ilium 16.5 years after diagnosis. However, Evans and Luna [7] also describe a patient who developed skin metastases 8 years following initial treatment. The patient died 11 years later of unknown causes with stable metastatic disease. Thomas et al. [30] described a woman with an orbital mass and multiple skin metastases 15 years after her primary diagnosis. We believe this is the first report of metastatic LGPA to the femur and scalp. Our patient developed DM to the lungs, femur, and scalp. The bone metastases were completely responsive to RT. His lung metastases have been present for 10 years, and are currently asymptomatic and stable in size. Thus, patients with metastatic disease may still have long-term survival with stable disease.
Table 3.
PLGA and LGPA with distant metastases
| Author | Sex | 1° location | Interval to DM (year) | Location of metastatic disease | Histology |
|---|---|---|---|---|---|
| Slootweg [6]a | M | Hard palate | 17 | Lung, vertebra | Extensive papillary architecture |
| Tanaka [29] | F | Soft palate | 18 | 2 lung masses | |
| Thomas [30] | F | Floor of mouth | 15 | Orbit, skin | 13 year after primary treatment, minor papillary component; 15 year after primary treatment, more than 75% cribiform architecture, with no papillary component |
| Castle [12] | Palate | Lung | |||
| Evans [7]a | 16.5 | Ilium | One of these three cases had papillary areas of more than focal extent | ||
| 8 | Skin, subcutis | ||||
| 24 | Lung | ||||
| Hannen [31] | F | Palate | 3 | Pleura | |
| Lee [28] | F | Lung | |||
| Pogodzinski [25] | F | Soft palate | 10 | Lung | |
| Current Reporta | M | Hard palate | 13 | Lung, femur, scalp | Initial tumor, 10% papillary component; 22 year after primary treatment, less than 5% of the tumor had a papillary component, more than 90% was solid with filigree cribiforming, and 25% necrotic |
aDiagnosed as LGPA
Conclusion
PLGA is the second most common minor salivary gland malignancy, characterized by architectural diversity, cytological uniformity, and indolent clinical behavior. Papillary and solid areas are part of the histological spectrum of PLGA, but extensive solid and/or papillary areas are rare. The category of LGPA is certainly justified as it suggests a greater potential for local recurrence and regional metastases. It will be important to adopt uniform diagnostic criteria for LGPA; therefore we recommend a cut-off of 10% papillary formation to distinguish LGPA from PLGA.
References
- 1.Seifert G, Sobin L, Thackray A, editors. Histological typing of salivary gland tumours. 2. New York: Springer; 1991. [Google Scholar]
- 2.Batsakis J, Pinkston G, Luna M, et al. Adenocarcinomas of the oral cavity: a clinicopathologic study of terminal duct carcinomas. J Laryngol Otol. 1983;97(9):825–35. doi: 10.1017/S0022215100095062. [DOI] [PubMed] [Google Scholar]
- 3.Freedman P, Lumerman H. Lobular carcinoma of intraoral minor salivary gland origin. Report of 12 cases. Oral Surg Oral Med Oral Pathol. 1983;56(2):157–66. doi: 10.1016/0030-4220(83)90282-7. [DOI] [PubMed] [Google Scholar]
- 4.Mills S, Garland T, Allen M. Low-grade papillary adenocarcinoma of palatal salivary gland origin. Am J Surg Pathol. 1984;8(5):367–74. doi: 10.1097/00000478-198405000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Allen M, Fitz-Hugh G, Marsh WL. Low-grade papillary adenocarcinoma of the palate. Cancer. 1974;33:153–8. doi :10.1002/1097-0142(197401)33:1<153::AID-CNCR2820330123>3.0.CO;2-A. [DOI] [PubMed]
- 6.Slootweg P, Muller H. Low-grade adenocarcinoma of the oral cavity. A comparison between the terminal duct and the papillary type. J Craniomaxillofac Surg. 1987;15(6):359–64. doi: 10.1016/s1010-5182(87)80083-5. [DOI] [PubMed] [Google Scholar]
- 7.Evans H, Luna M. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow-up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24(10):1319–28. doi: 10.1097/00000478-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Colmenero C, Patron M, Burgueno M, et al. Polymorphous low-grade adenocarcinoma of the oral cavity: a report of 14 cases. J Oral Maxillofac Surg. 1992;50(6):595–600. doi: 10.1016/0278-2391(92)90440-B. [DOI] [PubMed] [Google Scholar]
- 9.Buchner A, Merrell P, Carpenter W. Relative frequency of intra-oral minor salivary gland tumors: a study of 380 cases from northern california and comparison to reports from other parts of the world. J Oral Pathol Med. 2007;36(4):207–14. doi: 10.1111/j.1600-0714.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 10.Waldron C, el-Mofty S, Gnepp D. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1998;66(3):323–33. doi: 10.1016/0030-4220(88)90240-X. [DOI] [PubMed] [Google Scholar]
- 11.Yih W, Kratochvil F, Stewart J. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg. 2005;63(6):805–10. doi: 10.1016/j.joms.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Castle J, Thompson L, Frommelt R, et al. Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86(2):207–19. doi :10.1002/(SICI)1097-0142(19990715)86:2<207::AID-CNCR4>3.0.CO;2-Q. [PubMed]
- 13.Vincent S, Hammond H, Finkelstein M. Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1994;77(1):41–7. doi: 10.1016/S0030-4220(06)80105-2. [DOI] [PubMed] [Google Scholar]
- 14.Cho K, Jung J, Cho D, et al. Primary polymorphous low-grade adenocarcinoma of lung treated by sleeve bronchial resection: a case report. J Korean Med Sci. 2007;22(2):373–6. doi: 10.3346/jkms.2007.22.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagao T, Gaffey T, Kay P, et al. Polymorphous low-grade adenocarcinoma of the major salivary glands: report of three cases in an unusual location. Histopathology. 2004;44(2):164–71. doi: 10.1111/j.1365-2559.2004.01799.x. [DOI] [PubMed] [Google Scholar]
- 16.Cinamon U, Maly B, Elidan J. Polymorphous low-grade adenocarcinoma of the submandibular gland. Otolaryngol Head Neck Surg. 2000;123(3):337–8. doi: 10.1067/mhn.2000.108200. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Indo H, Takasaki T, et al. A rare case of intraosseous polymorphous low-grade adenocarcinoma (PLGA) of the maxilla. Dentomaxillofac Radiol. 2001;30(3):184–7. doi: 10.1038/sj.dmfr.4600596. [DOI] [PubMed] [Google Scholar]
- 18.Pelkey T, Mills S. Histologic transformation of polymorphous low-grade adenocarcinoma of salivary gland. Am J Clin Pathol. 1999;111(6):785–91. doi: 10.1093/ajcp/111.6.785. [DOI] [PubMed] [Google Scholar]
- 19.Simpson R, Pereira M, Ribeiro A, et al. Polymorphous low-grade adenocarcinoma of the salivary glands with transformation to high-grade carcinoma. Histopathology. 2002;41(3):250–9. doi: 10.1046/j.1365-2559.2002.01439.x. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Ordonez B, Linkov I, Huvos A. Polymorphous low-grade adenocarcinoma of minor salivary glands: a study of 17 cases with emphasis on cell differentiation. Histopathology. 1998;32(6):521–9. doi: 10.1046/j.1365-2559.1998.t01-2-00410.x. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell D, Eveson J, Ord R. Polymorphous low-grade adenocarcinoma of minor salivary glands—a report of three cases. Br J Oral Maxillofac Surg. 1989;27(6):494–500. doi: 10.1016/S0266-4356(89)80008-7. [DOI] [PubMed] [Google Scholar]
- 22.Evans H, Batsakis J. Polymorphous low-grade adenocarcinoma of minor salivary glands. A study of 14 cases of a distinctive neoplasm. Cancer. 1984;53(4):935–42. doi :10.1002/1097-0142(19840215)53:4<935::AID-CNCR2820530420>3.0.CO;2-V. [DOI] [PubMed]
- 23.Tsang Y, Tung Y, Chan J. Polymorphous low grade adenocarcinoma of the palate in a child. J Laryngol Otol. 1991;105(4):309–11. doi: 10.1017/s0022215100115713. [DOI] [PubMed] [Google Scholar]
- 24.Sung M, Kim K, Kim J, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2003;129(11):1193–7. doi: 10.1001/archotol.129.11.1193. [DOI] [PubMed] [Google Scholar]
- 25.Pogodzinski M, Sabri A, Lewis J, et al. Retrospective study and review of polymorphous low-grade adenocarcinoma. Laryngoscope. 2006;116(12):2145–9. doi: 10.1097/01.mlg.0000243200.35033.2b. [DOI] [PubMed] [Google Scholar]
- 26.Aberle A, Abrams A, Bowe R, et al. Lobular (polymorphous low-grade) carcinoma of minor salivary glands. A clinicopathologic study of 20 cases. Oral Surg Oral Med Oral Pathol. 1985;60(4):387–95. doi: 10.1016/0030-4220(85)90261-0. [DOI] [PubMed] [Google Scholar]
- 27.Slootweg P. Low-grade adenocarcinoma of the oral cavity: polymorphous or papillary? J Oral Pathol Med. 1993;22(7):327–30. doi: 10.1111/j.1600-0714.1993.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee V, McCaughan B, Scolyer R. Polymorphous low-grade adenocarcinoma in the lung: a case report. Int J Surg Pathol. 2004;12(3):287–92. doi: 10.1177/106689690401200313. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka F, Wada H, Inui K, et al. Pulmonary metastasis of polymorphous low-grade adenocarcinoma of the minor salivary gland. Thorac Cardiovasc Surg. 1995;43(3):178–80. doi: 10.1055/s-2007-1013795. [DOI] [PubMed] [Google Scholar]
- 30.Thomas K, Cumberworth V, McEwan J. Orbital and skin metastases in a polymorphous low grade adenocarcinoma of the salivary gland. J Laryngol Otol. 1995;109(12):1222–5. doi: 10.1017/S0022215100132517. [DOI] [PubMed] [Google Scholar]
- 31.Hannen E, Bulten J, Festen J, et al. Polymorphous low grade adenocarcinoma with distant metastases and deletions on chromosome 6q23-qter and 11q23-qter: a case report. J Clin Pathol. 2000;53(12):942–5. doi: 10.1136/jcp.53.12.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crean S, Bryant C, Bennett J, et al. Four cases of polymorphous low-grade adenocarcinoma. Int J Oral Maxillofac Surg. 1996;25(1):40–4. doi: 10.1016/S0901-5027(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 33.Le Q, Birdwell S, Terris D, et al. Postoperative irradiation of minor salivary gland malignancies of the head and neck. Radiother Oncol. 1999;52(2):165–71. doi: 10.1016/S0167-8140(99)00084-5. [DOI] [PubMed] [Google Scholar]