Abstract
In an effort to understand the unusual cytogenetic damage earlier encountered in the Yanomama Indians, plasma samples from 425 Amerindians representing 14 tribes have been tested for hemagglutination inhibition antibodies to the human JC polyoma virus and from 369 Amerinds from 13 tribes for hemagglutination inhibition antibodies to the human BK polyoma virus. There is for both viruses highly significant heterogeneity between tribes for the prevalence of serum antibody titers ≥1/40, the pattern of infection suggesting that these two viruses only relatively recently have been introduced into some of these tribes. Some of these samples, from populations with no known exposure to the simian polyoma virus SV40, also were tested for antibodies to this virus by using an immunospot assay. In contrast to the findings of Brown et al. (Brown, P., Tsai, T. & Gajdusek, D. C. (1975) Am. J. Epidemiol. 102, 331–340), none of the samples was found to possess antibodies to SV40. In addition, no significant titers to SV40 were found in a sample of 97 Japanese adults, many of whom had been found to exhibit elevated titers to the JC and BK viruses. This study thus suggests that these human sera contain significant antibody titers to the human polyoma viruses JC and BK but do not appear to contain either cross-reactive antibodies to SV40 or primary antibodies resulting from SV40 infection.
In 1969, we undertook to determine a baseline for cytogenetic damage in the cultured lymphocytes of individuals from two remote villages of Yanomama Amerindians, with the expectation of low levels of damage in this unacculturated, nonindustrialized population. To our surprise, 23 among a total of 4,969 cells scored showed a picture of extreme cytogenetic damage (1). We later termed these abnormal cells “rogue cells,” now arbitrarily defined as cells containing five or more exchange-type aberrations for which precise karyotypic identification of the origin of the aberrant chromosomes is usually impossible (2). Within the next several decades, similar cells were reported in cytogenetic studies of selected populations in England, Japan, and the former Soviet Union (2–11), albeit, with one exception (5), never with a frequency approaching the original observation.
Because the simian polyoma virus 40 (SV40) had been shown to produce similar damage in cultured human fibroblasts (12–15), the possible role in these cytological findings of infection with two well known human polyoma viruses, the JC virus (JCV) and the BK virus (BKV), was investigated (16). It was found that antibody titers against these two viruses were significantly elevated in persons in whom rogue cells were detected, the anti-JCV titers more so than the anti-BKV titers. Furthermore, inoculation of cultured human fetal brain cells with JCV produced chromosomal damage in the early post-inoculation cell divisions similar to that produced by SV40 in the early divisions of inoculated cultured human fibroblasts. On the basis of these observations, we hypothesized that a newly acquired infection with JCV (or, possibly, BKV) or reactivation of an existing infection was at least one cause of the appearance of rogue cells in the peripheral circulation (16).
The cytogenetic studies of the Yanomama were only one aspect of a much broader multidisciplinary study of various Amerindian tribes of Central and South America carried out between 1962 and 1986. In the course of these studies, a plasma bank encompassing samples from some 16 different and widely scattered tribes was established. The primary objective of the present study has been to return to the population in which rogue cells were first discovered and explore the frequency and distribution of seropositives for the two human polyoma virus now seen as possible causative agents. We report the results of antibody detection in samples from 425 individuals from the Yanomama and 13 other tribes with respect to anti-JCV and 369 individuals from the Yanomama and 12 other tribes with respect to BKV. It is argued that the frequency and distribution of positive titers to JCV and BKV and the level of the positive titers are consistent with the thesis that these viruses are only now reaching some of these populations, with our original cytogenetic observations possibly attributable to a first or early experience of these two Yanomama villages with the virus. These findings create an interesting paradox because the recent studies of Agostini et al. (17) suggest a quite high frequency of the Asian type of JCV infection in certain North American Amerindian tribes.
The second objective of this study stems from the fact that Brown et al. (18) reported that, among a collection of sera samples from remote, unacculturated tribal populations “with no possibility of having received any SV40-contaminated vaccine or having contacted SV40-infected primates” (ref. 18, p. 337), 14 of 40 sera with BK hemagglutination inhibition (HI) antibody titers ≥80 exhibited significant titers of SV40 neutralizing antibody, as did 6 of 111 sera with titers ≤20. The sample included an unspecified number of Amerindians. These positive findings were regarded as spurious because of serological cross-reactivity. Recently, however, reports of the detection with the PCR technique of SV40 DNA in certain human tumor tissues has reopened the discussion of whether SV40 may have infected some human populations (19–25). Others, however, using a highly sensitive plaque reduction assay, have failed to detect antibodies to SV40 or detect SV40 DNA in similar human tumors (26). Furthermore, no increase in the incidence of various cancers has been observed in the United States after administration of an early polio vaccine accidentally contaminated with SV40 (27, 28). Currently, discussion of the possible presence of SV40 DNA in some human tumors is vigorous (29). The present collection offers an opportunity to attempt to reexamine previous observations (18) regarding serological cross-reactivity within the polyoma virus family. To this end, we have tested the plasma of 165 members of the present sample for the presence of antibodies to SV40. We also have tested, for contrast, a sample of 97 adult Japanese previously found with the HI technique to average rather high titers for antibodies to JCV and BKV (16) for the presence of antibodies to SV40. The results of these tests also do not support either the thesis of serological cross-reactivity between simian SV40 and the human JC and BK viruses used in these tests or the thesis that SV40 has infected these populations.
SUBJECTS AND METHODS
Subjects.
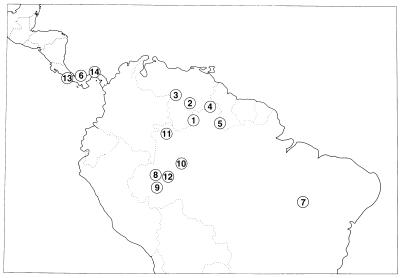
The locations of the 14 tribes from which the plasma samples were obtained are shown in Fig. 1. The two samples of Pano-speakers are presented as representing separate tribes because of the marked heterogeneity within this linguistic subdivision. Our publications on these tribes (see Table 1) reference evidences of contact and admixture with non-Amerindians as a surrogate measure of contact with cultures known to be infected with JCV and BKV.
Figure 1.
Locations in lower Central and northern South America of the 14 Amerindian tribes whose plasma was tested for antibodies to JCV and BKV. Numbers correspond to those given in Table 1.
Table 1.
The HI antibody titers against JCV and BKV in 14 Amerindian tribes of Central and South America
| Tribe | Reference | JCV titer
|
BKV titer
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <1/40 | 1/40 | 1/80 | ≥1/160 | Total | <1/40 | 1/40 | 1/80 | ≥1/160 | Total | ||
| Yanomama | 30 | 129 | 34 | 14 | 6 | 183 | 122 | 23 | 4 | 1 | 150 |
| 70.49% | 18.58% | 7.65% | 3.28% | 43.06% | 81.33% | 15.33% | 2.67% | 0.67% | 40.65% | ||
| Makiritare | 31 | 17 | 1 | 1 | 2 | 21 | 15 | 3 | 2 | 1 | 21 |
| 80.95% | 4.76% | 4.76% | 9.52% | 4.94% | 71.43% | 14.29% | 9.52% | 4.76% | 5.69% | ||
| Piaroa | 32 | 21 | 2 | 0 | 0 | 23 | |||||
| 91.30% | 8.70% | 0.0% | 0.0% | 5.41% | |||||||
| Macushi | 33 | 2 | 5 | 8 | 5 | 20 | 0 | 2 | 10 | 8 | 20 |
| 10.0% | 25.0% | 40.0% | 25.0% | 4.71% | 0.0% | 10.0% | 50.0% | 40.0% | 5.42% | ||
| Wapishana | 33 | 5 | 6 | 6 | 4 | 21 | 2 | 6 | 8 | 5 | 21 |
| 23.81% | 28.57% | 28.57% | 19.05% | 4.94% | 9.52% | 28.57% | 38.10% | 23.81% | 5.69% | ||
| Guaymi | 34 | 6 | 9 | 8 | 1 | 24 | 3 | 7 | 8 | 6 | 24 |
| 25.0% | 37.5% | 33.33% | 4.17% | 5.65% | 12.5% | 29.17% | 33.33% | 25.0% | 6.50% | ||
| Kraho | 35 | 10 | 8 | 1 | 1 | 20 | 2 | 4 | 7 | 7 | 20 |
| 50.0% | 40.0% | 5.0% | 5.0% | 4.71% | 10.0% | 20.0% | 35.0% | 35.0% | 5.42% | ||
| Northern Pano | 36 | 0 | 7 | 3 | 2 | 12 | 0 | 4 | 6 | 2 | 12 |
| 0.0% | 58.33% | 25.0% | 16.67% | 2.82% | 0.0% | 33.33% | 50.0% | 16.67% | 3.25% | ||
| Southern Pano | 36 | 3 | 3 | 3 | 1 | 10 | 3 | 0 | 4 | 3 | 10 |
| 30.0% | 30.0% | 30.0% | 10.0% | 2.35% | 30.0% | 0.0% | 40.0% | 30.0% | 2.71% | ||
| Ticuna | 37 | 1 | 5 | 4 | 8 | 18 | 1 | 2 | 11 | 4 | 18 |
| 5.56% | 27.78% | 22.22% | 44.44% | 4.24% | 5.56% | 11.11% | 61.11% | 22.22% | 4.88% | ||
| Baniwa | 36 | 2 | 12 | 2 | 0 | 16 | 0 | 2 | 9 | 5 | 16 |
| 12.5% | 75.0% | 12.5% | 0.0% | 3.76% | 0.0% | 12.5% | 56.25% | 31.25% | 4.34% | ||
| Kanamari | 36 | 1 | 7 | 7 | 3 | 18 | 0 | 6 | 11 | 1 | 18 |
| 5.56% | 38.89% | 38.89% | 16.67% | 4.24% | 0.0% | 3.33% | 61.11% | 5.56% | 4.88% | ||
| Boruca | 38 | 0 | 3 | 12 | 3 | 18 | 0 | 2 | 5 | 11 | 18 |
| 0.0% | 16.67% | 66.67% | 16.67% | 4.24% | 0.0% | 11.11% | 27.78% | 61.11% | 4.88% | ||
| Cuna | 38 | 4 | 12 | 5 | 0 | 21 | 0 | 4 | 10 | 7 | 21 |
| 19.05% | 57.14% | 23.81% | 0.0% | 4.94% | 0.0% | 19.05% | 47.62% | 33.33% | 5.69% | ||
| Totals | 201 | 114 | 74 | 36 | 425 | 148 | 65 | 95 | 61 | 369 | |
| 47.29% | 26.82% | 17.41% | 8.47% | 100.0% | 40.11% | 17.62% | 25.75% | 16.53% | 100.0% | ||
The ages of these Indians are seldom a matter of exact record and, for the most part, have been estimated in the field. For the purposes of this study, it should be emphasized that these are “young” populations. For instance, in the Yanomama, on whom the most complete demographic studies have been performed, the average estimated age for the total population was 15 (39). Because blood samples were seldom drawn from children <5 years of age, the age of the average Yanomama in the sample was ≈20 years, with very few individuals >50, and this same figure is generally applicable to the members of the other tribes sampled.
Methods.
The presence of antibodies against JCV and BKV was determined as described in detail in Neel et al. (16), using the HI technique. With respect to testing for the presence of antibodies to SV40, the methodology of Heberling and Kolter (40) was used. It should be noted that SV40 does not hemagglutinate erythrocytes as the human and rodent polyoma viruses do. In brief, viral antigen was prepared from SV40-infected monkey cells, was titrated by plaque assay, and was blotted onto 0.45-μm pore size nitrocellulose sheets. The nitrocellulose sheets were submerged into nonfat dry milk or other blotting agents in PBS containing 0.01% antifoam A (Sigma) and 0.0001% merthiolate. The sheet was placed on blotting paper saturated with PBS-Tween 20. Sera were diluted in PBS and were applied by saturating absorbent paper strips onto the nitrocellulose sheet over the test antigen. The samples were submerged in goat antihuman IgG conjugated with alkaline phosphatase for 1 hr at 37°C. After thorough washings, the alkaline phosphatase substrate, naphthol AS-MX phosphate with fast red, was applied, and the color reaction was monitored. Controls consisted of uninfected cell culture antigens and known positive and negative sera. The assay was done by the Simian Diagnostic Laboratory, Virus Reference Laboratory, San Antonio, TX, which uses such assays for screening colonies of nonhuman primates for SV40 infection. A negative sample was defined as absence of reactivity on the blotted filter at a 1:5 dilution.
RESULTS
Tables 1 and 2 present the basic findings with respect to JCV and BKV. The tribes are listed in the order in which the samples were collected between 1966 and 1986. Note that, for the Piaroa, data are available for JCV antibodies but not for BKV. Some 53% of the samples were characterized by what conventionally are considered to be significant titers against JCV (≥1/40), and 60% were characterized by similar titers against BKV. However, inspection of the data suggests so much tribal heterogeneity that an average value across tribes is of little meaning. For instance, all tested members of four of the more acculturated tribes (Baniwa, Kanamori, Boruca, and Cuna) exhibited significant titers to BKV. This impression is confirmed by a χ2 test for heterogeneity (see Table 2). Because of a paucity of entries in some cells of Table 1, for the analysis, all titers ≥1/40 have been grouped. The statistical computations used the sas 6.07 (SAS Institute, Carey, NC). A value of 0.5 has been substituted in cells with zero entries. There is enormous heterogeneity in the tribal data with respect to presence of significant titers.
Table 2.
An analysis of the heterogeneity of the data of Tables 1 and 2 with particular reference to the apparent degree of contact with non-Amerindians
| Analysis | JCV
|
BKV
|
||||||
|---|---|---|---|---|---|---|---|---|
| Tribes | χ2 | df | P | Tribes | χ2 | df | P | |
| Total heterogeneity | 1–14 | 152.54 | 13 | <0.01 | 1–14 (minus 3) | 209.23 | 12 | <0.001 |
| Within least contacted | 1–3 | 5.20 | 2 | 0.07 | 1–2 | 1.13 | 1 | 0.287 |
| Within most contacted | 4–14 | 26.16 | 10 | 0.005 | 4–14 | 12.84 | 10 | 0.233 |
| Σ least contacted vs. Σ most contacted | 1–3 vs. 4–14 | 133.28 | 1 | <0.001 | 1–2 vs. 4–14 | 205.03 | 1 | <0.001 |
It is notable that, even when positive, the titers are usually low. Thus, of the 224 persons with positive JCV titers, only 12 of the titers exceeded 1/320, and, for BKV, the comparable value was 2 among 221. By contrast, in a population of 100 relatively young Japanese (average age 23.9 ± 4.5 years) tested in the same laboratory, 28 of the 80 positive titers for JCV exceeded 1/320, as did 13 of the 80 positive titers for BKV (16). The difference is highly significant for both JCV (χ2 = 45.33, df = 1, P < 0.001) and BKV (χ2 = 29.21, df = 1, P < 0.001).
Degree of Acculturation and Seropositivity.
On the hypothesis that an important cause of this heterogeneity could be the degree of contact with non-Amerindians, we have divided the tribes into two groups, namely, “less contacted” and “more contacted.” This is a somewhat subjective decision, reached on the basis of the historical record, field notes, and the results of genetic typings that would reveal ethnic admixture. Some documentation for the assignment of tribes in this respect will be found in the references given in Table 1. It is important to recognize that even the less contacted tribes may over the years have experienced small and sporadic contacts with non-Indians (compare the account in ref. 41, regarding one of the most isolated tribes, the Yanomama). Treating tribes 1, 2, 3 as less contacted for the JCV studies and 1 and 2 as the less contacted for the BKV studies, a further heterogeneity χ2 analysis yields the results shown in Table 2. Again, all values ≥1/40 have been grouped for purposes of analysis. The “least contacted” tribes are, for both JCV and BKV, homogeneous whereas the “most contacted” tribes are homogeneous for BKV but heterogeneous for JCV. The final contrast involves the pooled least contacted tribes against the pooled more contacted tribes for the two viruses. Both contrasts result in relatively enormous χ2 values (Table 2). With respect to JCV, the respective percentages of the titer <1/40 group for the less and more contacted are 73.6 and 17.2, respectively, whereas for BKV, the corresponding values are 80.1 and 5.6. We consider this finding a strong suggestion of the role of contact with non-Indians in the frequency of positive titers for both viruses.
The Question of Geographic Heterogeneity in Antibody Titers Among the Yanomama.
Only with respect to the Yanomama, for whom JCV typings have been performed on 175 individuals and for BKV on 146, do the data permit an exploration of intratribal heterogeneity in titers. These persons were drawn from 33 villages. Because, however, the number of persons typed per village varies from 1 to 25, some grouping of villages is necessary for a statistical analysis. Ward (ref. 42; see also ref. 43) has published a map of the distribution of the Yanomama villages contacted in our studies and has determined that a genetic cluster analysis appears to partition the Yanomama into six numbered groups, occupying contiguous geographic areas. The Yanomama appear to have expanded in a centrifugal manner both their numbers and territory in the past century; these six clusters are essentially descent groups. For present purposes, we have combined Yanomama groups 1 and 2 (eastern Yanomama), 3 and 4 (northwestern Yanomama), and 5 and 6 (southwestern Yanomama). This results in samples of 41, 107, and 27 persons for JCV and 35, 84, and 27 persons for BKV, as shown in Table 3. Again grouping all titer values ≥1/40 for analytical purposes, the heterogeneity χ2 values for the data of Table 3 are as follows: for JCV, χ2 = 9.35, df = 2, and P = 0.009; and for BKV, χ2 = 7.83, df = 2, P = 0.020. For both JCV and BKV, this heterogeneity is the result of a higher frequency of positive responders in the Eastern (Brazilian) portion of the tribal distribution.
Table 3.
Geographic heterogeneity in the distribution of anti-JCV and anti-BKV titers in the Yanomama
| Titer | Area
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Northwest
|
Southwest
|
East
|
Total
|
|||||
| JCV | BKV | JCV | BKV | JCV | BKV | JCV | BKV | |
| <1/40 | 29 | 31 | 80 | 71 | 12 | 17 | 121 | 119 |
| 1/40 | 10 | 4 | 15 | 12 | 9 | 7 | 34 | 23 |
| 1/80 | 2 | 0 | 8 | 0 | 4 | 3 | 14 | 3 |
| ≥1/160 | 0 | 0 | 4 | 1 | 2 | 0 | 6 | 1 |
| Total | 41 | 35 | 107 | 84 | 27 | 27 | 175 | 146 |
Given the similarity in the two geographical distributions, under certain assumptions we may choose to regard the village data on JCV and BKV as independent tests of the hypothesis that these two human polyoma viruses are being introduced to the Yanomama more from the east than west, and we may combine the two χ2 values. The critical assumption is that the viruses are being introduced independently of one another. A less critical assumption is that the epidemiologies of the two viruses are, for practical purposes, identical. The combined χ2 = 17.28, df = 4, and P = 0.0017. Given the limited interaction between distant Yanomama villages, the data are consistent with multiple points of entry of the two viruses into the tribe, but with relatively more positive contacts to the Brazilian east than to the Venezuelan west.
JCV and BKV as Fellow Travelers.
In a previous publication, we reported that, in a Japanese population that appears to be hyperendemic with respect to JCV and BKV infection, there was no significant intra-individual correlation between the titers against these two viruses (16). This test implicitly assumed universal but separate exposures and, if positive, would have suggested cross-reactivity between the respective antibodies. Such a test would be inappropriate for these Amerindian populations if the thesis that the two viruses only now are being introduced is correct because, under these circumstances, it would confound exposure to the virus with response to the virus. We have, however, examined the tribal data for apparent differences in the frequency of persons with significant titers against the two viruses. The only tribe with a noteworthy discrepancy is the Kraho. Although in most tribes the frequencies of positives (titer ≥1/40) for JCV and BKV are quite similar, here there was a notable discrepancy, with 10 of 20 persons positive for JCV but 18 of the same 20 persons positive for BKV (χ2 = 7.62, df = 1, P = 0.006.). We thus conclude, assuming similar susceptibilities to the two viruses, that, in Central and South America in general, the two viruses have traveled together and exposees are equally susceptible to both viruses.
Studies on Antibodies to SV40.
Inasmuch as none of the present Amerindian populations could have had contact with the macaque primate hosts to SV40, and none were known to have been vaccinated against poliomyelitis with a vaccine cultivated on monkey cells, the present study appears to involve an ideal population in which to follow up the observations of Brown et al. (18) regarding cross-reactivity between antibodies to JC and BK, on the one hand, and SV40, on the other. Of the 425 Amerindian sera tested for antibodies to JCV and BKV, 165 also were tested for antibodies to SV40 by using the dot immunobinding assay of Heberling and Kolter (40). None of them exhibited a titer ≥1/5. Inasmuch as the titers in those Amerindians found positive to JCV and BKV were, in general, low or moderate, these findings were not considered a strong test for cross-reactivity. Accordingly, the study was extended to the sample of 199 Japanese adults described in Neel et al. (16) in which 67% of the subjects exhibited HI anti-JCV titers ≥1/40, and 44% exhibited titers ≥1/320. For BKV, 54% of the same sample exhibited anti-BKV titers ≥1/40, and 17% exhibited titers ≥1/320. None of the subset of 97 individuals tested exhibited anti-SV40 titers ≥1/5.
DISCUSSION
The Question of the Time Depth of JCV and BKV Infection in South American Indians.
The pattern of seropositivity for JCV and BKV in South American Indians observed in this study raises the possibility that these two viruses were not present in this region before contact with the Western world. In addition, Brown et al. (18), using the HI technique, have reported relatively low rates of infection in seven Indian tribes of Brazil and Paraguay with respect to BK antibodies and, in a subset of three of these tribes, also low rates of infection with respect to JCV. Furthermore, all nine of the Brazilian Ewarhoyana that they tested lacked antibodies to both JCV and BKV. Candeias et al. (44) examined three tribes of the Upper Xingu region of Brazil for antibodies to these two viruses with the HI technique, finding a low frequency of positive responders to BKV in all three of the tribes but an absence of positive responders to JCV in two of the tribes (39 and 66 tests). All of the tribes in which antibodies were not detected had experienced very limited contacts with non-Indians. These findings would seem to support the hypothesis that these two viruses were not present in many, if any, South American Indian tribes before contact with representatives of Western civilization.
Sugimoto et al. (45) have subdivided JCV into nine subtypes on the basis of the nucleotide composition of a 610-bp sequence from the VT-intergenic regions of the virus. With their terminology, the predominant subtype in Mongolia and surrounding regions, the presumed area of origin for the ancestors of the American Indian (46), is type 2. Recently, Agostini et al. (17) have isolated JCV DNA from the urine of 66.2% of 68 Navaho Indians from New Mexico and 56.0% of 25 Flathead Indians from Montana. Of the 59 isolates of DNA typed, 54 were type 2, rather convincing evidence of the Asiatic origin of the ancestors of these two tribes. No serological studies for JCV antibodies were conducted on these two tribes. However, in Japan, where, in various studies of urban adults, the frequency of persons with anti-JCV titers ≥1/40 varied between 66 and 90% (refs. 16, 47, and 48 and quoted from ref. 49), Kitamura et al. (50) found that 37% of a sample of 82 urban adults were excreting JCV DNA in their urine. Thus, the findings of Agostini et al. (17) imply a high level of seropositivity for this clastogenic virus in these two tribes of North American Indians.
If the hypothesis that the JCV only now is being introduced into the South American tribes under study is correct, this finding would seem to require that the JCV was somehow lost from the ancestors of the South American Indians as they migrated south through Central America. Alternatively, the hypothesis of a recent introduction of JCV into South American Indians may be incorrect, our observations simply indicating a different epidemiology of the JCV in unacculturated tribes as compared with highly contacted tribes. The data also permit a “mixed” explanation: in view of the demonstration quoted above of the Asiatic type of JCV in the Navaho of New Mexico, the particularly high frequency of seropositives in the highly acculturated tribes of Central America (see Table 1 and Fig. 1) might reflect both an extension into Central America of the JCV known to be present in North America and the results of extensive Indian contacts with Caucasoids and Negroids in that region. Fortunately, in time, the decision between these various explanations should be clear. In the JCV classification of Sugimoto et al. (45), the predominant type encountered in Europeans has been designated “1” while the predominant type encountered in Africans has been designated “3.” Accordingly, when JCV DNA ultimately is isolated from the urine or lymphocytes of members of the tribes we have studied, it would be expected to be types 1 (European) or 3 (African) if the virus only now is being introduced but type 2 (Asiatic) if the viral infection predates contact with persons of European or African origin.
There are, however, some unusual aspects to the epidemiology of JCV that must now be brought under consideration. From studies of viral subtypes within nuclear families, Kitamura et al. (51) and Kunitake et al. (52) conclude that, in Japan, viral transmission is from parent to child in approximately half of the infections, the other half of the infections originating outside the nuclear family. From the failure to detect in Okinawans born during the occupation of Okinawa by U.S. troops the JCV subtypes that make up the majority of infections in Americans, Kato et al. (ref. 53, p. 875) conclude that JCV “is rarely transmitted between human populations.” Our data do not contravene that suggestion because there are no data on the events that led to the postulated introduction of JCV into the Amerindians. On the other hand, the intimacy of life in a village of recently contacted Amerindians exceeds the imagination of most urban dwellers. Many Indian villages consist of members of only three or four extended genealogies, within which opportunities for intrafamilial transmission of the virus should have abounded in the past if the viruses were indeed present. Furthermore, Indian women often intermittently nurse sister’s babies from an early age onwards, providing an additional possible route of viral transmission. The fact that the frequency of positives is so low in some villages and tribes in which the virus is present thus reinforces our suggestion that the virus only recently has been introduced to these groups and the argument of Kato et al. (53) that the virus may not be readily transmitted.
Some Questions Raised by the Original Observation of Rogue Cells in Two Yanomama Villages.
As noted in the introduction, our original experience with rogue cells stems from cytogenetic studies in 1969 of the inhabitants of two Yanomama villages (08XY, 11ABC) located in the Parima mountain range near the Brazil–Venezuela border. This area is regarded as the traditional heartland of the Yanomama, from which they have expanded in all directions in the past century. The data on the Yanomama presented in this paper includes tests on six samples from one of these villages, collected at the time of the chromosome studies—all six samples exhibited titers <1/40.
We are thus faced with the paradox that the villages in which rogue cells—thought to be the result of a relatively recently introduced virus—first were observed by us are among the most remote in the entire tribe and the antibody titers in the few plasma samples available from collections at the time of the chromosome studies are nonsignificant. The relatively sparse data on JCV transmission, on its pattern of periodic reactivation, and on the early serological response to an infection render critical thinking on the significance of these observations difficult. In casting about for a possible explanation for a recent introduction of the virus into this region we found, however, that truth may be stranger than fiction. Two missionaries (and their families), representing the New Tribes Mission, had built homes close to one of these villages within the year preceding the fieldwork there of J.V.N. From them, we learned that, 7 years earlier, in 1962, the Brazilian Air Force, as one aspect of a program to create airfields in proximity to its borders, had flown earth-moving equipment and a contingent of troops into this undulating savannah country, with the objective of creating a satisfactory perimeter airstrip. They were followed shortly by a New Tribes Mission family. In this region, the boundary between Brazil and Venezuela is defined as demarcating the watershed for the Orinoco River (Venezuela) and the Amazon River (Brazil). Two years after the airstrip was established, a proper geological survey determined that the airstrip was in Venezuela, and the Brazilians and the mission family of necessity withdrew. There is no documentation of contacts of this group with the Yanomama, but it would be unusual if there were none, these contacts with the potential for the introduction of the two viruses. With the withdrawal of the Brazilians, this area had not known non-Indian contacts until the arrival of the present missionaries. But to explain our observations concerning rogue cells in this region of low anti-JCV titers, we must postulate that the virus was only now becoming active in the subjects of our study, and the activity was so recent that those infected had not yet acquired substantial antibody levels. Otherwise stated, we must make the unlikely postulate that we had fortuitously selected for cytogenetic studies two villages in which the virus was just becoming established, in consequence of which we documented the cytogenetic response of a “virgin-soil” population to the virus. An alternate explanation of our findings is, of course, that JCV activity was not responsible for the original cytogenetic findings in the Yanomama.
The Findings Regarding SV40 in These Tribes.
The findings of Brown et al. (18) that, in tribal populations not believed to be exposed to SV40, approximately one-third of individuals with anti-BKV titers ≥1/80 exhibited significant anti-SV40 titers created the presumption of serological cross-reactivity between the antibodies to human and simian polyoma viruses. (An unspecified number of the subjects of Brown et al. also exhibited significant anti-JCV titers.) The present data, obtained with different serological techniques, lend no support to that presumption. None of the Amerindian sera showed a reaction to the SV40 capsid antigen when the immunoblot assay was used. It is not known, however, whether this assay is less sensitive than neutralization assays for SV40. Considering that some of the sera showed elevated titers to JCV, the lack of any detectable binding to the SV40 antigen was unexpected because there is a close amino acid homology between these papovaviruses (54). However, there is even greater amino acid homology between JCV and BKV, but studies of sera that show very high titers to JCV by no means predict similarly high titers to BKV. Also, in a separate assay format using ELISA developed specifically for JCV and BKV (data not shown), both the HI and ELISA methodologies revealed little cross-reactivity among the papovaviruses. Consequently, these data suggest that these populations of Amerindians, with no contact with the Asiatic macaques that are the natural host of SV40 or with human populations immunized with SV40-contaminated vaccine, do not show antibody to SV40. In addition, members of a Japanese population that had high titers to JCV and BKV (16) also did not react to the SV40 antigen. Although the HI assay used for detecting antibody to the human polyomaviruses differs from the immunospot assay used for SV40, both methods have been shown to correlate satisfactorily with the results of viral neutralization tests (40, 55). These data, therefore, suggest that, in future epidemiological studies of the possible introduction of SV40 into a human population, potential cross-reactivity of any anti-SV40 serum with JCV or BKV capsid protein should not be a problem. The data also suggests that neither remote populations such as the Amerinds described in this paper nor highly developed, urban populations such as the Japanese we studied previously (16) possess antibodies to SV40.
Elsewhere, we have suggested that the clastogenic effects, on some cell types, of infection with JCV may play a role in oncogenesis (16, 56). An obvious inference is that malignancies should be more common in populations with high JCV presence. Unfortunately, populations that appear to differ in JCV pressure, such as relatively uncontacted Amerindians as contrasted with urbanized, industrialized populations, will often differ in so many other ways from each other that it seems unlikely that critical inferences regarding the role of JCV in oncogenesis could be drawn from comparisons of such diverse populations, even if the relevant data could be collected.
Acknowledgments
We are indebted to Dr. Sharon Kardia for statistical advice and to Dr. H. Strickler for a critical review of the paper. We also acknowledge the expert technical assistance of R. Traub, R. Hamilton, and M. Gravel. J.V.N. acknowledges current support from National Institutes of Health Grant CA26803.
ABBREVIATIONS
- JCV
JC virus
- BKV
BK virus
- HI
hemagglutination inhibition
- SV40
simian virus 40
References
- 1.Bloom A D, Neel J V, Choi K W, Iida S, Chagnon N. Proc Natl Acad Sci USA. 1970;66:920–927. doi: 10.1073/pnas.66.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awa A A, Neel J V. Proc Natl Acad Sci USA. 1986;83:1021–1025. doi: 10.1073/pnas.83.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox D P, Robertson F W, Brown T, Whitehead A R, Douglas J D M. Undersea Biomed Res. 1984;11:193–204. [PubMed] [Google Scholar]
- 4.Tawn E J, Cartmel C L, Pyta E M T. Mutat Res. 1985;144:247–250. doi: 10.1016/0165-7992(85)90059-4. [DOI] [PubMed] [Google Scholar]
- 5.Neel J V, Awa A A, Kodama Y, Nakano M, Mabuchi K. Proc Natl Acad Sci USA. 1992;89:6973–6977. doi: 10.1073/pnas.89.15.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheid W, Weber J, Petrenko S, Traut H. Health Phys. 1993;64:531–534. doi: 10.1097/00004032-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Sevan’kaev A V, Tsyb A F, Lloyd D C, Zhloba A A, Moiseenko V V, Skrjabin A M, Climov V M. Int J Radiat Biol. 1993;63:361–367. doi: 10.1080/09553009314550481. [DOI] [PubMed] [Google Scholar]
- 8.Verschaeve L, Domracheva E V, Kuzuetsov S A, Nechai V V. Mutat Res. 1993;287:253–259. doi: 10.1016/0027-5107(93)90018-b. [DOI] [PubMed] [Google Scholar]
- 9.Bochkov N P, Katosova L D. Mutat Res. 1994;323:7–10. doi: 10.1016/0165-7992(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 10.Lazutka J R. Mutat Res. 1996;350:315–329. doi: 10.1016/0027-5107(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 11.Salomaa S, Sevan’kaev A V, Zhloba A A, Kumpusalo E, Mäkinen S, Lindholm C, Kumpusalo L, Kolmakow S, Nissinen A. Int J Radiat Biol. 1997;71:51–59. doi: 10.1080/095530097144418. [DOI] [PubMed] [Google Scholar]
- 12.Ray F A, Peabody D S, Cooper J L, Cram L S, Kraemer P M. J Cell Biochem. 1990;42:13–31. doi: 10.1002/jcb.240420103. [DOI] [PubMed] [Google Scholar]
- 13.Ray F A, Meyne J, Kraemer P M. Mutat Res. 1992;284:265–273. doi: 10.1016/0027-5107(92)90011-p. [DOI] [PubMed] [Google Scholar]
- 14.Stewart N, Bacchetti S. Virology. 1991;180:49–57. doi: 10.1016/0042-6822(91)90008-y. [DOI] [PubMed] [Google Scholar]
- 15.Ray F A, Kraemer P M. Carcinogenesis. 1993;14:1511–1516. doi: 10.1093/carcin/14.8.1511. [DOI] [PubMed] [Google Scholar]
- 16.Neel J V, Major E O, Awa A A, Glover T, Burgess A, Traub R, Curfman B, Satoh C. Proc Natl Acad Sci USA. 1996;93:2690–2695. doi: 10.1073/pnas.93.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agostini H T, Yanagihara R, Davis V, Ryschkewitsch C F, Stoner G L. Proc Natl Acad Sci USA. 1997;94:14542–14546. doi: 10.1073/pnas.94.26.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown P, Tsai T, Gajdusek D C. Am J Epidemiol. 1975;102:331–340. doi: 10.1093/oxfordjournals.aje.a112169. [DOI] [PubMed] [Google Scholar]
- 19.Bergsagel D J, Finegold M J, Butel J S, Kupsky W J, Garcea R L. N Eng J Med. 1992;326:988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- 20.Carbone M, Pass H I, Rizzo P, Marinetti M, Murio M D, Mew D J Y, Levine A S, Procopio A. Oncogene. 1994;9:1781–1790. [PubMed] [Google Scholar]
- 21.Carbone M, Pass H I, Rizzo P, Procopio A, Giuliano M, Pass H I, Gebhardt M C, Mangham C, Hansen M, Malkin D F, et al. Oncogene. 1996;13:527–535. [PubMed] [Google Scholar]
- 22.Martini F, Iaccheri L, Lazzarin L, Carinci P, Corallini A, Gerosa M, Iuzzolino P, Brodano B G, Tongnon M. Cancer Res. 1996;56:4820–4825. [PubMed] [Google Scholar]
- 23.DeLuca A, Baldi A, Esposito V, Howard C M, Bagella L, Rizzo P, Caputi M, Pass H I, Giordano G G, Baldi F, et al. Nat Med. 1997;3:913–916. doi: 10.1038/nm0897-913. [DOI] [PubMed] [Google Scholar]
- 24.Lednicky J A, Stewart A R, Jenkins J J, Finegold M J, Butel J S. Int J Cancer. 1997;72:791–800. doi: 10.1002/(sici)1097-0215(19970904)72:5<791::aid-ijc15>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Barbanti-Brodano G, Martini F, De Matei M, Lazzarin L, Corallini A, Tognon M. Adv Virus Res. 1998;50:69–99. doi: 10.1016/s0065-3527(08)60806-4. [DOI] [PubMed] [Google Scholar]
- 26.Strickler H D, Goedert J J, Fleming M, Travis W D, Williams A E, Rabkin C S, Daniel R W, Shah K V. Cancer Epidemiol Biomarkers Prev. 1996;5:473–475. [PubMed] [Google Scholar]
- 27.Mortimer E A, Jr, Lepow M L, Gold E, Robbins F C, Burton G J, Fraumeni J L., Jr N Eng J Med. 1981;305:1517–1518. doi: 10.1056/NEJM198112173052507. [DOI] [PubMed] [Google Scholar]
- 28.Strickler H D, Rosenberg P S, Devesa S S, Hertel J, Fraumeni J F, Jr, Goedert J J. JAMA J Am Med Assoc. 1998;279:292–295. doi: 10.1001/jama.279.4.292. [DOI] [PubMed] [Google Scholar]
- 29.Brown F, Lewis A M Jr, editors. Simian Virus 40 (SV40): A Possible Human Polyomavirus. New York: Karger; 1997. , pp. xii and 412. [Google Scholar]
- 30.Neel J V. Annu Rev Genet. 1978;12:365–413. doi: 10.1146/annurev.ge.12.120178.002053. [DOI] [PubMed] [Google Scholar]
- 31.Gershowitz H, Layrisse M, Layrisse Z, Neel J V, Brewer C, Chagnon N, Ayres M. Am J Hum Genet. 1970;22:515–525. [PMC free article] [PubMed] [Google Scholar]
- 32.Tanis R J, Neel J V, Dovey H, Morrow M. Am J Hum Genet. 1973;25:655–676. [PMC free article] [PubMed] [Google Scholar]
- 33.Neel J V, Tanis R J, Migliazza E C, Spielman R S, Salzano F, Oliver W J, Morrow M, Bachofer S. Hum Genet. 1977;36:81–107. doi: 10.1007/BF00390440. [DOI] [PubMed] [Google Scholar]
- 34.Tanis R J, Neel J V, de Arauz R T. Am J Hum Genet. 1977;29:419–430. [PMC free article] [PubMed] [Google Scholar]
- 35.Salzano F M, Neel J V, Gershowitz H, Migliazza E C. Am J Phys Anthropol. 1977;47:337–347. doi: 10.1002/ajpa.1330470214. [DOI] [PubMed] [Google Scholar]
- 36.Mohrenweiser H, Neel J V, Mestriner M A, Salzano F M, Migliazza E, Simoes A L, Yoshihara C M. Am J Phys Anthropol. 1979;50:237–246. doi: 10.1002/ajpa.1330500212. [DOI] [PubMed] [Google Scholar]
- 37.Neel J V, Gershowitz H, Mohrenweiser H W, Amos B, Kostyu D D, Salzano F M, Mestriner M A, Lawrence D, Simoes A L, Smouse P E, et al. Ann Hum Genet. 1980;44:37–54. doi: 10.1111/j.1469-1809.1980.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 38.Barrantes R, Smouse P E, Mohrenweiser H W, Gershowitz H, Azofeifa J, Arias T D, Neel J V. Am J Hum Genet. 1990;46:63–84. [PMC free article] [PubMed] [Google Scholar]
- 39.Neel J V, Weiss K M. Am J Phys Anthropol. 1975;42:25–51. doi: 10.1002/ajpa.1330420105. [DOI] [PubMed] [Google Scholar]
- 40.Heberling R L, Kalter S S. J Clin Microbiol. 1986;23:109–113. doi: 10.1128/jcm.23.1.109-113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson R B. Yanomami Warfare: A Political History. Santa Fe, NM: School of American Research Press; 1995. , pp. xiv and 449. [Google Scholar]
- 42.Ward R H. Ann Hum Genet. 1972;36:21–43. doi: 10.1111/j.1469-1809.1972.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 43.Tanis R E, Ferrell R E, Neel J V, Morrow M. Ann Hum Genet. 1974;38:179–190. doi: 10.1111/j.1469-1809.1974.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 44.Candeias J A N, Baruzzi R G, Pripas S, Iunes M. Rev Saude Publica. 1977;11:510–514. [Google Scholar]
- 45.Sugimoto C, Kitamura T, Guo J, Al-Ahdal M N, Shchelkunov S N, Otova B, Ondrejka P, Chollet J-Y, El-Safi S, Ettayebi M, et al. Proc Natl Acad Sci USA. 1997;94:9191–9196. doi: 10.1073/pnas.94.17.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neel J V, Biggar R J, Sukernik R I. Proc Natl Acad Sci USA. 1994;91:10737–10741. doi: 10.1073/pnas.91.22.10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taguchi F, Kajioka J, Miyamura T. Microbiol Immunol. 1982;26:1057–1064. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 48.Mashiko J, Nakamura K, Shinozaki T, Araki K, Fujii R, Yasui K, Ogiwara H. Teikyo Med J. 1982;5:299. [Google Scholar]
- 49.Walker D L, Frisque R J. In: The Papovaviridae, Vol. 1: The Polyomaviruses. Salzman N P, editor. New York: Plenum; 1986. pp. 327–377. [Google Scholar]
- 50.Kitamura T, Aso Y, Kuniyoshi N, Hara K, Yogo Y. J Infect Dis. 1990;161:1128–1133. doi: 10.1093/infdis/161.6.1128. [DOI] [PubMed] [Google Scholar]
- 51.Kitamura T, Kunitake T, Guo J, Tominaga T, Kawabe K, Yogo Y. J Clin Microbiol. 1994;32:2359–2363. doi: 10.1128/jcm.32.10.2359-2363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunitake T, Kitamura T, Guo J, Taguchi F, Kawabe K, Yogo Y. J Clin Microbiol. 1995;33:1448–1451. doi: 10.1128/jcm.33.6.1448-1451.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato A, Kitamura T, Sugimoto C, Ogawa Y, Nakazato K, Nagashima K, Hall W W, Kawabe K, Yogo Y. Arch Virol. 1997;142:875–882. doi: 10.1007/s007050050125. [DOI] [PubMed] [Google Scholar]
- 54.Frisque R J, Bream G L, Canella M T. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrews C A, Daniel R W, Shah K V. In: Polyomaviruses and Human Neurological Disease. Sever J L, Madden D L, editors. New York: Liss; 1983. pp. 133–141. [Google Scholar]
- 56.Neel J V. Am J Hum Genet. 1998;63:489–497. doi: 10.1086/301954. [DOI] [PMC free article] [PubMed] [Google Scholar]