Abstract
Histopathological findings in cases of hairy leukoplakia (HL) are not exclusive to this lesion. A total of 36 tissue samples from patients previously diagnosed with HL based solely on morphological aspects were used in this study. Our purpose was to confirm the presence of Epstein–Barr virus (EBV) in these tissue samples by in situ hybridization (ISH), and to compare the detection of EBV with specific histopathological findings observed in each case. Among the 36 specimens, 80.55% were EBV positive, confirming the previous clinical and histhophatological diagnosis. None of the histopathological findings analyzed correlated with the presence or absence of EBV. This shows that a definitive diagnosis of HL cannot be established based on histopathological findings alone. Because there are many important implications on the establishment of definitive diagnosis of HL, the detection of EBV by ISH is obligatory.
Keywords: Hairy leukoplakia, EBV, In situ hybridization, Histopathological features
Introduction
The Epstein–Barr virus (EBV) belongs to the human herpesvirus family (HHV-4), and infects approximately 90% of the world’s adult population asymptomatically [1, 2]. EBV is the causative agent of infectious mononucleosis, and is associated with hairy leukoplakia (HL) and certain lymphoid and epithelial cancers such as Burkitt’s lymphoma, immunoblastic lymphoma, Hodgkin’s lymphoma, and nasopharyngeal cancer [3–6].
Hairy leukoplakia is a benign lesion of the oral mucosa that is observed in immunosuppressed patients. Clinically, it presents as a white patch, corrugated, and painless, that cannot be scraped off. It is more commonly seen along the sides of the tongue [7, 8].
The etiopathogenesis of HL is associated with the replication of EBV in superficial epithelial cells [6, 9]. It was initially described as being exclusive to AIDS patients [7, 8, 10]. Later, however, HL was diagnosed in immunosuppressed HIV-negative patients, particularly in organ transplant recipients [11–13].
Differential diagnosis is based on the exclusion of irritative hyperkeratosis, pseudomembranous candidiasis, lichen planus, white sponge nevus, idiopathic HL or tobacco-associated HL, and benign migratory glossitis [14, 15].
The histopathological features of HL are not exclusive to this lesion, and may include hyperkeratosis, epithelial hyperplasia, ballooning degeneration, acanthosis, and mild or moderate inflammatory infiltrate [10, 14, 16]. For this reason, the criteria adopted for making a final diagnosis of HL have been the subject of debate. Earlier studies suggested that the analysis of both clinical features and histopathological findings was enough to establish a diagnosis [17]. After EBV was identified as the causative agent of HL [18], some authors began to suggest that its genetic material should be detected in epithelial cells for final diagnosis [19, 20]. Nowadays it is accepted that the definitive diagnosis of OHL currently relies upon such evidence of EBV in lesional tissue [21].
The EBV can be detected by various techniques such as polymerase chain reaction, in situ hybridization (ISH), immunohistochemistry, immunocytochemistry, and electron microscopy [22–25]. The gold standard, however, is ISH for diagnosis of OHL [19, 20].
These techniques cannot always be employed in a pathology laboratory either because of financial issues or limited laboratory facilities. This has often led to histological diagnoses of HL, which are based solely on the morphological characteristics of the lesion [20]. The Service of Surgical Pathology of the School of Dentistry of the University of São Paulo (FOUSP) has been establishing diagnoses of suspected HL based on the histological changes observed in hematoxylin-eosin-stained sections.
The main purpose of our study was to detect the presence of EBV in lesions histopathologically diagnosed as suspected HL at the Service of Surgical Pathology of the Department of Oral Pathology-FOUSP. The secondary objective of this study was to compare the presence of EBV and the main histopathological features of HL in order to identify the morphological indicators of HL in hematoxylin-eosin-stained sections.
Material and Methods
A total of 38 paraffin-embedded specimens were used in this study. The specimens were obtained at the Service of Surgical Pathology, Discipline of Oral Pathology, Department of Stomatology, School of Dentistry, University of São Paulo. Said specimens were taken from patients who had been diagnosed with HL or suspected HL based solely on microscopic examination using routine staining methods.
The data on sex, race, age, anatomical site of the lesion, and HIV status of patients were collected for analysis. The histopathological slides were re-available for two oral pathologists in order to verify the presence of histological features of HL, namely:
Hyperparakeratosis
Acanthosis
Epithelial hyperplasia
Presence of koilocyte-like cells (koilocytosis)
Secondary infection by Candida sp
Minimal or absent inflammation in the subjacent connective tissue.
The presence or absence of the aforementioned histological features was checked. Then, the frequency of each finding was compared with the results obtained after ISH for the detection of EBV.
The association between the histopatological features and EBV detection was tested by Fisher exact test. A significance level of P < 0.05 was used.
In situ Hybridization
Among 38 specimens morphologically analyzed, 36 had histological features compatible with HL. These 36 specimens were sliced into 5 μm sections and mounted on slides that had been previously coated with a solution of 10% 3-aminopropyltriethoxysilane (Sigma Chemical Co., MO, USA) in 100% ethanol, and oven-dried at 37°C for 24 h.
Slides were deparaffinized in xylene (30 min at 59°C, and 20 min at 25°C) and rehydrated through graded ethanol (absolute, 95 and 85%). Slides were immersed in a solution of 10% ammonium hydroxide in 95% ethanol for 10 min to remove formalin pigment. Slides were then washed for 10 min in tap water and immersed twice in distilled water.
The reaction for the detection of EBV was carried out using the PNA ISH Detection Kit (DakoCytomation, Glostrup, Denmark). This kit contained negative and positive control probes and all the reagents necessary for the reaction, except for the specific probe. Lymphoid tissue sections were used as positive control. These sections were kindly donated by the Service of Pathology of the Adolfo Lutz Institute.
The slides were placed in a humidity chamber. A total of 150 μl of diluted proteinase K (1:10 in buffer solution—10 mM TRIS and NaCl, pH 7.6) was added to each section, and specimens were incubated for 25 min at room temperature. The slides were immersed twice in distilled water (three minutes each time), immersed in 95% ethanol for 10 s, and left in the humidity chamber for five minutes to dry.
After pretreatment with proteinase K, the hybridization step began. Two drops of fluorescein-conjugated PNA probe (EBER-Y5200, DakoCytomation Inc., CA, USA) were added to each tissue section. Sections were then covered with coverslips. The slides, inside the humidity chamber, were incubated at 55°C for 1 h and 30 min.
The coverslips were removed and the slides were immersed in preheated stringent wash solution, diluted 1:60 (concentrated Stringent Wash 50X, DAKO, S3500), and incubated at 55° for 25 min. Slides were then briefly immersed in TBS (10 mM TRIS and NaCl, pH 7.6).
The slides were placed again in the humidity chamber and two to three drops of antibody (Anti-FITC/AP) were added to each section, which were incubated for 30 min at room temperature. The antibody was rinsed with TBS (pH 7.5). Slides were immersed twice in TBS (3 min each time) and then immersed twice in distilled water (1 min each time).
The slides were placed in the humidity chamber and two to three drops of substrate [5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium (NBT)] were added to each section. Sections were incubated for 45 min at room temperature. The substrate was removed by immersing slides in distilled water. The slides were then washed for 5 min with tap water.
The sections were counterstained with Fast-red (DakoCytomation Inc., CA, USA) for 1 min at room temperature, washed for 5 min with tap water and immersed in distilled water. The sections were then dehydrated in increasing concentrations of ethanol and xylene and mounted with Permount mounting medium (Fisher Scientific, NJ, USA). Upon microscopic examination, the presence of EBV was confirmed when a dark blue or black color was observed at the site of hybridization (nucleus).
Results
Among the 36 tissue samples subjected to ISH for the detection of EBV, 29 (80.55%) were positive.
Among the patients analyzed, 23 (63.88%) were male and 13 (36.11%) were female. The mean age was 33 years. The data on the HIV status of patients, obtained from the charts analyzed, are shown in Table 1.
Table 1.
Data on HIV status
| HIV status | Total number of patients (n = 36) | EBV-positive patients (n = 29) | EBV-negative patients (n = 07) |
|---|---|---|---|
| HIV positive | 25 (69.44%) | 24 (82.75%) | 01 (14.3%) |
| HIV negative | 1 (2.8%) | 1 (3.45%) | 0 (0%) |
| Unknown | 10 (27.7%) | 4 (13.8%) | 6 (85.75%) |
According to the information on the charts, HL was most often found along the lateral borders of the tongue (29 patients—80.55%), and sometimes on the inside of the cheek, other areas of the tongue, or retromolar area. The information regarding the site of the lesion is shown in Table 2. Fisher’s exact test revealed no statistically significant relation between the presence of EBV along the lateral borders of the tongue and at other sites (P = 0.525).
Table 2.
Location of the lesions
| Location of lesions | Total number of patients (n = 36) | EBV-positive patients (n = 29) | EBV-negative patients (n = 07) |
|---|---|---|---|
| Lateral border of the tongue | 29 (80.55%) | 23 (79.31%) | 6 (85.71%) |
| Inside of the cheek | 3 (8.3%) | 3 (10.34%) | 0 |
| Other areas of the tongue | 3 (8.3%) | 1 (3.45%) | 2 (28.57%) |
| Retromolar area | 1 (2.77%) | 1 (3.44%) | 0 |
Fisher’s exact test P > 0.05
The correlation between histopathological findings observed by the authors with the results of the ISH is presented in Table 3. These are no significant differences in the frequency of typical HL histological features between EBV positive and negative cases.
Table 3.
Histopathological features
| Histopathological features | Total number of patients (n = 36) | EBV-positive patients (n = 29) | EBV-negative patients (n = 7) |
|---|---|---|---|
| Hyperparakeratosis | 36 (100%) | 29 (100%) | 7 (100%) |
| Acanthosis | 30 (83.3%) | 28 (96.55%) | 2 (28.57%) |
| Koilocyte-like cells | 36 (100%) | 29 (100%) | 7 (100%) |
| Presence of Candida sp | 10 (27.8%) | 9 (31.03%) | 1 (14.3%) |
| Minimal or absent inflammation in the subjacent connective tissue | 27 (75%) | 24 (82.75%) | 3 (42.85%) |
| Epithelial hyperplasia | 35 (97.22%) | 29 (100%) | 6 (85.71%) |
Fisher’s exact test for comparison of proportions P > 0.05
Discussion
The diagnosis of HL is a controversial issue. Some authors believe diagnosis can be made based on clinical features alone; others believe it can be made based on clinical and histopathological or cytopathological features; and there are authors who believe that it is necessary to detect EBV, the etiological agent of HL, to confirm diagnosis. This can be done by applying molecular biology techniques to biopsied tissue [20, 26].
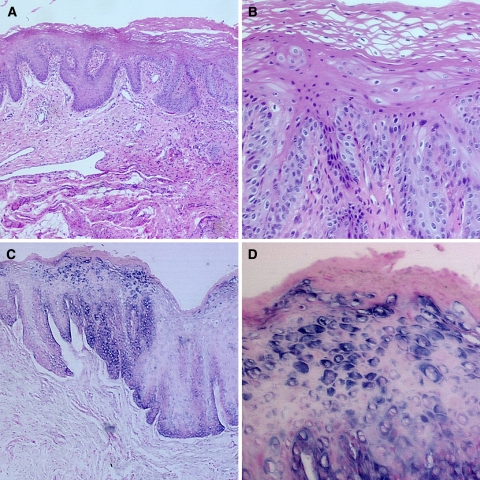
The diagnoses of HL made at our Service of Surgical Pathology were based solely on the morphological findings observed. Therefore, the objective of our study was to assess the accuracy of these diagnoses. ISH detected the presence of EBV in 29 of the 36 specimens analyzed. This means that 19.45% of the patients had been misdiagnosed with HL (Fig. 1).
Fig. 1.
Histopathological features and ISH of HL: (a) Histopathological features include acanthosis, hyperparakeratosis, koilocyte-like cells and inflammatory infiltrate in the subjacent connective tissue (H&E/magnification of 100×); (b) Koilocyte-like cells or balloon cells (H&E/magnification of 400×); (c) (100×), and (d) (400×). ISH showing the presence of EBV
None of the histopathological findings analyzed in the present study correlated with the presence of EBV. This shows that a definitive diagnosis of HL cannot be made based on the histopathological findings alone.
Some studies have been carried out to determine which histopathological findings should be present for the diagnosis of HL. These studies have considered the most common features of the lesion and compared these features with the presence of EBV, detected by ISH [20, 27].
The most common histopathological features of HL include hyperparakeratosis, acanthosis, epithelial hyperplasia, presence of koilocyte-like cells, presence of Candida sp, and minimal or absent inflammation in the subjacent connective tissue [28, 29]. These findings are observed in most cases of HL, but are not exclusive to this disease. Hence, a diagnosis of HL cannot be made based on morphological findings alone [35–37].
The most common histopathological features observed in our study were similar to those found in other studies [20, 27, 30], except for the presence of hyphae of Candida sp. Candida was found in 27.7% of the specimens analyzed in this study, which is below the average of 50% found in other studies [30]. Since the emergence of AIDS, the diagnosis of HIV-related oral lesions has been important to the diagnosis, monitoring, and staging of the syndrome. Highly active antiretroviral therapy (HAART) became available in 1996, resulting in a substantial decrease in the incidence and severity of opportunistic infections of the oral cavity [31–33].
The detection and correct diagnosis of HL are especially important because this condition is related to the immune competence of patients. HL is not exclusively associated to AIDS, this lesion is related with immunosuppression in general, especially in transplanted HIV-negative patients [11–13]. In our results, we diagnosed one case of HL in an HIV-negative renal transplant recipient (Table 1).
In HIV positive patients without anti HIV therapy the detection of HL can determine the start of medication. Moreover HAART should be administered to HIV-positive children who are diagnosed with HL, regardless of their CD4+ T lymphocyte count [34]. In patients receiving antiretroviral therapy, the presence of HL may suggest that the therapy is not working, either because the drug is ineffective or because the patient has not adhered to treatment. In patients whose HIV status is unknown, a definitive diagnosis of HL contributes to the diagnosis of HIV infection. In this context we believe that final diagnosis of HL should always be based on both histopathological examination and ISH. This will yield more reliable results, which can help to determine the HIV status of a patient.
Our study included cases diagnosed with suspected HL at unusual sites, such as the inside of the cheek (three patients) and the retromolar area (one patient), where clinical diagnosis is extremely difficult. Among the EBV-positive cases confirmed by ISH, no statistically significant differences were observed between the different anatomical sites. Cases in which EBV was not detected included all the different anatomical sites considered, and differential diagnosis is necessary for the correct management of the patient.
Final diagnosis of HL is extremely important because the lesion is related to the immune competence of patients. The use of complementary techniques to confirm the diagnosis of HL is fundamental to the clinical and therapeutic management of this lesion. Our results permitted us to change the routine of our laboratory for diagnosis of HL suggestive lesions.
References
- 1.Faulkner GC, Krajewski AS, Crawford DH. The ins and outs of EBV infection. Trends Microbiol. 2000;8(4):185–9. doi: 10.1016/S0966-842X(00)01742-X. [DOI] [PubMed] [Google Scholar]
- 2.Rickinson AB, Kieff E. Epstein-Barr virus. Fields virology, 3. Philadelphia: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 3.Cruchley AT, Murray PG, Niedobitek G, Reynolds GM, Williams DM, Young LS. The expression of the Epstein-Barr virus nuclear antigen (EBNA-I) in oral hairy leukoplakia. Oral Dis. 1997;3(Suppl 1):S177–S9. doi: 10.1111/j.1601-0825.1997.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 4.Walling DM, Etienne W, Ray AJ, Flaitz CM, Nichols CM. Persistence and transition of Epstein-Barr virus genotypes in the pathogenesis of oral hairy leukoplakia. J Infect Dis. 2004;190:387–95. doi: 10.1086/421708. [DOI] [PubMed] [Google Scholar]
- 5.Walling DM, Flaitz CM, Adler-Storthz K, Nichols CM. A non-invasive technique for studying oral epithelial Epstein-Barr virus infection and disease. Oral Oncol. 2003;13:436–44. doi: 10.1016/S1368-8375(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 6.Raab-Traub N, Webster-Cyriaque J. Epstein-Barr virus infection and expression in oral lesions. Oral Dis. 1997;3(Suppl 1):S164–S70. doi: 10.1111/j.1601-0825.1997.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 7.Greenspan D, Greenspan JS, Conant M, Petersen V, Silverman S, Jr, Souza Y. Oral hairy leukoplakia in male homosexuals: evidence of association with both pappilomavirus and a herpes-group virus. Lancet. 1984;2(8407):831–4. doi: 10.1016/S0140-6736(84)90872-9. [DOI] [PubMed] [Google Scholar]
- 8.Greenspan JS, Greenspan D, Lennette ET, Abrams DI, Conant MA, Petersen V, et al. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS associated lesion. New Eng J Med. 1985;313:1564–71. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 9.Walling DM, Flaitz CM, Nichols M. Epstein-Barr virus replication in oral hairy leukoplakia: response, persistence, and resistance to treatment with valacyclovir. J Infect Dis. 2003;188:883–90. doi: 10.1086/378072. [DOI] [PubMed] [Google Scholar]
- 10.Greenspan JS, Greenspan D. Oral hairy leukoplakia: diagnosis and management. Oral Surg Oral Med Oral Pathol. 1989;67:396–403. doi: 10.1016/0030-4220(89)90381-2. [DOI] [PubMed] [Google Scholar]
- 11.Greenspan D, Greenspan JS, Souza YG, Levy JA, Ungar AM. Oral hairy leukoplakia in an HIV-negative renal transplant recipient. J Oral Pathol Med. 1989;18:32–4. doi: 10.1111/j.1600-0714.1989.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 12.Macleod RI, Long LQ, Soames JV. Oral hairy leukoplakia in an HIV-negative renal transplant patient. Br Dent J. 1990;169:208–9. doi: 10.1038/sj.bdj.4807318. [DOI] [PubMed] [Google Scholar]
- 13.Seymour PA, Thomason JM, Nolan A. Oral lesions in organ transplant patients. J Oral Pathol Med. 1997;26:297–304. doi: 10.1111/j.1600-0714.1997.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 14.Migliorati CA, Jones AC, Baughman PA. Use of cytology in the diagnosis of hairy leukoplakia. Oral Surg Oral Med Oral Pathol. 1993;76(6):704–10. doi: 10.1016/0030-4220(93)90038-6. [DOI] [PubMed] [Google Scholar]
- 15.Triantos D, Porter SR, Scully C, Teo CG. Oral hairy leukoplakia: clinicopathologic features, pathogenesis, diagnosis, and clinical significance. Clin Infect Dis. 1997;25:1392–6. doi: 10.1086/516131. [DOI] [PubMed] [Google Scholar]
- 16.Walling DM, Flaitz K, Hosein FG, Montes-Walters M, Nichols CM. Effect of Epstein-Barr virus replication on Langerhans cells in pathogenesis of oral hairy leukoplakia. J Infect Dis. 2004;184:1656–63. doi: 10.1086/383132. [DOI] [PubMed] [Google Scholar]
- 17.Shiboski CH. Epidemiology of HIV-related oral manifestations in women: a review. Oral Dis. 1997;3(Suppl 1):18–27. doi: 10.1111/j.1601-0825.1997.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 18.Souza Y, Greenspan D, Hammer M. Demonstration od Epstein-Barr virus DNA in the epithelial cells of hairy leukoplakia. J Dent Res. 1986;65:765. [Google Scholar]
- 19.EC-Clearinghouse on Oral Problems and Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus. Classification and diagnostic criteria for oral lesions in HIV infection. J Oral Pathol Med 1993;22(7):289–91. [PubMed]
- 20.Greenspan JS, Souza YG, Regezi JA, Daniels TE, Greenspan D, MacPhail LA, et al. Comparison of cytopathic changes in oral hairy leukoplakia with in situ hybridization for EBV DNA. Oral Dis. 1998;4:95–9. doi: 10.1111/j.1601-0825.1998.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 21.Crawford DH. Biology and disease associations of Epstein-Barr virus. Phil Trans R Soc Lond B. 2001;356:461–73. doi: 10.1098/rstb.2000.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt-Westhausen A, Gelderblom HR, Neuhaus P, Reichart PA. Epstein-Barr virus in lingual epithelium of liver transplant patients. J Oral Pathol Med. 1993;22:274–6. doi: 10.1111/j.1600-0714.1993.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 23.Mabruk MJEMF, Flint SR, Coleman DC, Sheils O, Toner M, Atkins GJ. A rapid microwave-in situ hybridization method for the definitive diagnosis of oral hairy leukoplakia: comparison with immunohistochemistry. J Oral Pathol Med. 1996;25:170–6. doi: 10.1111/j.1600-0714.1996.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 24.Mabruk MJEMF, Flint SR, Toner M, Balluz I, Coleman D, Sullivan D, et al. In situ hybridization and the polymerase chain reaction (PCR) in the analysis of biopsies and exfoliative cytology specimens for definitive diagnosis of oral hairy leukoplakia (OHL) J Oral Pathol Med. 1994;23:302–8. doi: 10.1111/j.1600-0714.1994.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 25.Walling DM, Ling PD, Gordadze AV, Montes-Walters M, Flaitz CM, Nichols CM. Expression of Epstein-Barr virus latent genes in oral epithelium: determinants of the pathogenesis of oral hairy leukoplakia. J Infect Dis. 2004;190:396–9. doi: 10.1086/422039. [DOI] [PubMed] [Google Scholar]
- 26.Braz-Silva PH, Ortega KL, Rezende NP, Nunes FD, Magalhães MHCG. Detection of Epstein-Barr virus (EBV) in the oral mucosa of renal transplant patients. Diagn Cytopathol. 2006;34(1):24–8. doi: 10.1002/dc.20380. [DOI] [PubMed] [Google Scholar]
- 27.Southam JC, Felix DH, Wray D, Cubie HA. Hairy leukoplakia—a histological study. Histopathol. 1991;19:63–7. doi: 10.1111/j.1365-2559.1991.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 28.Schiodt M, Greenspan D, Daniels TE, Greenspan JS. Clinical and histologic spectrum or oral hairy leukoplakia. Oral Surg Oral Med Oral Pathol. 1987;64:716–20. doi: 10.1016/0030-4220(87)90174-5. [DOI] [PubMed] [Google Scholar]
- 29.Fraga-Fernández J, Benito C, Lizaldez EB. Oral hairy leukoplakia: a histopathologic study of 32 cases. Am J Dermatol. 1990;12(6):571–8. [PubMed] [Google Scholar]
- 30.Reichart PA, Langford A, Gelderblom HR, Pohle HD, Becker J, Wolf H. Oral hairy leukoplakia: observations in 95 cases ans review of the literature. J Oral Pathol Med. 1989;18:410–5. doi: 10.1111/j.1600-0714.1989.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 31.Eyeson JD, Tenant-Flowers M, Cooper DJ, Johnson NW, Warnakulasuriya KAAS. Oral manifestations of an HIV positive cohort in the era of highly active anti-retroviral therapy (HAART) in south London. J Oral Pathol Med. 2002;31:169–74. doi: 10.1034/j.1600-0714.2002.310308.x. [DOI] [PubMed] [Google Scholar]
- 32.Tappuni AR, Flemming GJP. The effect of antiretroviral therapy on the prevalence of oral manifestations in HIV-infected patients: A UK study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:623–8. doi: 10.1067/moe.2001.118902. [DOI] [PubMed] [Google Scholar]
- 33.Marcus M, Maida CA, Freed JR, Younai F, Coulter ID, Der-Martirosian C, et al. Oral white patches in a national sample of medical HIV patients in the era of HAART. Community Dent Oral Epidemiol. 2005;33:99–106. doi: 10.1111/j.1600-0528.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Gomez FJ, Flaitz C, Catapano P, Murray P, Milnes AR, Dorenbaum A. Classification, diagnostic criteria, and treatment recomendations for orofacial manifestations in HIV-infected pediatric patients. J Clin Pediatr Dent. 1999;23(2):85–96. [PubMed] [Google Scholar]
- 35.Fisher DA, Daniels TE, Greenspan JS. Oral hairy leukoplakia unassociated with human immunodeficiency virus: pseudo oral hairy leukoplakia. J Am Acad Dermatol. 1992;27(2 Pt 1):257–8. doi: 10.1016/s0190-9622(08)80732-3. [DOI] [PubMed] [Google Scholar]
- 36.Green TL, Greenspan JL, Greenspan D, Souza YG. Oral lesions mimicking hairy leukoplakia: a diagnostic dilemma. Oral Surg Oral Med Oral Pathol. 1989;67(4):422–6. doi: 10.1016/0030-4220(89)90385-X. [DOI] [PubMed] [Google Scholar]
- 37.Braz-Silva PH, Ortega KL, Resende NPM, Magalhães MHCG. Detection of Epstein-Barr virus (EBV) by in situ hybridization in lesions like oral hairy leukoplakia. Mod Pathol. 2006;19(Suppl 3):150. [Google Scholar]