Abstract
Eosinophilic angiocentric fibrosis (EAF) is a rare, benign condition of unknown etiology involving the sinonasal tract and the upper respiratory airways, and rarely, larynx, and orbit. We report four cases of EAF identified, in three women and one man, aged 31, 57, 27, and 51 years, respectively. The patients complained of sinonasal obstructive symptoms of long duration, nasal masses, epiphora, and/or proptosis. Histologically, all cases demonstrated a dense fibrotic stroma with a perivascular “onion-skin” whorling pattern, and a dense inflammatory infiltrate consisting of lymphocytes, plasma cells, eosinophils, and some neutrophils. In addition, one patient demonstrated modest acute neutrophilic inflammation with focal endothelial proliferation. No patient exhibited clinical or histological evidence of Wegener's granulomatosis, granuloma faciale, Kimura's disease, and malignant lymphomas. Surgical excision was performed in all cases, and to date, medical therapy has been of limited help. The clinical and histopathological features and differential diagnoses of this underreported EAF condition are discussed.
Keywords: Eosinophilic angiocenteric fibrosis, Sinonasal tract, Fibrosis, Eosinophils
Introduction
Eosinophilic angiocenteric fibrosis (EAF) is a rare condition of unknown etiology. It was first described by Holmes and Panje in 1983 [1]. Two years later, Roberts and McCann reported two cases of female patients with an unusual stenosing lesion involving the upper respiratory tract and gave a descriptive diagnosis: eosinophilic angiocenteric fibrosis (EAF) [2]. A review of the English literature reveals 28 additional cases of EAF involving the sinonasal tract have been reported [3–22]. EAF typically presents in young to middle-aged females as a slowly progressive upper airway obstruction in association with a submucosal inflammatory, fibrosing tumor-like lesion. Histologically, the lesion is characterized by a perivascular, eosinophil-rich inflammatory infiltrate and progressive fibrosis leading to a characteristic whorling, ‘onion-skin’-type pattern [1]. The unresolving fibrosis and consequent stenosis requires surgical intervention. We report an additional four cases of EAF centered around the nasal cavity and review of pertinent literature available on the previously reported cases.
Materials and Methods
Four patients diagnosed with eosinophilic angiocenteric fibrosis from 1998 to 2008 and originating in the head and neck region were identified from a review of the files of the Department of Pathology at Massachusetts General Hospital, Virginia Commonwealth University Health System and University Health Network of University of Toronto. Clinical records and surgical pathology reports together with follow-up information was reviewed.
Results
Patient Information
The patients included three females and one male who ranged in age from 27 to 57 years (Table 1). The most common presentation was a submucosal or soft tissue mass in the upper respiratory tract (sinonasal cavity) and orbit, progressive nasal obstruction, and epiphora. One patient (case #2) had a history of allergy to mold, dust and ragweed, decreased sense of smell and proptosis. The other patient (case#3) had a history of cocaine abuse and nasal septal perforation (Fig. 1).
Table 1.
Summary of clinical features of our four cases of sinonasal eosinophilic angiocenteric fibrosis
| Case # | Age/sex | Clinical symptoms | Site | Laboratory tests | Radiological findings | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|
| 1 | 31/F | Right epiphora, left orbital mass | Sinuses (left maxillary & ethmoid), orbit | ANCA, p29, MPO | Not done | Multiple biopsies, steroids, single resection | Rec. × 3,4 years; SOB |
| 2 | 57/M | Nasal congestion, epiphora, proptosis, decreased sense of smell | Nose (bilateral lateral wall), multiple sinuses, lacrimal gland | ANCA, p29, MPO, ANA, anti-DNA, peripheral eosinophilia | Mass involving lacrimal gland and multiple sinus cavities | Steroids, single resection | Rec. × 2 years; SOB |
| 3 | 27/F | Nasal obstruction | Nose (bilateral lateral wall) | ANA, ANCA | Not done | Biopsy | Lost to follow-up |
| 4 | 51/F | Nasal mass, obstruction & epiphora | Nose (right lateral wall) | Not done | Mass involving right nasal cavity, medial canthus and nasolacrimal duct | Single resection (debulking) | Persistent mass; small increase in size |
Rec., recurrence; SOB, shortness of breath
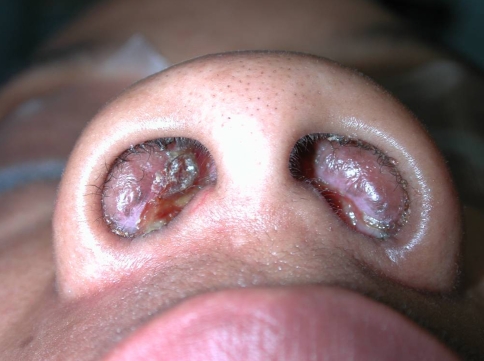
Fig. 1.
A 27-years-old woman affected with bilateral nasal obstructing lesion
Pathologic Findings
The masses ranged from 2.3 to 5.0 cm in size. Histologically, all cases demonstrated a dense fibrotic stroma with a perivascular ‘onion-skin’-type whorling pattern and an inflammatory infiltrate rich in eosinophils, lymphocytes, and plasma cells (Fig. 2). In addition, case #3 demonstrated modest acute inflammation and focal endothelial proliferation. No vasculitis, granuloma formation, giant-cell histiocytic reaction, or necrosis was identified in any of these cases.
Fig. 2.
Nasal mass biopsy. (a) Inflammatory infiltrate consisting of lymphocytes, plasma cells, and eosinophils. (b) Perivascular fibrosis and eosinophil-rich inflammatory infiltrate (hematoxylin and eosin; ×400). (c) Thick collagen bundle with perivascular fibrosis (‘onion-skin’-type whorling pattern)
Immunohistochemical Studies
Immunohistochemical studies were performed on only case #4. CD31 and CD34 positive endothelial cells were seen within the sclerotic nodule. Perivascular, mixed T-cell and B-cell lymphocytic population was demonstrated by CD5 and CD20 immunohistochemical stains, respectively.
Flow Cytometric Studies
Lymphoma work-up by flow cytometry was performed on two cases (#1&2) and revealed polyclonal kappa and lambda positive, CD19 positive B cells, as well as CD4 and CD8 positive T-cells. No evidence of monoclonal B- or unusual T-cell population was identified.
Immunological Studies
Indirect immunofluorescence testing for anti-neutrophil cytoplasmic antibodies (ANCA) performed in three patients (cases #1–3) was negative. In addition, ELISA for antibodies to p29 (proteinase 3) and myeloperoxidase (MPO) performed in two patients (cases #1&2) was also negative. In case #2, cryoglobulin, ANA, and anti-DNA were also negative.
Radiologic Findings
Radiographic evaluation by computed tomography (CT) was available in all the cases and showed expanding soft tissue masses in the nasal turbinate extending into the sinuses (Fig. 3). All lesions, except the case #4, extended into the orbital soft tissue.
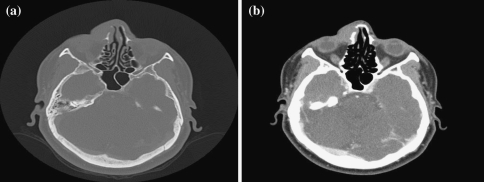
Fig. 3.
A 51-year-old woman with contrast head CT-images. (a) With bone window showing no bone erosion. (b) Soft tissue mass along the right nasal bone extending medially into the nasal cavity
Follow-up and Treatment
In case #1, the patient was on steroid therapy and underwent surgical excision of the lesion. The lesion recurred after 4 years, and recently, the patient has developed shortness of breath and pulmonary nodularity. In case #2, the lesion was excised and the patient has been on Prednisone to relieve nasal congestion. He, too, has developed pulmonary symptoms.
Discussion
EAF is a rare benign lesion of the sinonasal and the upper respiratory tract, and rarely, subglottis and orbit [2, 24–27]. Since 1983, when it was first described by Holmes and Panje [1], there have been only 31 cases of EAF involving the sinonasal tract reported within the English literature to date (Table 2). It’s etiology is unknown, although trauma and allergy have been implicated [2, 9]. Women are more commonly affected than men, but the reason is unknown. The symptoms are non-specific in all cases, and includes nasal obstruction, epistaxis, breathing difficulties, epiphora and less likely, proptosis; however, they tend to be chronic and progressive [1–23]. Radiographic evaluations are also usually non-specific and shows clouding and opacification of the nasal cavity and sinuses with or without bony erosion.
Table 2.
Literature review of reported cases of sinonasal eosinophilic angiocenteric fibrosis
| Report | Age/sex | Clinical symptoms | Site | Related findings | Treatment | EV | OS | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Holmes and Panje [1] | 49/M | Nasal obstruction | Nose (lateral wall) | Granuloma faciale | Intra-lesional triamcinolone, single resection | PD | ||
| Roberts and McCann [2] | 27/F | Nasal congestion | Nose (septum & lateral wall) | None | Antihistamine, nasal & systemic steroids, multiple resection | + | + | PD |
| 59/F | Nasal congestion | Nose (septum & lateral wall) | Granuloma faciale, urticaria | Intra-lesional steroids, multiple resection | + | + | PD | |
| Roberts and McCann [3] | 54/F | Nasal stuffiness | Nose (septum) | None | Multiple resections, radiotherapy | N/A | ||
| 50/F | Nasal stuffiness | Nose (lateral wall) | None | Multiple resections | PD | |||
| Altemani et al. [4] | 54/F | Nasal obstruction | Nose (septum & lateral wall) | Allergic rhinitis | Multiple resections | + | + | PD |
| Matai et al. [5] | 51/M | Nasal obstruction | Nose (septum) | Hay fever, prior nasal trauma | Single resection | + | + | FD |
| Thompson and Heffner [6] | 28/M | Nasal obstruction, epistaxis, pain | Nose (septum & lateral wall), maxillary sinus | None | Nasal & systemic steroids, single resection | + | + | PD |
| 49/F | Nasal obstruction | Nose (septum) | None | Nasal & systemic steroids, single resection | + | + | PD | |
| 64/F | Nasal obstruction | Nose (septum & lateral wall), maxillary sinus | None | Nasal & systemic steroids, multiple resection | + | + | PD | |
| Burns et al. [7] | 38/M | Nasal obstruction & swelling | Nose (septum & lateral wall) | Granuloma faciale | Intra-lesional & systemic steroids, multiple resection, dapsone, YAG laser | + | + | PD |
| Loane et al. [8] | 42/M | Nasal obstruction, post-nasal drip | Nose (septum) | Wegener’s granulomatosis, asthma | Immunosuppression, multiple resections | + | N/A | |
| Pereira et al. [9] | 52/M | Nasal obstruction | Nose (septum) | Allergic rhinitis | Partial resection | + | + | PD |
| Owa et al. [10] | 41/M | Nasal obstruction | Nose (septum) | None | Single resection | + | FD | |
| Goldman [11] | 50/F | Nasal obstruction | Nose (septum & lateral wall) | None | Intra-lesional steroids, multiple resection | PD | ||
| Tabaee et al. [12] | 79/M | Nasal congestion, swelling, tenderness | Nose (septum & lateral wall) | Granuloma faciale, c-ANCA, Squamous cell cancer, prior nasal surgery | Single resection | + | Resolution at 1 year follow-up | |
| Onder and Sungur [13] | 45/M | Nasal obstruction | Nose (septum) | None | Single resection | + | N/A | |
| Chinell et al. [14] | 31/F | Nasal obstruction | Nose (septum) | Granuloma faciale | Biopsy and dapsone | + | PD | |
| Nguyen et al. [15] | 31/F | Nasal obstruction | Nose (septum) | None | Single resection | + | N/A | |
| Narayan and Douglas-Jones [16] | 72/F | Nasal obstruction & swelling | Nose (septum & lateral wall) | Granuloma faciale | Biopsy | + | PD | |
| Paun et al. [17] | 37/F | Epistaxis, epiphora | Nose (lateral wall) | Granuloma faciale, pencillin-sensitive | Multiple resections, oral steroids and azathioprine | PD | ||
| 68/M | Nasal obstruction | Nose (septum) | None | Single resection | + | + | FD | |
| 57/F | Nasal obstruction | Nose (septum), orbit | None | Single resection, dapsone, hydroxychloroquine, azathioprine, systemic steroids | + | + | PD | |
| 58/F | Nasal obstruction | Nose (lateral wall) | None | Single resection | + | FD | ||
| Yung et al. [18] | 66/F | Recurrent epistaxis | Nose (septum) | Granuloma faciale | Biopsy | + | + | PD |
| 45/F | Recurrent epistaxis, nasal obstruction | Nose (septum & lateral wall), maxillary sinus | Granuloma faciale | Biopsy, oral prednisolone, azathioprine | + | + | PD | |
| Holme et al. [19] | 72/F | Nasal obstruction | Nose (septum & lateral wall) | Granuloma faciale | Biopsy, pulsed dye laser, dapsone and clofazimine | + | PD | |
| Slovik et al. [20] | 45/M | Nasal obstruction | Nose | Rheumatoid arthritis, chronic inflammatory bowel disease | Biopsy | PD | ||
| Clauser et al. [21] | 31/M | Nasal obstruction | Nose (septum) | None | Single resection | + | + | PD |
| Watanabe and Moriwaki [22] | 51/M | Nasal obstruction | Nose (septum) | None | Single resection | − | − | FD |
| Nigar et al. [23] | 67/F | Nasal obstruction & swelling | Nose (lateral wall), gingiva | Granuloma faciale | Single resection | + | PD | |
| Present paper | 31/F | Epiphora, orbital mass | Sinuses (maxillary, ethmoid), orbit | None | Multiple biopsies, steroids, single resection | − | + | PD |
| 57/M | Nasal congestion, epiphora, proptosis | Nose (lateral wall), multiple sinuses, lacrimal gland | Allergic rhinitis, peripheral eosinophilia | Steroids, single resection | − | + | PD | |
| 27/F | Nasal obstruction | Nose (lateral wall) | Cocaine abuse | Biopsy | + | + | Lost to follow-up | |
| 51/F | Nasal mass, obstruction & epiphora | Nose (lateral wall) | None | Single resection | − | + | PD |
EV, eosinophilic vasculitis; OS, onion skinning; Blank, pathology not reported; PD, persistent disease; FD, free of disease; N/A, not available
The histology of EAF is pathognomonic and is characterized by perivascular inflammatory cell infiltration with progressive fibrosis around small vessels, leading to a characteristic ‘onion-skin’-type pattern [2]. Eosinophils are the predominant inflammatory cells. The main histologic differential diagnosis includes lesions with prominent eosinophilic infiltrates (Table 3). Absence of geographic necrosis, necrotizing vasculitis, and granulomatous inflammation excludes Wegener’s granulomatosis (WG) and Churg-Strauss syndrome (CS). Blood test positive for c-ANCA and p-ANCA supports the diagnosis of WG and CS, respectively. Absence of dense lymphoid aggregates with prominent germinal centers excludes Kimura’s disease. Some authors have suggested an association between EAF and granuloma faciale, a benign cutaneous disease of unknown etiology characterized by sharply circumscribed plaques and skin nodules, with a predilection for the facial region [28]. While there are some histopathologic features in common among these diseases, granuloma faciale lacks the ‘onion-skin’ pattern of collagen whorling around a central vessel that is characteristic of EAF. The debate, therefore, continues as to whether EAF is a mucosal variant of granuloma faciale, or whether they are distinct entities [9, 12, 18]. Nasal polyps and chronic sinusitis are also included in the differential diagnosis, however, the presence of progressive inflammatory process should warrant further clinical, radiological and histologic evaluation.
Table 3.
Differential diagnosis of eosinophilic angiocentric fibrosis in sinonasal and upper respiratory tract
| Disease | Sites | Clinical presentation | Laboratory tests | Histopathological features |
|---|---|---|---|---|
| Eosinophilic angiocenteric fibrosis | Upper respiratory tract, lacrimal sac, orbit | Nasal obstruction, epiphora, proptosis | None | Dense fibrosis with perivascular ‘onion-skin’ whorling pattern, eosinophil-rich inflammatory infiltrate |
| Wegener’s granulomatosis | Upper respiratory tract, lungs, kidneys | Nasal pain, stuffiness, rhinitis, hearing loss, saddle-nose deformity (later stage) | Cytoplasmic (antiproteinase 3) c-ANCA positive (85%) |
Geographic zones of bionecrosis, foreign-body giant cells, granuloma (rare) |
| Churg-Strauss syndrome | Upper and lower respiratory tract, skin, kidney, gastrointestinal tract, heart, nerve | Asthma, sinusitis | Blood eosinophilia, perinuclear (anti-myeloperoxidae) p-ANCA positive | Fibrinoid necrosis, extravascular granulomas with eosinophilia |
| Kimura’s disease | Skin (head and neck) | Subcutaneous nodules, lymphadenopathy, salivary gland enlargement | Blood eosinophilia, raised ESR and serum IgE levels | Dense lymphoid aggregates with prominent germinal centers, fibrous tissue |
| Granuloma faciale | Skin (face) | Plaques and nodules | None | Polymorphous infiltrate (N, E) in the dermis, fibrosis, Grenz zone |
ANCA, antineutrophil cytoplasmic antibodies; ESR, erythrocyte sedimentation rate; N, neutrophils; E, eosinophils
Based on the literature to date, the most common treatment modality of EAF is surgical resection. The recurrence rate is extremely high, with persistence of disease seen following most nasal resections [2–4, 6, 7, 9, 11]. Other treatment modalities, including local and systemic corticosteroid therapy have been tried with minimal clinical resolution of disease [2, 6, 7, 17, 18, 25]. No definitive treatment of choice has been recognized, and the etiology of EAF remains elusive despite the consistency of pathological findings in the described EAF cases.
In conclusion, EAF is a rare, benign, progressive fibro-inflammatory lesion of unknown etiology with predilection for the upper respiratory tract, especially the sinonasal tract. It is a diagnosis of exclusion. Surgical resection is the treatment of choice, though multiple procedures are often required. Though a possible association between GF and EAF, and WG and EAF have been reported, further case reports of this rare lesion are necessary before the etiology, pathogenesis and management can be clearly defined [1, 2, 7, 8, 12, 14–18, 23].
References
- 1.Holmes DK, Panje WR. Intranasal granuloma faciale. Am J Otolaryngol. 1983;4:184–6. doi: 10.1016/S0196-0709(83)80041-6. [DOI] [PubMed] [Google Scholar]
- 2.Roberts PF, McCann BG. Eosinophilic angiocentric fibrosis of the upper respiratory tract: a mucosal variant of granuloma faciale? A report of three cases. Histopathology. 1985;9(11):1217–25. doi: 10.1111/j.1365-2559.1985.tb02801.x. [DOI] [PubMed] [Google Scholar]
- 3.Roberts PF, McCann BG. Eosinophilic angiocentric fibrosis of the upper respiratory tract: a postscript. Histopathology. 1997;31(4):385–6. [PubMed] [Google Scholar]
- 4.Altemani AM, Pilch BZ, Sakano E, et al. Eosinophilic angiocentric fibrosis of the nasal cavity. Mod Pathol. 1997;10(4):391–3. [PubMed] [Google Scholar]
- 5.Matai V, Baer S, Barnes S, et al. Eosinophilic angiocentric fibrosis. J Laryngol Otol. 2000;114(7):563–4. doi: 10.1258/0022215001906183. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LD, Heffner DK. Sinonasal tract eosinophilic angiocentric fibrosis. A report of three cases. Am J Clin Pathol. 2001;115(2):243–8. doi: 10.1309/7D97-83KY-6NW2-5608. [DOI] [PubMed] [Google Scholar]
- 7.Burns BV, Roberts PF, Carpentier J, et al. Eosinophilic angiocentric fibrosis affecting the nasal cavity. A mucosal variant of the skin lesion granuloma faciale. J Laryngol Otol. 2001;115(3):223–6. doi: 10.1258/0022215011907037. [DOI] [PubMed] [Google Scholar]
- 8.Loane J, Jaramillo M, Young HA, et al. Eosinophilic angiocentric fibrosis and Wegener’s granulomatosis: a case report and literature review. J Clin Pathol. 2001;54(8):640–1. doi: 10.1136/jcp.54.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira EM, Millas I, Reis-Filho JS, et al. Eosinophilic angiocentric fibrosis of the sinonasal tract: report on the clinicopathologic features of a case and review of the literature. Head Neck. 2002;24(3):307–11. doi: 10.1002/hed.10041. [DOI] [PubMed] [Google Scholar]
- 10.Owa AO, Boyle S, Gallimore AP. Eosinophilic angiocentric fibrosis as a cause of nasal obstruction. Rhinoplasty. 2002;40(1):41–3. [PubMed] [Google Scholar]
- 11.Goldman NC. Angiocentric eosinophilic fibrosis. Otolaryngol Head Neck Surg. 2003;128(3):445–6. doi: 10.1067/mhn.2003.22. [DOI] [PubMed] [Google Scholar]
- 12.Tabaee A, Zadeh MH, Proytcheva M, et al. Eosinophilic angiocentric fibrosis. J Laryngol Otol. 2003;117(5):410–3. doi: 10.1258/002221503321626500. [DOI] [PubMed] [Google Scholar]
- 13.Onder S, Sungur A. Eosinophilic angiocentric fibrosis: an unusual entity of the nasolacrimal tract. Arch Pathol Lab Med. 2004;128(1):90–1. doi: 10.5858/2004-128-90-EAF. [DOI] [PubMed] [Google Scholar]
- 14.Chinell PA, Kawashita MY, Sotto MN, et al. Grauloma faciale associated with sinonasal tract eosinophilic angiocentric fibrosis. Acta Derm Venereol. 2004;84(6):486–7. [PubMed] [Google Scholar]
- 15.Nguyen DB, Alex JC, Calhoun B. Eosinophilic angiocenteric fibrosis in a patient with nasal obstruction. Ear Nose Throat J. 2004;83(3):183–4. [PubMed] [Google Scholar]
- 16.Narayan J, Douglas-Jones AG. Eosinophilic angiocentric fibrosis and granuloma faciale: analysis of cellular infiltrate and review of literature. Ann Otol Rhinol Laryngol. 2005;114(1 Pt 1):35–42. doi: 10.1177/000348940511400107. [DOI] [PubMed] [Google Scholar]
- 17.Paun S, Lund VJ, Gallimore A. Nasal fibrosis: long-term follow up of four cases of eosinophilic angiocentric fibrosis. J Laryngol Otol. 2005;119(2):119–24. doi: 10.1258/0022215053419989. [DOI] [PubMed] [Google Scholar]
- 18.Yung A, Wachsmuth R, Ramnath R, et al. Eosinophilic angiocentric fibrosis—a rare mucosal variant of granuloma faciale which may present to the dermatologist. Br J Dermatol. 2005;152(3):574–6. doi: 10.1111/j.1365-2133.2005.06439.x. [DOI] [PubMed] [Google Scholar]
- 19.Holme SA, Laidler P, Holt PJ. Concurrent granuloma faciale and eosinophilic angiocentric fibrosis. Br J Dermatol. 2005;153(4):851–3. doi: 10.1111/j.1365-2133.2005.06864.x. [DOI] [PubMed] [Google Scholar]
- 20.Slovik Y, Putterman M, Nash M, et al. Eosinophilic angiocentric fibrosis of sinonasal tract in a male patient with chronic bowel inflammation. Am J Rhinol. 2006;20(1):91–4. [PubMed] [Google Scholar]
- 21.Clauser L, Mandrioli S, Polito J, et al. Eosinophilic angiocentric fibrosis. J Craniofac Surg. 2006;17(4):812–4. doi: 10.1097/00001665-200607000-00040. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe N, Moriwaki K. Atypical eosinophilic angiocentric fibrosison nasal septum. Auris Nasus Larynx. 2006;33(3):355–8. doi: 10.1016/j.anl.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Nigar E, Dhillon R, Carr E, et al. Eosinophilic angiocentric fibrosis and extrafacial granuloma faciale. Histopathology. 2007;51(5):729–31. doi: 10.1111/j.1365-2559.2007.02840.x. [DOI] [PubMed] [Google Scholar]
- 24.Fageeh NA, Mai KT, Odell PF. Eosinophilic angiocentric fibrosis of the subglottic region of the larynx and upper trachea. J Otolaryngol. 1996;25(4):276–8. [PubMed] [Google Scholar]
- 25.Leibovitch I, James CL, Wormald PJ, et al. Orbital eosinophilic angiocentric fibrosis case report and review of literature. Ophthalmology. 2006;113(1):148–52. doi: 10.1016/j.ophtha.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 26.Valenzuela AA, Whitehead KJ, Brown I, Sullivan TJ. Eosinophilic angiocentric fibrosis: an unusual entity producing complete lacrimal duct obstruction. Orbit. 2006;25(2):159–61. doi: 10.1080/01676830600674544. [DOI] [PubMed] [Google Scholar]
- 27.Kiratli H, Onder S, Yildiz S, et al. Eosinophilic angiocentric fibrosis of the orbit. Clin Experiment Ophthalmol. 2008;36(3):274–6. doi: 10.1111/j.1442-9071.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- 28.Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51(2):269–73. doi: 10.1016/j.jaad.2003.11.071. [DOI] [PubMed] [Google Scholar]