Abstract
Background
We examined the ability of inhibitors of the synthesis or actions of 20-HETE, metabolite of arachidonic acid, to inhibit proliferation of human renal carcinoma cell lines.
Materials and Methods
786-O and 769-P cells were exposed to either 10 μM HET0016 (selective inhibitor of 20-HETE synthesis), 10 μM WIT002 (20-HETE antagonist), or vehicle. Subsequently, we assessed the effect of WIT002 on tumor growth in vivo using an ectopic mouse model of clear-cell renal carcinoma.
Results
Addition of HET0016 and WIT002 inhibited the proliferation of 786-O and 769-P human renal cell carcinoma lines. HET0016 and WIT002 had little effect on the proliferation of primary cultures of normal human proximal tubule epithelial cells. WIT002 (10 mg/Kg, s.c.) administered daily to athymic nude mice implanted subcutaneously with 786-O cells reduced the growth of the tumors by 84 % compared to vehicle (p<0.001).
Conclusion
20-HETE is required for proliferation of human renal epithelial cancers.
Keywords: Renal Adenocarcinoma, CYP4A, 20-hydroxyeicosatetraenoic acid, eicosanoids, xenograft model
Renal cell carcinoma comprises 90-95% of all neoplasms of the kidney (12) and is the sixth leading cause of cancer deaths. Renal cell carcinomas are highly proliferative and metastatic and very resistant to conventional treatment (chemotherapy, hormonal therapy and radiotherapy). Renal clear cell carcinomas grow rapidly and are associated with very poor patient survival (16). Thus, there is a tremendous need for the development of new treatment strategies. Recent studies have implicated a potential role for cytochrome P450 metabolites of arachidonic acid in the pathogenesis of several forms of cancer. Cytochrome P450 enzymes of the 4A and 4F families (CYP4A and CYP4F) catalyze omega-hydroxylation of arachidonic acid (AA) to form 20-hydroxyeicosatetraenoic acid (20-HETE) (8). 20-HETE stimulates mitogenic and angiogenic responses both in vitro and in vivo (9). Inhibitors of the synthesis of 20-HETE have been reported to block the angiogenic response of a variety of growth factors including VEGF, FGF, EGF in vivo and the proliferation U235 and 9L gliosarcoma cells in vitro and the growth of these tumors in vivo (4-6). Since renal epithelial cells normally express high levels of enzymes of the CYP4A and CYP4F families and avidly produce 20-HETE when incubated with arachidonic acid it is likely that renal cell carcinomas retain the ability to produce 20-HETE. Thus, in the present study we examined the effects of an inhibitor of the synthesis of 20-HETE and a 20-HETE antagonist on the proliferation of renal adenocarcinoma cells in vitro and tumor growth in vivo.
Materials and Methods
Cell culture
Human renal cell adenocarcinoma lines 786-O and 769-P were obtained from ATCC and grown according to the supplier recommendations. Normal primary cultured human renal proximal tubule epithelial cells (RPTC) were obtained from Cambrex Bio Science and were cultivated according to manufacturer protocols using REGM Bullit Kit.
20-HETE and EETs measurements using LC/MS/MS
The renal cells were collected and homogenized in 1 ml of a 0.1 M KPO4 buffer containing 5 mM MgCl2 and 1 mM EDTA. The homogenate was incubated for 30 min at 37°C with a saturating concentration of arachidonic acid (40 μM) and 1 mM NADPH. The reaction was stopped by acidification with formic acid to pH 3.5 and extracted twice with 3 mls of ethyl acetate after the addition of 2 ng of an internal standard d6-20-HETE. The organic phase was dried under nitrogen. The metabolites of AA were separated by HPLC on a Betabasic C18 column (150×2.1 mm, 3 μm; Thermo Hypersil-Keystone, Bellefonte, PA) at a flow rate of 0.2 mL/min using an isocratic elution with 51:9:40:0.01 mixture of acetonitrile: methanol:water:acetic acid for 30 minutes followed by a step gradient to 68:13:19:0.01 acetonitrile:methanol:water:acetic acid for 15 minutes. The effluent was ionized using negative ion electrospray and peaks eluting with a mass/charge ratio (m/z) of 319>245 (20-HETE), 319>301 (HETEs and EETs), 337>319 (DiHETEs), or 325>251 (internal standard) were monitored using an Applied Biosystems API 3000 LC/MS/MS. The ratio of ion abundance in the peaks of interest versus that seen in the internal standard were determined and compared with standard curves generated over a range from 0.2 to 10 ng for 20-HETE and from 1.0 to 10 ng for the other metabolites.
Real-time RT-PCR
Total RNA was isolated using TRIzol (Invitrogen) following standard manufacturer's instruction. Reverse transcription of mRNA was carried out using iScript cDNA synthesis kit (BioRad Laboratories). cDNA was diluted 50-fold and a 2 microliter sample used in duplicate real-time PCR experiments using iQ SYBR Green (BioRad Laboratories) and Mx3000P real-time PCR system (Stratagene). Reaction cycling parameters were as follows: 1 cycle of 3 minutes at 95°C followed by 45 cycles of 10 seconds 95°C and 45 seconds at 58°C. LinRegPCR software was used to calculate PCR efficiencies (11) and geNORM software used to determine normalization genes (15). Primer sequences were: CYP4A11 – TGCTGATCAAGGCAGTTCAG (F) and GCTCCT GGTCCTGTTGG (R), CYP4A22 – GATCAAGGCAGCTCAG CTCT (F) and GTAGCTCCTGGTCGTGTTGG (R), CYP4F2 – AAGCACCCAGAATACCAGGA (F) and TCATGCACATGGTCAGGAAG (R), CYP4F3 – CTGTCGGCAGGAGGTACAAG (F) and CCTCAGGCTCTCCTTAATGC (R). Human housekeeping gene primer sets for b2m and hprt1 were purchased from RealTimePrimers.com.
Ectopic mouse model of clear cell renal carcinoma
Experiments were carried out on 6-week immunodefficient athymic nude mice weighing 20-26g (Homozygous mice, strain code 194, Charles River Laboratories, Wilmington MA). Animals were acclimated for 1 week prior to injection with renal cell carcinoma. Immediately before each implantation, the cells were trypsinized, counted and resuspended in 10% serum containing RPMI media. The concentration of cells was adjusted to 40 millions/ml and 4 million cells/animal were injected subcutaneously. The cells were allowed to grow for 7-15 days until the size of the tumors reached approximately 0.1 cm3 The mice then received daily s.c. injections of WIT002 (10 mg/kg/day in 200 μl) in an isotonic NaPO4 buffer (pH 9.0) or vehicle (0.1 M NaPO4 solution pH 9.0). The diameter of the tumor was measured on every 3-4 days for 2 weeks using precision calibers. Tumor volume was calculated using the formula: volume=4/3πr3 as previously described (1). At the end of the experiment the mice were euthanized with CO2 and the tumors were excised to confirm the diameter measurements.
Results
Inhibition of 20-HETE synthesis decreases 786-O and 769-P cell proliferation
Recently, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/Apo-2 ligand has received a lot of attention because the majority of tumor cell lines tested in vitro were sensitive to recombinant TRAIL, whereas it displayed minimal cytotoxicity towards normal cell lines (2;3). As demonstrated in Figure 1 we studied the effect of 20-HETE inhibitors on a TRAIL-sensitive (769-P) and TRAIL-insensitive (786-O) renal carcinoma cell lines. In these experiments the medium was changed to serum-free containing the mitogens EGF or Endothelin-1 (ET-1). Both EGF and ET-1 are known to play important role in the growth of renal cancer cells. We found that HET0016 [N-hydroxy-N′-(4-butyl-2 methylphenyl)formamidine], a selective inhibitor of the synthesis of 20-HETE reduced the proliferation of renal cell carcinoma 786-O and 769-P cells by 66% and 40%, respectively (Figure 1).
Figure 1.
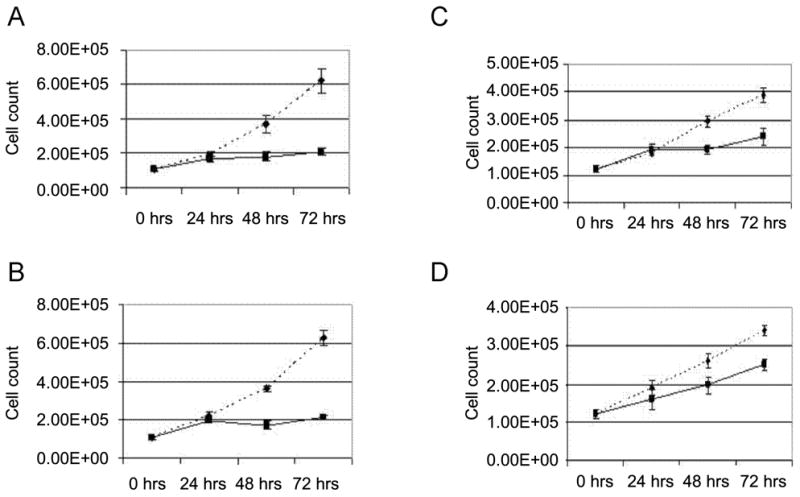
HET0016 inhibits proliferation of 786-O and 769-P renal adenocarcinoma cells. Equal numbers of either 786-O (A, B) or 769-P (C, D) cells were plated and next day (0 hrs) transferred to serum-free medium containing either EGF (A, C) or ET-1 (B, D). Cells were exposed either to 10 μM HET0016 (solid lane), or vehicle (dotted lane). Cell counting was performed at the day of transfer to serum free medium (0 hrs) and 24, 48 and 72 hours thereafter. Medium was changed to fresh containing mitogens and drugs every 24 hours. Data presented are characteristic experiment from at least two separate experiments, each performed in triplicate.
Inhibition of 20-HETE signaling decreases 786-O and 769-P cell proliferation
We also examined the effects of the 20-HETE antagonist 20-6,15 HEDE on the proliferation of the TRAIL-sensitive (769-P) and TRAIL-resistant (786-O) renal carcinoma cells (Figure 2 A, B, C, D). 20-6,15 HEDE completely inhibited the proliferation of both cell lines whether proliferation was stimulated by ET-1 or EGF.
Figure 2.
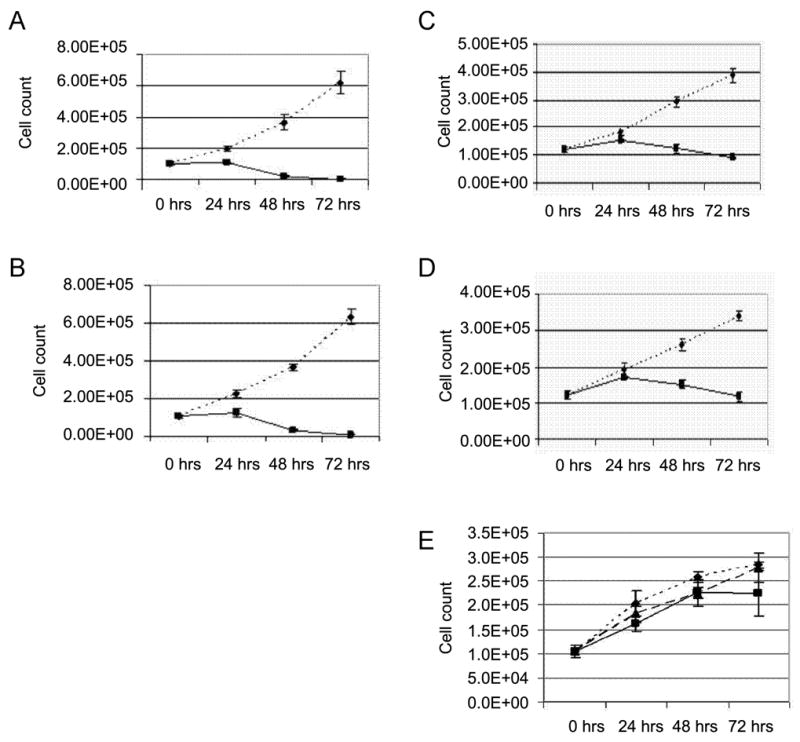
WIT002 inhibits proliferation of 786-O and 769-P renal adenocarcinoma cells (A-D), but HET0016 and WIT002 fail to inhibit proliferation of normal renal epithelial cells RPTC (E). Equal numbers of 786-O (A, B) or 769-P (C, D) cells were plated and next day (0 hrs) transferred to serum-free medium containing either EGF (A, C) or ET-1 (B, D). Cells were exposed either to 10 μM WIT002 (solid lane), or vehicle (dotted lane). Cell counting was performed at the day of transfer to serum free medium (0 hrs) and 24, 48 and 72 hours thereafter. Medium was changed to fresh containing mitogens and drugs every 24 hours. Data presented are characteristic experiment from at least two separate experiments, each performed in triplicate. (E) Equal numbers of RPTC cells were plated and next day (0 hrs) transferred to medium containing either vehicle (diamonds, dotted lane), HET002 (squares, dotted lane) or WIT002 (triangles, solid lane). Concentrations of HET0016 and WIT002 were 10 μM. Cell counting was performed at the day of transfer to serum free medium (0 hrs) and 24, 48 and 72 hours thereafter. Data presented are characteristic experiment from at least two separate experiments, each performed in triplicate.
HET0016 and WIT002 are not cytostatic to normal proximal tubule epithelial cells
Experiments were also performed using early passages of human primary renal proximal tubule epithelial cells (RPTC) to access the direct cytotoxic effects of HET0016 and 20-6,15 HEDE. As illustrated in Figure 2E, both HET0016 and WIT002 had little effect on the proliferation of normal human renal proximal tubular cells.
Renal adenocarcinoma cells express Cyp4F2 and Cyp4F3 mRNAs
We examined renal adenocarcinoma cells 786-O and 769-P for the presence of mRNAs encoding 4 human Cyp450 isoforms: CYP4A11, CYP4A22, CYP4F2 and CYP4F3. The relative mRNA expression levels of CYP4F2 was about 3 times higher in 786-O cells when compared to 769-P cells, as detected by real-time RT-PCR. The levels of CYP4F3 mRNA did not differ significantly in 786-O and 769-P cells, whereas CYP4A11 and CYP4A22 mRNAs were not detected.
Effects of HET0016 on the metabolism of arachidonic acid in renal carcinoma cells
The results of these experiments are presented in Figure 3. LC/MS/MS analysis revealed the presence of 15-, 12-, 5-HETEs and 14,15-, 11,12-, 8,9-EETs, but not 20-HETE in renal adenocarcinoma cells 786-O (Figure 3A). Incubation of the cells for 30 minutes with AA increased the levels of 20-HETE and DiHETEs, whereas the levels of EETs, 15-, 12- and 5-HETEs were not altered (Figure 3B). Addition of HET0016 (10 μM) selectively reduced the formation of 20-HETE in 786-O cells incubated with AA (Figure 3C).
Figure 3.
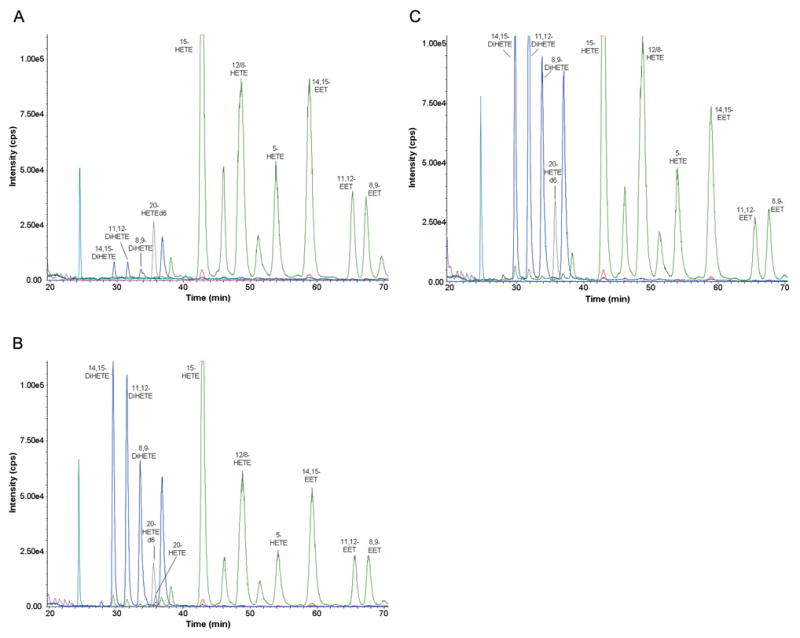
Liquid Chromatography/Mass Spectroscopy profiling of eicosanoids in 786-O renal adenocarcinoma cells. 786-O cells were plated, harvested, pelleted, and resuspended in serum-free medium and quickly frozen in liquid N2. LC/MS analysis was performed as described in Materials and Methods. Profiling of eicosanoids was performed either without incubation with arachidonic acid (A), or after 30 min incubation with arachidonic acid in the absence (B) or presence (C) of HET0016.
WIT002 suppresses renal adenocarcinoma cell proliferation in vivo
The effect of the 20-HETE antagonist, WIT002 on the growth of 786-O clear cell renal carcinoma was assessed in ectopic mouse model of renal tumor (Figure 4). The growth of tumors was significantly suppressed by WIT002 administered daily to athymic nude mice implanted subcutaneously with cells 786-O. Tumor growth was inhibited by 84% ± 12%, where 84 is average and 12 is SEM. It is of note that in these experiments WIT002 treatment started only after the tumor was seeded for 7-14 days and was relatively large 0.1 cm. Thus, WIT002 was effective at arresting the growth of a fairly advanced tumor.
Figure 4.
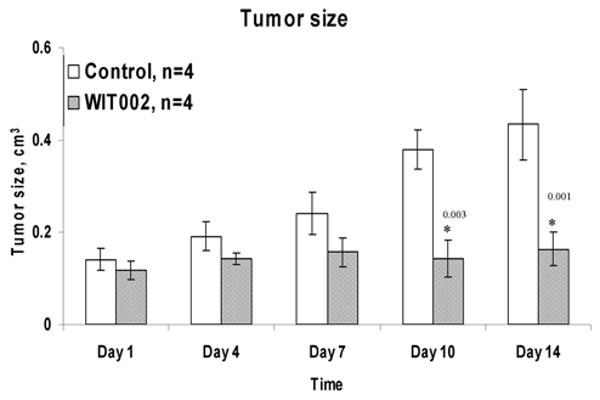
WIT002 decreases tumor growth in ectopic mouse model of clear cell renal carcinoma. During the treatment period the mice had their tumor size measured at different time points by a precision hand measuring ruler (obtained from Scienceware) and tumor volume was calculated as described in Materials and Methods.
Discussion
Preceding studies have indicated that enzymes of the CYP4A and CYP4F families are expressed in normal human renal tissue and it avidly produces 20-HETE (13). However, no one has studied whether renal clear cell carcinomas retain the ability to produce 20-HETE. In the current study we found that renal adenocarcinoma cells expressed CYP4F isoforms and produce 20-HETE. Moreover, inhibition of 20-HETE synthesis by HET0016 and an administration of an analog of 20-HETE that interferes with its acting did suppress proliferation of cultured renal carcinoma cells in vitro. It was previously reported that 5-Lipoxygenase inhibitors attenuate growth of human RCC (7) whereas COX-2 inhibitors have only slight anti-proliferative effects against RCC (17). Our data is the first indication that another class of enzymes, which utilizes arachidonic acid, the CYP4F family of proteins, is playing important role in regulation of renal carcinoma growth.
The molecular mechanisms of 20-HETE signaling in the proliferation of epithelial cancer cells are unknown, Nevertheless, it has been shown that enforced overexpession of CYP4A and, correspondingly, generation of 20-HETE in human glioma cells in vitro is accompanied by increased signaling via ERK cascade and enhanced expression of cyclin D1/2 and VEGF (4). When epithelial cells, which participate in formation of renal cysts, were genetically modified to overproduce CYP4A12, it caused a four- to five-fold increase in cell proliferation compared with control cells, and this increase was completely abolished when 20-HETE synthesis was inhibited (10). Long term inhibition of 20-HETE signaling was accompanied by decrease of EGFR phosphorylation in mouse kidneys suggesting a possibility of coupling 20-HETE with transactivation of EGFR (10). Aberrant signaling of multiple members of the epidermal growth factor receptor (EGFR) family often causes uncontrolled proliferation of cancer cells. It is of note that transactivation of EGFR by prostaglandins, products of Cox-2 activity, was reported in a number of cancer cells (14). Whether 20-HETE, product of CYP450 enzymes, is capable of EGFR transactivation remains to be determined.
Acknowledgments
NIH research grants RO1 DK 41684 (Sorokin), RO1 HL 022563 (Sorokin), HL 029587 (Roman), HL 036279 (Roman) and Medical College of Wisconsin New Interdisciplinary Research Grant (Sorokin). The authors thank Jacob Huh (Medical College of Wisconsin) for his participation in the early stages of this project.
References
- 1.Chao C, Goluszko E, Lee YT, Kolokoltsov AA, Davey RA, Uchida T, Townsend CM, Jr, Hellmich MR. Constitutively active CCK2 receptor splice variant increases Src-dependent HIF-1 alpha expression and tumor growth. Oncogene. 2007;26:1013–1019. doi: 10.1038/sj.onc.1209862. [DOI] [PubMed] [Google Scholar]
- 2.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 3.Griffith TS, Fialkov JM, Scott DL, Azuhata T, Williams RD, Wall NR, Altieri DC, Sandler AD. Induction and regulation of tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Res. 2002;62:3093–3099. [PubMed] [Google Scholar]
- 4.Guo AM, Sheng J, Scicli GM, Arbab AS, Lehman NL, Edwrads PA, Falck JR, Roman RJ, Scicli AG. Expression of CYP4A1 in U251 Human Glioma Cell Induces Hyperproliferative Phenotype in vitro and Rapidly Growing Tumors in vivo. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.108.140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo M, Roman RJ, Falck JR, Edwards PA, Scicli AG. Human U251 glioma cell proliferation is suppressed by HET0016 [N-hydroxy-N′-(4-butyl-2-methylphenyl)formamidine], a selective inhibitor of CYP4A. J Pharmacol Exp Ther. 2005;315:526–533. doi: 10.1124/jpet.105.088567. [DOI] [PubMed] [Google Scholar]
- 6.Guo M, Roman RJ, Fenstermacher JD, Brown SL, Falck JR, Arbab AS, Edwards PA, Scicli AG. 9L Gliosarcoma Cell Proliferation and Tumor Growth in Rats Are Suppressed by N-Hydroxy-N′-(4-butyl-2-methylphenol) Formamidine (HET0016), a Selective Inhibitor of CYP4A. J Pharmacol Exp Ther. 2006;317:97–108. doi: 10.1124/jpet.105.097782. [DOI] [PubMed] [Google Scholar]
- 7.Matsuyama M, Yoshimura R, Mitsuhashi M, Tsuchida K, Takemoto Y, Kawahito Y, Sano H, Nakatani T. 5-Lipoxygenase inhibitors attenuate growth of human renal cell carcinoma and induce apoptosis through arachidonic acid pathway. Oncol Rep. 2005;14:73–79. [PubMed] [Google Scholar]
- 8.McGiff JC, Carroll MA. Cytochrome P450-dependent arachidonate metabolites, renal function and blood pressure regulation. Adv Prostaglandin Thromboxane Leukot Res. 1991;21B:675–682. [PubMed] [Google Scholar]
- 9.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 10.Park F, Sweeney WE, Jia G, Roman RJ, Avner ED. 20-HETE Mediates Proliferation of Renal Epithelial Cells in Polycystic Kidney Disease. J Am Soc Nephrol. 2008;208:1929–1939. doi: 10.1681/ASN.2007070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 12.Sachdeva K. Renal Cell Carcinoma. eMedicine from WebMD. 1-7-2008. Ref Type: Electronic Citation. [Google Scholar]
- 13.Simpson AE. The cytochrome P450 4 (CYP4) family. Gen Pharmacol. 1997;28:351–359. doi: 10.1016/s0306-3623(96)00246-7. [DOI] [PubMed] [Google Scholar]
- 14.Sorokin A. Eicosanoids and resistance of cancer cells to chemotherapeutic agents. In: Bonavida B, editor. Sensitization of Cancer Cells for Chemo/Immuno/Radio-therapy. Humana Press; 2008. pp. 133–156. [Google Scholar]
- 15.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss RH, Lin PY. Kidney cancer: identification of novel targets for therapy. Kidney Int. 2006;69:224–232. doi: 10.1038/sj.ki.5000065. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura R, Matsuyama M, Kawahito Y, Takemoto Y, Tsuchida K, Kuratsukuri K, Segawa Y, Shinnka T, Sano H, Nakatani T. The effects of cyclooxygenase-2 inhibitors on urological cancer cells. Int J Mol Med. 2004;13:789–793. [PubMed] [Google Scholar]


