Abstract
Purpose
A major mechanism of resistance to chlorethylnitrosureas and methylating agents involves the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT). We sought to determine the dose of oral lomeguatrib, a pseudosubstrate inactivator of MGMT, required to render active protein undetectable 12 hours after dosing in prostate, primary central nervous system (CNS) and colorectal cancer patients.
Experimental Design
Lomeguatrib was administered orally as a single dose (20 to 160mg) approximately 12 hours prior to tumour resection. Dose escalation was projected to continue until grade 2 toxicity or complete inactivation of tumour MGMT was encountered. Total MGMT protein levels were quantified by enzyme linked immunosorbent assay (ELISA), and active protein by biochemical assay. MGMT promotor methylation was determined in glioblastoma DNA by methylation-specific PCR.
Results
Thirty seven patients were dosed with lomeguatrib, and 32 informative tumour samples obtained. Mean total MGMT level varied between tumour types: prostate cancer 554±404 fmol/mg protein (±SD), CNS tumours 87.4±40.3 fmol/mg protein, colorectal cancer 244±181 fmol/mg protein. MGMT promoter hypermethylation did not correlate with total protein expression. Consistent total MGMT inactivation required 120mg lomeguatrib in prostate and colorectal cancers. Complete consistent inactivation in CNS tumours was observed only at the highest dose of lomeguatrib, 160mg.
Conclusions
Total MGMT inactivation can be achieved in prostate, primary CNS and colorectal cancers with a single administration of 120 or 160mg lomeguatrib. The dose needed did not correlate with mean total MGMT protein concentrations. Lomeguatrib 120-160mg/day should be administered to achieve total MGMT inactivation in future studies.
Keywords: lomeguatrib, DNA repair, O6-methylguanine-DNA methyltransferase, phase I, pharmacodynamic
Introduction
Chlorethylnitrosoureas such as 1, 3-bis- (2-chloroethyl)-1-nitrosurea (BCNU) and methylating agents such as temozolomide are cytotoxic by virtue of adducts formed at the O6-position of guanine (1-3). Resistance to these O6-alkylating agents can be conferred by the repair protein O6-methylguanine-DNA methyltransferase (MGMT), which removes the alkyl group from the O6 position on guanine in a stoichiometric, auto-inactivating reaction (3, 4). It covalently transfers the alkyl group to its active site cysteine either before chloroethylguanine initiated DNA interstrand cross-links can form or before O6-methylguanine: thymine (O6-MG: T) mispairing results from further rounds of replication. MGMT is unique in its ability to remove DNA adducts independently rather than via multi-enzyme complexes, as is the case in most other DNA repair systems.
Lomeguatrib [6-(4-bromo-2-thienyl) methoxy purin-2-amine] is an orally bioavailable potent pseudosubstrate for MGMT. The drug was developed with the aim of inactivating MGMT, rendering cells more sensitive to the cytotoxic effects of O6-alkylating agents. Covalent transfer of the bromothenyl group on lomeguatrib to the active site cysteine inactivates MGMT. Lomeguatrib has shown promising activity in sensitising a variety of human tumour xenografts to the growth inhibitory effects of O6-alkylating agents, including temozolomide and BCNU, at the expense of only limited additional toxicity (5, 6).
Following a phase I study of combination treatment with lomeguatrib and temozolomide (7), a randomised phase II study using this combination in over 100 patients with metastatic melanoma has been reported (8). Patients were treated with 40 to 80mg lomeguatrib with 125mg/m2 temozolomide or 200mg/m2 temozolomide alone orally on days 1 to 5 every 28 days for up to 6 cycles. The lomeguatrib dose was selected based upon tumour depletion at 4 hours in 6 melanoma patients included in the phase I trial. The efficacy of combination treatment with lomeguatrib and temozolomide was found to be similar to that of temozolomide alone in terms of response rates and median time to disease progression (13.5 % vs. 17.3 % and 65.5 vs. 68 days respectively). This may have been due to the scheduling of lomeguatrib, which permitted rapid recovery of tumour MGMT. Tumour biopsies from patients showed early recovery of MGMT activity, within 24 hours, even when 60 or 80mg of lomeguatrib was given daily (8).
Data are needed on the effects of lomeguatrib in other tumour types to determine the doses best used in tumour site specific combination studies. Our study focused on four tumour types, primary breast, prostate and CNS cancers and primary or secondary colorectal cancers. These were selected on the basis of results from work on human xenograft models, which showed that lomeguatrib enhanced the anti-tumour effects of temozolomide in these tumour types (9,10). Tumour types were also chosen to provide a range of MGMT activities, according to the literature (11).
The primary aim of this study was to determine the dose of oral lomeguatrib required to render active MGMT undetectable 12 hours after dosing in primary breast, prostate and CNS tumours and primary or secondary colorectal tumours as measured by biochemical assay. The 12 hour time point was chosen based upon pharmacokinetic and pharmacodynamic results from our phase I trial of lomeguatrib with temozolomide (7). Given that some of the tumours might not have expressed the MGMT protein and would have shown no activity even before lomeguatrib dosing, it was also necessary to quantify total MGMT protein expression (both active and inactive) and for this we used an antibody-based method. In this way, we could estimate the extent to which MGMT had been inactivated by lomeguatrib treatment. We also evaluated the safety of lomeguatrib as a single agent. In CNS tumours, MGMT promoter methylation status was determined in DNA extracted from tumour aliquots to assess the relationship with MGMT protein expression levels, and to determine intra-tumoural heterogeneity in methylation status.
Patients and Methods
Eligibility
Patients due to undergo elective surgery for removal of a primary breast, prostate or CNS tumour or primary or secondary colorectal cancer were identified from the 2 clinical centres. To be eligible for the study, patients needed to be aged 18 or over with elective surgery for tumour removal scheduled within 14 days. Histological or cytological confirmation of cancer was required for breast and colorectal patients. In the absence of histological or cytological confirmation of cancer for CNS and prostate patients, strong radiological suspicion or a PSA diagnosis (≥ 20 μg/l) were necessary respectively. Patients whose subsequent histology did not confirm a primary CNS (defined as glioblastoma multiforme or anaplastic astrocytoma) or prostate cancer were excluded from analysis of tumour MGMT inactivation.
This study was conducted under the auspices of Cancer Research UK in accordance with the principles of the International Conference on Harmonisation of Good Clinical Practice guidelines and the Declaration of Helsinki. The trial was approved by the Cancer Research UK Independent Ethics Committee and the Local Research Ethics Committee of each trial centre. All patients enrolled in the study gave written informed consent.
Treatment and dose escalation
Lomeguatrib (KuDOS Pharmaceuticals Ltd, Cambridge, UK) was administered orally approximately 12 hours prior to tumour resection. Patients were requested to fast for two hours pre- and post-dosing and advised to swallow the lomeguatrib capsules whole so as not to interfere with the enteric coating. Five dose levels of lomeguatrib were to be studied in each tumour type: 20, 40, 80, 120 and 160mg. These were selected to include the dose range found from phase I work to completely deplete MGMT in a variety of solid tumours, i.e. 40 to 80mg/day (7). Dose levels above and below this range were included to take into account the different levels of MGMT expression in the tumours studied in anticipation of the possibility that different doses of lomeguatrib might be required to engender complete MGMT depletion (10, 11). The dose range also reflected data from studies with the MGMT pseudosubstrate O6-benzylguanine, showing that approximately three times the dose of inactivator is required to deplete MGMT in tumour as in peripheral blood mononuclear cells (PBMCs) (12).
Initially, patients were enrolled in cohorts of 3 to allow an assessment of the safety of each lomeguatrib dose. If any of the 3 patients had detectable active tumour MGMT escalation continued. In the event of all 3 patients exhibiting no active protein, a further 3 patients were to be recruited at that dose level to confirm the observation. Dose escalation was to cease in the event of an adverse event greater than grade 1, except for grade 2 nausea and vomiting, that was probably, possibly, or almost certainly related to lomeguatrib. As safety data emerged from other studies dose escalation was permitted immediately any patient showed active residual tumour MGMT. Due to poor recruitment, the breast cancer arm of the study was discontinued.
Toxicity and response evaluation
Pre-treatment evaluations were performed in the 2 weeks before the anticipated date of surgery. These involved a complete medical history and physical examination including performance status and vital signs. The results of pre-operative baseline blood tests performed within the same time interval documenting bone marrow, renal and hepatic function, glucose, uric acid and bone chemistry were recorded from patient notes. Patients were assessed for safety and toxicity from the time of informed consent until end of treatment evaluation 28 days after lomeguatrib administration. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria version 2.0. Patients were formally assessed preoperatively as outlined above, again on the day after surgery and 28 days after dosing. Post-operative assessments involved a physical examination including vital signs. Full blood count and biochemistry profile were performed and adverse events and concurrent medication recorded. If any adverse events attributable to lomeguatrib occurred, the patient was followed up monthly until resolution of the events. The safety profile at each dose level was reviewed before the next group of patients was recruited. In the event that surgery was delayed such that the tumour could not be removed within 6-18 hours of the lomeguatrib dose, that patient was replaced. All tumour samples were analysed to confirm that tumour tissue was removed at operation, and that the histology conformed to that under investigation in the protocol. Patients with no tumour found, or inappropriate histology were replaced.
Pharmacodynamics
At the time of surgery, approximately 12 hours after lomeguatrib dosing, between 1 and 6 representative samples from the tumour were collected and immediately frozen on dry ice prior to storage at −70°C before determination of MGMT activity and total protein (13, 14). Samples obtained less than 6 hours after dosing, or more than 18 hours afterwards were excluded from analysis. Ten millilitres of blood were collected in a universal tube containing 100 μl 0.5 M EDTA for PBMC isolation and analysis of MGMT activity (13). The pharmacodynamic effect of lomeguatrib was assessed by measuring active and total MGMT protein in each tumour type to determine the degree of MGMT inactivation. In tumours where more than one sample was provided for analysis the mean value was calculated. The MGMT activity assay was biochemical, measuring the transfer from a DNA substrate of radio-labelled methyl groups to MGMT protein in the sample under standard conditions (13). Total MGMT protein (active and inactive) was also determined by a validated ELISA to confirm protein expression in the tumour prior to lomeguatrib administration, and to allow calculation of the degree of MGMT inactivation (13). Only samples showing evidence of MGMT protein expression (i.e greater than lower limit of quantitation (LLOQ), i.e. >30fmol/mg protein) by this method were eligible for analysis. MGMT activity was reported as completely inactivated if the level of active MGMT was less than 32.5 Fmoles per mL of tissue sonicate. MGMT activity was also measured in post treatment PBMC samples as a confirmation of lomeguatrib ingestion.
MGMT promoter methylation status
Given the emerging role of tumour MGMT promoter methylation in selecting glioblastoma patients for chemotherapy (14) we extracted DNA from CNS tumour sonicates using a Qiagen Blood and Cell Culture DNA mini kit (Qiagen Limited, West Sussex, UK). Our method was similar to that of Hegi (14), but did not require a 2 stage PCR as we had large amounts of DNA available. Briefly, bisulphite conversion of DNA (500ng) was performed using EZ DNA Methylation-Gold Kit from Zymo Research (Cambridge Bioscience, Cambridge, UK). PCR amplification was undertaken using 0.8uM concentrations of the following primers (synthesised by MWG, Eurofins Genetic Services Ltd, Ebersberg, Germany): methylation specific primers forward: 5′-TTTCGACGTTCGTAGGTTTTCGC; reverse: 5′-GCACTCTTCCGAAAACGAAACG; and unmethylated DNA primers: forward: 5′-TTTGTGTTTTGATGTTTGTAGGTTTTTGT; reverse: 5′-AACTCCACACTCTTCCAAAAACAAAACA, with 200uM deoxynucleoside triphosphates. PCR Conditions were: 97°C for 5mins, 80°C hold, add 0.2ul Promega Go Taq DNA Polymerase (Promega Ltd, Chilworth Research Centre, United Kingdom), then 35 cycles of 95°C for 30secs, 59°C for 30secs and 72°C for 30secs, followed by 72°C for 10mins and 4°C hold. Controls for the PCR reactions were Intergen CpGenome Universally Methylated DNA as positive control and Promega Human Male DNA and ddH2O as negative controls. Methylation patterns were scored as indicated. PCR products were run on a 2% agarose gel stained with ethidium brmide and visualised with UV. A 100bp marker ladder was loaded to enable size estimation. If methylation specific primers gave a product of the correct size then the sample DNA was considered to be methylated, and if unmethylated primer gave a product it was considered unmethylated.
Results
Patients and dose escalation
Of the forty patients enrolled in the study, 37 received lomeguatrib. A patient with colorectal cancer liver metastases had their hepatectomy cancelled due to the diagnosis of new lung metastases, and a prostate cancer patient declined participation after registration. The third patient was not dosed pre-operatively in error.
All 37 patients administered lomeguatrib were evaluable for toxicity, but only 32 patients provided tumour specimens informative in assessing MGMT inactivation. Two patients had tumour samples taken that had no detectable MGMT, active or inactive. Tumour material taken from a third patient was insufficient for analysis and tumour was not provided from a fourth. A fifth patient with radiological evidence of a CNS tumour was found to have primary CNS lymphoma, and was excluded from analysis.
The number of patients with each tumour type administered lomeguatrib at each dose level is given in Table 1. All of the patients had tumour excised within the permitted 6 to 18 hour window after lomeguatrib dosing, with the interval ranging from 8 hour to 17 hours 40 minutes. The median time to tumour sampling was 12 hours in CNS tumours, but slightly longer for the other two tumour types (Table 1). There was no correlation between the extent of MGMT inactivation and the time to tumour sampling (data not shown).
Table 1.
Effect of lomeguatrib on tumour MGMT*
| Primary CNS Tumour |
Colorectal Cancer |
Prostate Cancer |
|||||
|---|---|---|---|---|---|---|---|
| Number | 9 | 14 | 8 | ||||
|
Median time (h) between dosing
and tumour removal (range) |
12 (8.0 – 14.5) | 14.5 (11.75 – 17.67) | 13.83 (12.67 – 16.25) | ||||
|
Mean total MGMT (fmol/mg
protein) ± SD |
89.9 ± 44.5 | 244 ± 181 | 554 ± 404 | ||||
|
Range of total MGMT (fmol/mg
protein) |
38 - 165 | 47 - 547 | 47 - 1136 | ||||
|
Patients with total MGMT inactivation |
Mean % MGMT inactivation (range) |
Patients with total MGMT inactivation |
Mean % MGMT inactivation (range) |
Patients with total MGMT inactivation |
Mean % MGMT inactivation (range) |
||
|
Lomeguatrib
dose (mg) |
20 | 0/3 | 43 (25-70) | 0/3 | 82 (77-89) | 0/1 | 94 |
| 40 | 0/1 | 46 | 1/2 | 61 (22-100) | 1/1 | 100 | |
| 80 | 0/1 | 57 | 2/3 | 98 (93-100) | 2/3 | 98 (95-100) | |
| 120 | 0/2 | 73 (62-85) | 5/6 | 100 | 2/3 | 100 (99-100) | |
| 160 | 2/2 | 100 | |||||
MGMT – O6-methylguanine-DNA methyltransferase; SD – standard deviation; CNS – central nervous system
two patients with breast cancer were administered 20mg lomeguatrib, one provided an informative tumour biopsy specimen in which all the MGMT was inactivated.
MGMT depletion
With one exception, patients administered lomeguatrib exhibited very low or no active MGMT in PBMCs. One patient with a CNS tumour given 20mg of drug had 16.7fmol/μg DNA active MGMT, a finding more consistent with pre-treatment levels found in our other studies. The percentage of inactive tumour MGMT in this individual, at 25 %, was lower than that observed in the other 2 CNS patients at this dose level.
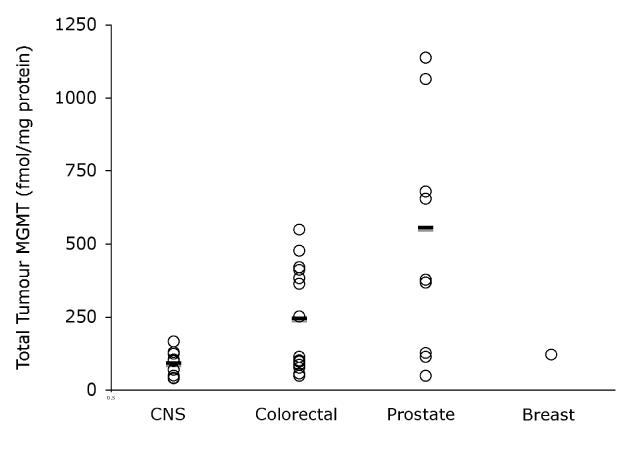
Total tumour MGMT varied considerably between tumour types (Figure 1) with prostate cancers having the highest levels (mean 554 +/− SD 404 fmol/mg protein) and CNS tumours the lowest (89.9 +/− 44.5 fmol/mg protein). Colorectal tumours showed intermediate levels of total protein: 244 +/− 181 fmol/mg protein).
Figure 1.
Total tumour MGMT (fmol/mg protein, open circles) and mean (—) by tumour type.
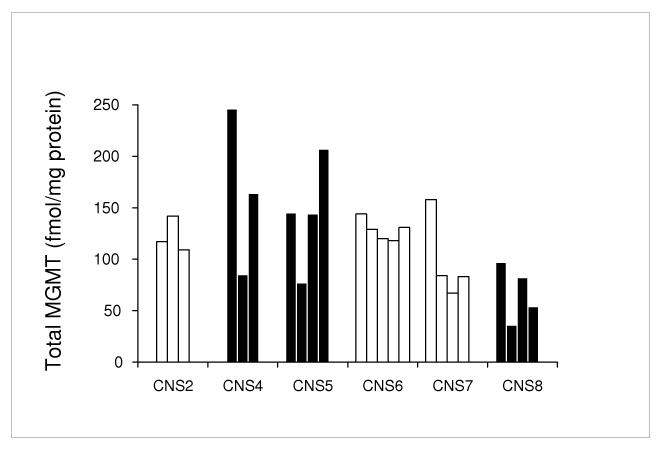
Across all three tumour types, increasing doses of lomeguatrib were associated with an increasing proportion of inactive MGMT in tumours (Table 1). There were marked differences in the proportion of MGMT that was inactive between the tumour types. These differences were most striking at the lowest dose level where 25-70 % of MGMT was inactive in CNS tumours, 77-89 % in colorectal cancer and 94 % in prostate cancer. MGMT protein levels in subdivided CNS tumour samples showed variable heterogeneity in total MGMT levels (Figure 2).
Figure 2.
Total MGMT protein and MGMT promoter methylation status (white bars unmethylated, black bars methylation present) in aliquots of CNS tumours from patients in the study.
MGMT promoter methylation
The MGMT promoter region in DNA isolated from all 6 of the CNS tumour extracts contained unmethylated DNA. Three of the tumours also contained methylated DNA. Multiple aliquots from individual tumours gave consistent results in all 6 cases (Figure 2).
Toxicity
Lomeguatrib was very well tolerated with only three grade 1 adverse events recorded that may have been related to the drug. These did not appear to be dose related and all resolved spontaneously. They included an abnormal sensation in the abdomen and two instances of raised liver enzymes (raised transaminases, gamma glutyltransferase and alkaline phosphatase) between 1 and 4 weeks after administration of the study drug.
A number of adverse events were recorded that were considered to be secondary to the patient’s surgery and not due to lomeguatrib administration. Of these, 5 episodes were serious by virtue of the need for, or prolongation of, hospitalisation of the patient. One patient experienced pain and blurred vision in their right eye (both grade 2), assessed as secondary to their right temporal craniotomy. There were two instances of grade 3 rises in alanine transaminase in patients following hepatic resections. One patient developed a grade 3 chest infection and a grade 4 pulmonary embolus and a further patient suffered a grade 3 ileus.
Discussion
The aim of this trial was to determine the dose of lomeguatrib that reliably inactivated tumour MGMT in patients with one of four cancers. The use of a pharmacodynamic end-point to determine the dose of lomeguatrib for use in future studies was based on the expectation that MGMT inactivation was likely to be achievable at doses well below the maximum tolerated dose of the drug. The range of lomeguatrib doses was selected based upon the findings of our phase I trial where 40 to 80mg of lomeguatrib given orally produced total MGMT inactivation in both tumour and PBMCs 4 hours after dosing (9), and by the constraints inherent in the 10mg capsule size available to us.
Complete MGMT inactivation was seen in primary CNS tumour biopsies after treatment with 160mg lomeguatrib, and was first observed in colorectal and prostate cancer with 40 mg lomeguatrib. However, consistent inactivation was only observed in these two tumour types with 120mg lomeguatrib. Very slow recruitment led to the abandonment of the breast cancer arm of the study, although the only patient given lomeguatrib (20mg) showed complete inactivation of MGMT. The originally specified end-point of the study, to define a dose of lomeguatrib that achieved total MGMT inactivation in 6 out of 6 samples for each tumour type, was not achieved mainly due to slow accrual and the imminent expiry of the lomeguatrib stock. Nevertheless, depletion was complete in two CNS and prostate and five colorectal patient tumours.
PBMC samples were used to check that ingestion and absorption of lomeguatrib had taken place. In all but one case absent or very low levels of active MGMT confirmed the administration of an MGMT inactivator. The exception was a patient with a CNS tumour given 20 mg lomeguatrib, and raises the possibility that not all the drug was absorbed. It may be that concurrent anti-epileptic medication (phenytoin 300mg daily) affected drug metabolism or that recovery of active MGMT was already in progress at the time of sampling, which was 12 hours and 25 minutes after lomeguatrib administration.
Substantial variation in MGMT protein levels were seen both between and within tumour types, as previously reported (11). The mean total MGMT levels in the 3 main tumour types assessed, with an increase from primary CNS tumours to colorectal cancer to prostate cancer, were in keeping with previous reports on xenograft models and clinical tumour sample series (10, 11).
This is the first study to report levels of active and inactive MGMT in tumour biopsies. We did not anticipate the variation in the proportion of inactive to total MGMT between the three tumour types studied. It may be that the basis for this observation is that tumour penetration of lomeguatrib differs between prostate, colorectal and CNS cancers. Alternatively, the proportion of active to inactive MGMT may inherently differ between the tumour types. The study design did not mandate the collection of tumour without lomeguatrib administration, which may have resolved this issue. If the effects of the inactivator are tissue specific, this has implications for the indications in which the drug should be evaluated. To date, results using daily doses of 40 to 80mg in melanoma and colorectal cancer have been disappointing, but our findings suggest that prostate cancer may be a better target for treatment.
If the proportion of active to inactive repair protein differs between tumours this has implications for the methods used to evaluate MGMT in clinical specimens. In particular, immunohistochemical techniques that fail to distinguish active from inactive protein will not provide an accurate assessment of relative repair capacity when compared across tumour types.
The proportion of MGMT that was inactive broadly increased with increasing doses of lomeguatrib in all three tumour types. We observed no correlation between the dose of lomeguatrib required for inactivation and mean total MGMT in the three cancers. Previous reports based on studies in cell culture, indicated that following its action on methylated DNA, MGMT is ubiquitinated and degraded by proteasome activity. We cannot exclude the possibility that such an event may have contributed to some elimination of lomeguatrib- inactivated MGMT. However, inactive MGMT was clearly abundant, so it may be that this is not a rapid effect in tumours, or perhaps that lomeguatrib- inactivated protein is not as effective a substrate for ubiquitination and proteasome degradation.
In keeping with previous findings, oral lomeguatrib was well tolerated at the dose levels studied (8, 9, 15, 16). It is extremely unlikely to have single agent anti-tumour activity and will only be used in combination with cytotoxic agents. To this end the results seen in primary brain tumours, the mainstay of treatment for which is temozolomide, are of particular interest. MGMT promoter methylation status is currently used in Europe to stratify patients to be treated with temozolomide alongside radiotherapy (14). In CNS tumour samples we observed the presence of unmethylated MGMT promoter in all and methylated promoter in half of the samples. There was no correlation between promoter methylation and protein expression, indeed the highest level of MGMT expression was seen in one of the samples that contained methylated DNA. This raises some doubts about the validity of CNS tumour MGMT promoter methylation as a predictor of MGMT expression levels, but might indicate that the observed correlation between MGMT promoter methylation and response to therapy is not attributable to MGMT expression.
Studies have been conducted with lomeguatrib and temozolomide in a variety of solid tumours in the phase I setting. The main toxicities seen with combination treatment were haematological, and required the standard single agent dose of temozolomide to be reduced by 40-60 % depending upon the duration of lomeguatrib administration. A regimen of oral lomeguatrib 40mg/day with temozolomide 125mg/m2 days 1 to 5 was suggested for further evaluation (9). The dose of temozolomide that could safely be administered with the higher levels of lomeguatrib required reliably to achieve complete MGMT inactivation in primary brain tumours will need to be established. Ranson et al established that MGMT recovers rapidly after treatment with lomeguatrib and temozolomide in patients with metastatic melanoma (8). However it may be that although 120 or 160mg doses of lomeguatrib are required to inactivate tumour MGMT lower doses are needed to maintain inactivation. We have no data to show how long inactivation lasts, and the short half life of lomeguatrib (1.5-2 hours) suggests that maintaining activation may require twice daily dosing.
The results of our study shed new light on previous trials with lomeguatrib in colorectal cancer. A phase II trial to evaluate lomeguatrib in combination with temozolomide in metastatic colorectal cancer has recently been reported (16). Patients received 40mg lomeguatrib with temozolomide (50-200mg/m2) daily by mouth for 5 days every 4 weeks. Despite consistent depletion of MGMT in PBMCs, no clinical responses to treatment were seen. This negative result can be explained by colorectal cancer being inherently insensitive to temozolomide, and MGMT not being a significant factor in this resistance. Deficient mismatch repair, for example, can lead to tolerance of methylation damage. Our results suggest that inadequate inactivation of MGMT in tumour, which was not measured in the trial, is an additional possible reason for the lack of effect observed.
In summary, total MGMT depletion can be achieved in primary CNS, colorectal and prostate cancers with a single administration of lomeguatrib. The doses required were 120mg for colorectal and prostate cancers and 160mg for CNS tumours. Lomeguatrib is well tolerated at these doses, which are recommended for future studies in these tumour types.
Statement of Translational Relevance.
The DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) is a major factor in resistance to chlorethylnitrosureas. Lomeguatrib is a small molecule inactivator of MGMT, with the potential to enhance the cytotoxicity of chemotherapy. This report describes a phase 0 trial to establish the biologically effective dose of lomeguatrib to use in future studies of the agent.
Acknowledgements
Supported by Cancer Research-UK. MM receives support from the NIHR Biomedical Research Centre, Oxford. Dedicated to the memory of R.Stanley McElhinney.
References
- 1.Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20:2388–99. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- 2.McElhinney RS, McMurry TB, Margison GP. O6-alkylguanine-DNA alkyltransferase inactivation in cancer chemotherapy. Mini Rev Med Chem. 2003;3:471–85. doi: 10.2174/1389557033487980. [DOI] [PubMed] [Google Scholar]
- 3.Middleton MR, Margison GP. Improvement of chemotherapy efficacy by inactivation of a DNA-repair pathway. Lancet Oncol. 2003;4:37–44. doi: 10.1016/s1470-2045(03)00959-8. [DOI] [PubMed] [Google Scholar]
- 4.Pegg AE. Properties of mammalian O6-alkylguanine-DNA transferases. Mutat Res. 1990;233:165–75. doi: 10.1016/0027-5107(90)90160-6. [DOI] [PubMed] [Google Scholar]
- 5.Middleton MR, Kelly J, Thatcher N, et al. O6-(4-bromothenyl)guanine improves the therapeutic index of temozolomide against A375M melanoma xenografts. Int J Cancer. 2000;85:248–52. doi: 10.1002/(sici)1097-0215(20000115)85:2<248::aid-ijc16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Middleton MR, Thatcher N, McMurry TB, McElhinney RS, Donnelly DJ, Margison GP. Effect of O6-(4-bromothenyl)guanine on different temozolomide schedules in a human melanoma xenograft model. Int J Cancer. 2002;100:615–7. doi: 10.1002/ijc.10532. [DOI] [PubMed] [Google Scholar]
- 7.Ranson M, Middleton MR, Bridgewater J, et al. Lomeguatrib, a potent inhibitor of O6-alkylguanine-DNA-Alkyltransferase: phase I safety, pharmacodynamic, and pharmacokinetic trial and evaluation in combination with temozolomide in patients with advanced solid tumours. Clin Cancer Res. 2006;12:1577–84. doi: 10.1158/1078-0432.CCR-05-2198. [DOI] [PubMed] [Google Scholar]
- 8.Ranson M, Hersey P, Thompson D, et al. Randomized trial of the combination of lomeguatrib and temozolomide compared with temozolomide alone in chemotherapy naive patients with metastatic cutaneous melanoma. J Clin Oncol. 2007;25:2540–5. doi: 10.1200/JCO.2007.10.8217. [DOI] [PubMed] [Google Scholar]
- 9.Clemons M, Kelly J, Watson AJ, et al. O6-(4-bromothenyl)guanine reverses temozolomide resistance in human breast tumour MCF-7 cells and xenografts. Br J Cancer. 2005;93:1152–6. doi: 10.1038/sj.bjc.6602833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middleton MR. Strategies to enhance the efficacy of alkylating agent chemotherapy [PhD] University of Manchester; Manchester: 2000. [Google Scholar]
- 11.Chen JM, Zhang YP, Wang C, Sun Y, Fujimoto J, Ikenaga M. O6-methylguanine-DNA methyltransferase activity in human tumors. Carcinogenesis. 1992;13:1503–7. doi: 10.1093/carcin/13.9.1503. [DOI] [PubMed] [Google Scholar]
- 12.Spiro TP, Gerson SL, Liu L, et al. O6-benzylguanine: a clinical trial establishing the biochemical modulatory dose in tumor tissue for alkyltransferase-directed DNA repair. Cancer Res. 1999;59:2402–10. [PubMed] [Google Scholar]
- 13.Watson AJ, Margison GP. O6-alkylguanine-DNA alkyltransferase assay. Methods Mol Biol. 2000;152:49–61. doi: 10.1385/1-59259-068-3:49. [DOI] [PubMed] [Google Scholar]
- 14.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Eng J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 15.Watson AJ, Middleton MR, McGown G, et al. O6-methylguanine-DNA methyltransferase depletion and DNA damage in patients with melanoma treated with temozolomide alone or with lomeguatrib. Br J Cancer. 2009;100:1250–6. doi: 10.1038/sj.bjc.6605015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan OA, Ranson M, Michael M, et al. A phase II trial of lomeguatrib and temozolomide in metastatic colorectal cancer. Br J Cancer. 2008;98:1614–8. doi: 10.1038/sj.bjc.6604366. [DOI] [PMC free article] [PubMed] [Google Scholar]