Abstract
Epithelioid hemangioendothelioma is an uncommon vascular tumor of soft tissue and bone that may rarely occur in the liver, lung and the head and neck. We present five new cases of epithelioid hemangioendothelioma of the head and neck region diagnosed and managed in one institution in order to define the phenotypic characteristics, podoplanin immunohistochemical staining and the biological outcome. Podoplanin is a transmembrane mucoprotein selectively expressed in lymphatic endothelium and recently in some vascular neoplasms. The patients were comprised of two male and three female patients ranging in age from 4 to 71 years. The lesions were found in the gingiva, submandibular region soft tissue, nasal cavity and tongue, and ranged in size from 0.7 to 2.5 cm. All tumors manifested infiltrative cords and nests of epithelioid cells with occasional spindle morphology in a myxoid stroma. Immunohistochemical analysis of vascular and epithelial markers showed strong and uniform cytoplasmic reactivity for podoplanin and variable intensity and staining of CD31 and lack of cytokeratin staining in tumor cells. Surgical treatment included simple and wide local excisions. Of the three patients with follow-up, one developed lymph node metastasis and one had no evidence of disease 10 months after surgery. The patient with multiple recurrences and LN metastases was additionally treated with chemotherapy and is under consideration for radiation therapy. Hemangioendothelioma of the head and neck is: (1) a low-grade malignancy with a tendency for local recurrence and regional lymph node metastasis, (2) complete excision with negative margins is the treatment of choice for localized disease and (3) podoplanin may be useful in differentiating epithelioid hemangioendothelioma from non-vascular tumors.
Keywords: Epithelioid hemangioendothelioma, Podoplanin, Immunohistochemistry, Biological outcome, Differential diagnoses
Introduction
Epithelioid hemangioendothelioma (EHE) is a generally low-grade vascular neoplasm, consisting of an epithelioid endothelial cell proliferation with distinctive myxohyaline stroma [1]. The tumor can arise in skin, bone or soft tissue or may have a primary parenchymal location, most commonly in the liver and lung [2–12]. Several examples at rare sites including the head and neck have previously been reported [13–18]. Regardless of origin, EHE may pose a diagnostic challenge due to the overlapping morphologic features with certain carcinomas, melanoma, and epithelioid sarcoma phenotypes [19–37]. This diagnostic difficulty may further be compounded by occasional expression of cytokeratin in these tumors [12]. The purpose of this study is to report five new cases of EHE in the head and neck region and to discuss the pertinent differential diagnoses and the expression of podoplanin in these tumors. To our knowledge, podoplanin expression in these neoplasms has not been previously investigated.
Materials and Methods
Five patients diagnosed with epithelioid hemangioendothelioma from 1996 to 2006 and originating in the head and neck region were identified from a review of the files of the Department of Pathology at M.D. Anderson Cancer Center. Clinical records and surgical pathology reports together with the follow-up information, were reviewed. In all cases, hematoxylin-and-eosin stained histologic preparations were available and were reviewed. In the four cases for which paraffin blocks were available, immunohistochemical studies were performed using the avidin–biotin-peroxidase complex method in a Dako AutoStainer (Carpinteria, CA). The primary antibodies used were mouse monoclonal antibodies to podoplanin (D2-40, Signet Laboratories, Dedham, MA, 1:50 dilution), CD31 (JC70A, Dako, 1:20), and cytokeratin (CAM 5.2, BD BioSciences, San Diego, CA, 1:25). The immunostaining was done using the LSAB2 peroxidase kit (Dako). To enhance the immunostaining for podoplanin and CD31, a heat epitope retrieval procedure was performed using a Black-and-Decker vegetable steamer. The buffer solution used was Tris-EDTA buffer, pH 8.0. Enzymatic pretreatment with 0.2% protease, type XXIV (Sigma Chemical Co., St. Louis, MO) in Tris buffer saline, pH 7.3, was used. The antigen–antibody immunoreaction was visualized using 3-amino-9-ethylcarbazole as chromogen. To evaluate the specificity of the antibodies, known positive and negative tissues were used as controls (Table 1).
Table 1.
Antibodies utilized in staining of head and neck hemangioendothelioma
| Antibody | Clone | Dilution | Source |
|---|---|---|---|
| Podoplanin | D2-40 | 1:50 | Signet |
| CD31 | JC 70 A | 1:20 | Dako |
| Cam 5.2 | Zym 5.2 | 1:25 | BD |
BD: Becton Dickenson
Results
Patient Information
The patients included two males and three females who ranged in age from 4 to 71 years. The most common presentation was a submucosal or soft tissue mass. The locations of the lesions were the gingiva in two cases, the nasal cavity, tongue and submandibular soft tissue, one each.
Pathologic Findings
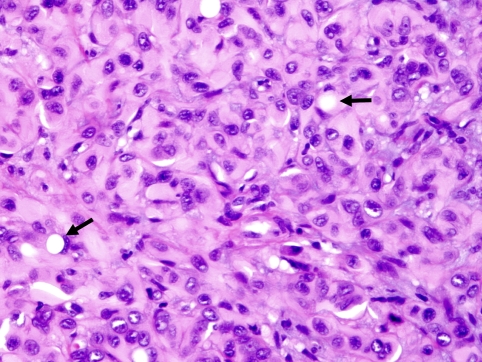
The tumors ranged from 0.7 to 2.5 cm in size. Histologically, the tumors appeared as encapsulated, predominantly epithelioid cellular proliferations with ill-defined boundaries (Fig. 1) embedded within a collagenous to fibromyxoid stroma. Cytologically, the tumor cells demonstrated moderate to ample eosinophilic to amphophilic cytoplasm with nuclei having irregular nuclear contours, coarse chromatin and prominent nucleoli (Fig. 1). A consistent feature in all cases was intracytoplasmic lumen formation in variable proportions (Fig. 2). Mitotic figures were rare. The pertinent pathologic features are summarized in Table 2.
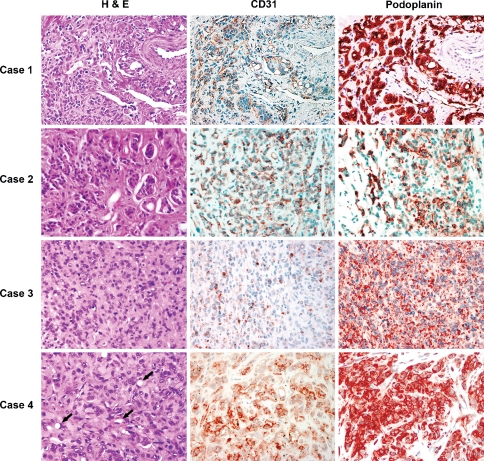
Fig. 1.
Four cases of EHE stained with hematoxylin and eosin (H&E), CD31 and podoplanin. H&E sections of all four cases show epithelioid morphology with intra-cytoplasmic lumina, readily apparent in Case 4 (arrows). CD31 shows patchy expression compared to the diffuse, homogenous expression of podoplanin
Fig. 2.
A high power micrograph of case #4 illustrating the epithelioid features and the intra-cellular vaculation of tumor cells (arrows)
Table 2.
Histopathologic findings in epithelioid hemangioendothelioma of head and neck
| Case # | Stroma | Pleomorphism | Mitoses (#/10 HPF) | Spindle features | Necrosis |
|---|---|---|---|---|---|
| 1 | Fibrotic | Absent | <1 | 30% | Absent |
| 2 | Myxoid | Focal | =2 | Absent | Absent |
| 3 | Fibrotic | Absent | =1 | <5% | Absent |
| 4 | Fibrotic | Absent | <1 | Absent | Absent |
| 5 | Fibrotic | Absent | <1 | <5% | Absent |
HPF = high power fields
Immunohistochemical Studies
Immunohistochemical studies were performed on four tumors with archival tissues. All four tumors showed strong and uniform membrane and/or cytoplasmic staining for podoplanin. CD 31 was also demonstrated in tumor cells and blood vessels but the staining was of variable intensity and heterogeneous (Fig. 1). Immunostaining for cytokeratin was negative in all four lesions.
Radiologic Findings
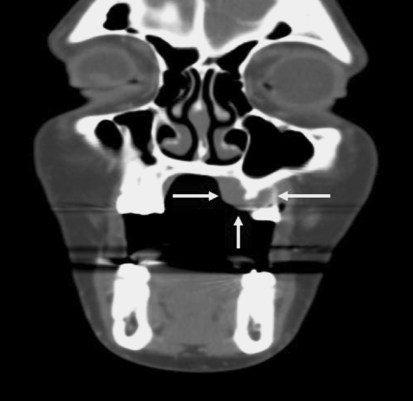
Radiographic evaluation by computed tomography (CT) was available in one case (case 3) which presented in the gingiva and showed underlying bone resorption of the maxillary cortex (Fig. 3).
Fig. 3.
Computed tomography (coronal view) of the maxillofacial area shows a soft tissue mass involving the left hard palate with erosion of the maxillary cortex (Case 3)
Follow-up and Treatment
In case #1, the patient was initially treated with local excision but recurred after 3 and 5 years and was subsequently treated by wider excision and he is disease free at 10 years of follow up. The lesion in case #3 was locally excised twice at 1-year intervals. The lesion recurred for the third time after 4 years and was treated by excision and maxillectomy. The patient recently presented with a fourth local recurrence and lymph node metastases 5 years and 4 months from initial diagnosis. Case #4 presented with concurrent lymph node metastases and was treated by wide local excision and lymph node dissection. The patient is currently disease free at 10 months following surgery. The clinical features of our five cases of EHE arising in the head and neck are summarized in Table 3.
Table 3.
Summary of clinical features of 5 cases of epithelioid hemangioendothelioma
| Case # | Age (Years) | Sex | Site | Size (cm) | Treatment | Follow-up |
|---|---|---|---|---|---|---|
| 1 | 4 | M | Nasal Cavity | 2.5 | Excision | Rec. × 2, 3 & 5 years, NED 10 years |
| 2 | 62 | F | Neck | 1.8 | NA | NA |
| 3 | 17 | F | Gingiva | 0.7 | Excision, Maxillectomy, Chemo and XRT | Rec. × 4, LN Mets (within 5 years) |
| 4 | 66 | F | Gingiva with LN Mets | NA | WLE & LN dissection | NED (10 months) |
| 5 | 71 | M | Tongue | 1.0 | Excision | NA |
Abbreviations: Chemo, Chemotherapy; Cm, Centimeter; F, Female; LN, Lymph node; M, Male; Mets, Metastases; Mo, Months; NA, Not available; NED, No evidence of disease; Rec. Recurrence; WLE, Wide local excision; XRT, Radiation therapy
Discussion
The present study, for the first time, shows that podoplanin may play a role in the differential diagnosis of epithelioid hemangioendotheliomas originating in the head and neck. In contrast to the heterogeneous expression of traditional endothelial markers, all tumors exhibited homogenous and uniform cytoplasmic staining by podoplanin. Podoplanin is an intracellular protein reported to be expressed in lymphatic endothelium, alveolar type I cells, osteoblasts and peritoneal mesothelial cells but not in normal vascular endothelial cells [38]. In contrast, conventional endothelial markers (CD31, CD34, Factor VIII antigen) are expressed by both lymphatic and blood vessel endothelial cells [39]. Recently, podoplanin has been reported to be expressed in vascular neoplasms including Kaposi sarcomas [40], Kaposiform hemangioendotheliomas [41], hobnail hemangiomas [42] and a subset of angiosarcomas [43, 44]. Although we contend that podoplanin expression in our cases and other vascular neoplasms suggest an aberrant expression during vascular tumorigenesis, the possibility that some of these lesions originate from lymphatic origin cannot be entirely ruled out.
Histopathologically, all of our lesions manifested similar bland epithelioid cytomorphologic features and infiltrative patterns with the characteristic collagenous and myxoid stroma typically seen in EHE. Other studies, however, have reported increased mitoses, necrosis, spindle atypia and pleomorphism in some EHE [2]. We attribute the difference between these results to be the inclusion of the epithelioid form of angiosarcoma in some of the previous reports. In this study, however, two patients had lymph node metastases and experienced multiple recurrences. The multiple recurrences in some of these tumors may be related to their critical location within the head and neck region and the undefined boundaries leading to incomplete or inadequate excision. Complete excision with wide margins is, therefore, the recommended primary treatment for these lesions [18].
EHE in the head and neck may pose diagnostic difficulties, especially with certain types of carcinoma and epithelioid-forming sarcomas [18, 36, 37, 43]. This may rarely be complicated by aberrant expression of cytokeratin in some of these cases [12, 35]. The differentiation of EHE from epithelioid angiosarcomas is based mainly on the presence of marked cytologic atypia and pleomorphism and high mitotic rate in the latter tumor. Significant anaplasia and high mitotic rate (>5/10 HPF) should exclude the diagnosis of EHE. Epithelioid sarcoma may also be considered in the differential diagnosis of EHE. The low mitotic activity (<5/50 HPF), minimal pleomorphism, lack of necrosis and the presence of intracytoplasmic vascular lumen formation should distinguish EHE from epithelioid sarcoma [37]. Since epithelioid sarcomas commonly express cytokeratin and other epithelial markers [37, 45] and because EHE may occasionally express these markers [2], podoplanin may further aid in differentiating these tumors.
Clinically, the patients’ characteristics in this study were similar to those previously reported (Table 4) [11, 14]. Tumors were most frequently located in the soft tissue of the head and neck, gingiva and tongue. Other sites previously cited included buccal mucosa, parotid gland, nasal cavity, floor of mouth, larynx and thyroid [21–27]. Treatment was mainly local excision with only one case also treated by radiation. Lymph node metastasis was encountered in only two of 43 cases previously reported and with the addition of two of our cases, a total of 4 of 48 (8.3%) cases had lymph node metastases. The collective data, including the present series, showed no mortality in patients with head and neck EHE. The availability of anti-angiogenic agents may play a role in future treatment of this entity. Radiation therapy is also under investigation as a treatment modality in patients with multiple recurrences and lymph node metastasis.
Table 4.
Clinicopathologic features of previously published epithelioid hemangioendotheliomas of the head and neck
| Reference (#) (Year) | #’s of Cases | Age/ Gender | Size (cm) | Site | Treatment | Follow-up |
|---|---|---|---|---|---|---|
| 2, 1997 | 5 | 30–65 years/4M-1F | 0.4–4.0 | ST, Cheek and Neck | SE | NED 42–60 months |
| 13, 1986 | 1 | NA/ M | NA | Gingiva | SE | NED 36 months |
| 14, 1986 | 1 | 4–67 years/7M-5F | 1.7–2.5 | Neck ST, Gingiva | WLE and SE | LN mets-2 cases, Rec.-1 case |
| 15, 1987 | 1 | 25 years/F | 1.0 | Palate | SE | NED 21 months |
| 16, 1987 | 1 | 4 years/M | NA | Gingiva | SE | NA |
| 17, 1991 | 2 | 36–45 years /1M-1F | 0.2–1.5 | Tongue, Gingiva | SE | Rec. 1 case |
| 18, 2007 | 9 | 6–53y years/8M-1F | 0.5–7.0 | Tongue, FOM, Oral Cavity | WLE | Rec.-3 Cases |
| NED 6–96 months | ||||||
| 19, 1995 | 1 | 7 years/F | 1.5 | Gingiva | WLE | NED 48 months |
| 20, 1996 | 1 | 46 years/M | 1.0 | ST, Cheek | SE | NED 36 months |
| 21, 1998 | 1 | 44 years/F | 3.7 | Thyroid | Subtotal Thyroidectomy | NED 24 months |
| 22, 1999 | 1 | 19 years/M | 1.5 | Larynx | SE | NED 36 months |
| 23, 2000 | 1 | 48 years/F | 2.0 | Parotid | NA | NA |
| 24, 2001 | 1 | 18 years/F | NA | Buccal Mucosa | SE | Rec. 9 months |
| Rec. WLE | NED 24 months | |||||
| 25, 2003 | 1 | 23 years/M | NA | Nasal Cavity | WLE | NED 12 months |
| 26, 2003 | 1 | 81 years/M | 2.0 | Parotid | Total Parotidectomy | NED 7 months |
| 27, 2004 | 1 | 28 years/M | NA | Parotid | Parotidectomy and LN dissection | NA |
| 28, 2005 | 1 | 25 years/M | NA | Nasal cavity | WLE | NA |
| 29, 2005 | 1 | 28 years/F | 0.6 | Gingiva | SE | NED 8 months |
| 30, 2006 | 1 | 34 years/F | 2.5 | ST, Neck | SE | NED 84 months |
Abbreviations: AWD: Alive With Disease; Cm: Centimeters; F: Female; LN: Lymph Node; M: Male; Mets: Metastases; NA: Not Available; NED: No Evidence of Disease; Rec: Recurrence; SE: Simple excision; ST: Soft Tissue; WLE: Wide Local Excision; #: Number; FOM: Floor of mouth
We conclude that epithelioid hemangioendothelioma of the head and neck is a low-grade malignancy with a tendency for local recurrence and regional lymph node metastases. Complete excision with negative margins is the treatment of choice and long-term follow up is recommended as local recurrences (25%) remain common. While lymph node metastases are rare, clinical monitoring should include lymph node evaluation. Podoplanin, in addition to histologic features, may be used to differentiate EHE from non-vascular neoplasms, such as poorly differentiated carcinomas and epithelioid sarcomas.
References
- 1.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma, a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–81. doi: 10.1002/1097-0142(19820901)50:5<970::AID-CNCR2820500527>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Mentzel T, Beham A, Calonje E, et al. Epithelioid hemangioendothelioma of skin and soft tissue: clinicopathologic and immunohistochemical study of 30 cases. AM J Surg Pathol. 1997;21:363–74. doi: 10.1097/00000478-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Ishak KG, Sesterhenn IA, Goodman ZD, et al. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol. 1984;15:839–52. doi: 10.1016/S0046-8177(84)80145-8. [DOI] [PubMed] [Google Scholar]
- 4.Hurley TR, Whisler WW, Clasen RA. Recurrent intracranial epithelioid hemangioendothelioma associated with multicentric disease of liver and heart. Case report. Neurosurgery. 1994;35:148–51. doi: 10.1227/00006123-199407000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Buchenroth M, Beus J, Bittinger F, et al. Solitary epithelioid hemangioendothelioma, a rare vascular tumor of the lung. Pneumologie. 1995;49:239–42. [PubMed] [Google Scholar]
- 6.Dail DH, Liebow AA, Gmelich JT, et al. Intravascular, bronchiolar and alveolar tumor of the lung (IVBAT): an analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer. 1983;51:452–64. doi: 10.1002/1097-0142(19830201)51:3<452::AID-CNCR2820510317>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Marchiano D, Fisher F, Hofstetter S. Epithelioid hemangioendothelioma of the heart with distant metastases. A case report and literature review. J Cardiovasc Surg. 1993;34:529–33. [PubMed] [Google Scholar]
- 8.Rosai J, Gold J, Landy R. The histiocytoid hemangiomas: a unifying concept embracing several previously described entities of skin, soft tissue, large vessels, bone and heart. Hum Pathol. 1979;10:707–30. doi: 10.1016/s0046-8177(79)80114-8. [DOI] [PubMed] [Google Scholar]
- 9.Suster S. Epithelioid and spindle cell hemangioendothelioma of the spleen. Report of a distinctive splenic vascular neoplasm of childhood. Am J Surg Pathol. 1992;16:785–92. doi: 10.1097/00000478-199208000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Suster S, Moran CA, Koss MN. Epithelioid hemangioendothelioma of the anterior mediastinum. Clinicopathologic, immunohistochemical, and ultrastructural analysis of 12 cases. Am J Surg Pathol. 1994;18:871–81. doi: 10.1097/00000478-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tsuneyoshi M, Dorfman HD, Bauer TW. Epithelioid hemangioendothelioma of bone, a clinicopathologic, ultrastructural and immunohistochemical study. Am J Surg Pathol. 1986;10:754–64. doi: 10.1097/00000478-198611000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Haelst U, Pruszczynski M, ten Cate L, et al. Ultrastructural and immunohistochemical study of epithelioid hemangioendothelioma of bone: co-expression of epithelial and endothelial marker. Ultrastruct Pathol. 1990;14:141–9. doi: 10.3109/01913129009025126. [DOI] [PubMed] [Google Scholar]
- 13.Ebo CM, Boever JA, Adriaens PA, et al. Hemangioendothelioma of the gingiva. Histopathologic and therapeutic considerations. J Clin Periodontal. 1986;13:11–8. doi: 10.1111/j.1600-051X.1986.tb01408.x. [DOI] [PubMed] [Google Scholar]
- 14.Ellis GL, Kratochvil FJ. Epithelioid hemangioendothelioma of the head and neck: a clinicopathologic report of twelve cases. Oral Surg Oral Med Oral Pathol. 1986;61:61–8. doi: 10.1016/0030-4220(86)90204-5. [DOI] [PubMed] [Google Scholar]
- 15.Moran WJ, Dobleman TJ, Bostwick DG. Epithelioid hemangioendothelioma (histiocytoid hemangioma) of the palate. Laryngoscope. 1987;97:1299–302. doi: 10.1288/00005537-198711000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Araujo VC, Marcucci G, Sesso A, et al. Epithelioid hemangioendothelioma of the gingiva: case report and ultrastructural study. Oral Surg Oral Med Oral Pathol. 1987;63:472–7. doi: 10.1016/0030-4220(87)90261-1. [DOI] [PubMed] [Google Scholar]
- 17.Marrogi AJ, Boyd D, El-Mofty SK, et al. Epithelioid hemangioendothelioma of the oral cavity: report of two cases and review of the literature. J Oral Maxillofac Surg. 1991;49:633–8. doi: 10.1016/0278-2391(91)90346-N. [DOI] [PubMed] [Google Scholar]
- 18.Sun ZJ, Zhang L, Zhang WF, et al. Epithelioid hemangioendothelioma of the oral cavity. Oral Dis. 2007;13:244–50. doi: 10.1111/j.1601-0825.2006.01281.x. [DOI] [PubMed] [Google Scholar]
- 19.Flaitz CM, McDaniel RK, Mackay B, et al. Primary intraoral epithelioid hemangioendothelioma presenting in childhood: review of the literature and case report. Ultrastruct Pathol. 1995;19:275–9. doi: 10.3109/01913129509064231. [DOI] [PubMed] [Google Scholar]
- 20.Kiryu H, Hashimoto H, Hori Y. Ossifying epithelioid hemangioendothelioma. J Cutan Pathol. 1996;23:558–61. doi: 10.1111/j.1600-0560.1996.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui MT, Evans HL, Ro JY, et al. Epithelioid hemangioendothelioma of the thyroid gland: a case report and review of literature. Histopathology. 1998;32:473–6. doi: 10.1046/j.1365-2559.1998.00384.x. [DOI] [PubMed] [Google Scholar]
- 22.Boscaino A, Errico ME, Orabona P, et al. Epithelioid hemangioendothelioma of the larynx. Tumori. 1999;85:515–8. doi: 10.1177/030089169908500618. [DOI] [PubMed] [Google Scholar]
- 23.Pigadas N, Mohamid W, McDermott P. Epithelioid hemangioendothelioma of the parotid salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:730–8. doi: 10.1067/moe.2000.106299. [DOI] [PubMed] [Google Scholar]
- 24.Orsini G, Fioroini M, Rubini C, et al. Epithelioid hemangioendothelioma of the oral cavity: report of case. J Oral Maxillofac Surg. 2001;23:31–5. doi: 10.1053/joms.2001.21007. [DOI] [PubMed] [Google Scholar]
- 25.Di Girolamo A, Giacomini PG, Coli A, et al. Epithelioid hemangioendothelioma arising in the nasal cavity. J Laryngol Otol. 2003;117:75–7. doi: 10.1258/002221503321046711. [DOI] [PubMed] [Google Scholar]
- 26.Amin KS, Mc Guff HS, Cashman SW, et al. Epithelioid hemangioendothelioma of the parotid gland with atypical features. Otolarygol Head Neck Surg. 2003;129:596–8. doi: 10.1016/S0194-5998(03)00726-5. [DOI] [PubMed] [Google Scholar]
- 27.Falvo F, Marzullo A, Catania A, et al. Epithelioid hemangioendothelioma of the parotid salivary gland: a case report. Chir Ital. 2004;56:457–62. [PubMed] [Google Scholar]
- 28.Tseng CC, Tsay SH, Tsai TL, et al. Epithelioid hemangioendothelioma of the nasal cavity. J Chin Med Assoc. 2005;68:45–8. doi: 10.1016/S1726-4901(09)70132-7. [DOI] [PubMed] [Google Scholar]
- 29.Chi AC, Weathers DR, Folpe AL, et al. Epithelioid hemangioendothelioma of the oral cavity: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:717–24. doi: 10.1016/j.tripleo.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 30.Rigby MH, Taylor SM, Bullock MJ, et al. Epithelioid hemangioendothelioma of the submandibular triangle. J Otolaryngol. 2006;35:194–5. [PubMed] [Google Scholar]
- 31.Napaki S, Stirling JW. Spindle and epithelioid (histiocytoid) hemangioendothelioma of cervical lymph nodes. Pathology. 2004;36:587–9. doi: 10.1080/00313020400010989. [DOI] [PubMed] [Google Scholar]
- 32.Casey MC, Lim C, Hickey MC. Case of the month: an unusual cause of neck pain. Br J Radiol. 2004;77:539–40. doi: 10.1259/bjr/46974640. [DOI] [PubMed] [Google Scholar]
- 33.Hristova EN, Krishnamurthy S, Ro JY, et al. Pulmonary epithelioid hemangioendothelioma with prominent signet ring cell features mimicking metastatic adenocarcinoma. Annals of Diag Pathol. 2003;7:160–4. doi: 10.1016/S1092-9134(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 34.Kim SS, Lee SH, Kim KH, et al. A case of epithelioid hemangioendothelioma. Korean J Derm. 2004;42:1366–8. [Google Scholar]
- 35.O’Connell JX, Kattapuram SV, Mankin JH, et al. Epithelioid hemangioendothelioma of bone: a tumor often mistaken for low-grade angiosarcoma or malignant hemangioendothelioma. Amer J of Surg Pathol. 1993;17:610–7. doi: 10.1097/00000478-199306000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Chase DR, Enzinger FM, Weiss SW, et al. Keratin in epithelioid sarcoma. An immunohistochemical study. Am J Surg Pathol. 1984;8:435–41. doi: 10.1097/00000478-198406000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma—like hemangioendothelioma. Am J Surg Pathol. 2003;27:48–57. doi: 10.1097/00000478-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Ordonez NG. Podoplanin: a novel diagnostic immunohistochemical marker. Adv in Anat Pathol. 2006;13:83–8. doi: 10.1097/01.pap.0000213007.48479.94. [DOI] [PubMed] [Google Scholar]
- 39.Sirgi KE, Wick MR, Swanson PE. B72.3 and CD34 immunoreactivity in malignant epithelioid soft tissue tumors: adjuncts in the recognition of endothelial neoplasms. Am J Surg Pathol. 1993;17:179–85. doi: 10.1097/00000478-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2–40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–40. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 41.Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2–40 immunohistochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454–60. doi: 10.1038/modpathol.3800444. [DOI] [PubMed] [Google Scholar]
- 42.Franke FE, Steger K, Marks A, et al. Hobnail hemangiomas (targetoid hemosiderotic hemangiomas) are true lymphangiomas. J Cutan Pathol. 2004;31:362–7. doi: 10.1111/j.0303-6987.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 43.Ordonez NG. D2–40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372–80. doi: 10.1016/j.humpath.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries, podoplanin as a specific marker for lymphatic endothelium. AM J Pathol. 1999;154:385–94. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2004;13:114–21. doi: 10.1097/00125480-200605000-00002. [DOI] [PubMed] [Google Scholar]