Abstract
When intracellular pathogens invade mammalian hosts, naïve CD8+ T cells differentiate into cytotoxic killers, which lyse infected target cells and secrete cytokines that activate intracellular microbicides. We now show that CD8+ T cells deficient in the transcription factors T-bet and eomesodermin (Eomes) fail to differentiate into functional killers required for defense against lymphocytic choriomeningitis virus. Instead, virus-specific CD8+ T cells lacking both T-bet and Eomes differentiate into an IL-17-secreting lineage, reminiscent of the helper T cell fate that has been implicated in autoimmunity and extracellular microbial defense. Upon viral infection, mice with T cells lacking both T-bet and Eomes develop a CD8+ T cell-dependent, progressive inflammatory and wasting syndrome characterized by multi-organ infiltration of neutrophils. T-bet and Eomes, thus, ensure that CD8+ T cells adopt an appropriate course of promoting intracellular rather than extracellular destruction.
Keywords: Th17, Tc17, IL-17, IL-21, IL-22, IL-23, RORγt, autoimmunity
The T-box transcription factors T-bet and Eomes function in a variety of immune cells to regulate the expression of genes involved in Type 1 immunity (1, 2). In CD4+ T cells, T-bet plays a critical role in driving induction of IFN-γ and other aspects of the Type 1 genetic program (3–7). T-bet also functions as a critical and non-redundant regulator of the development and function of natural killer T (NKT) cells (8). In contrast, both T-bet and Eomes are expressed in CD8+ T cells and natural killer (NK) cells, suggesting they might function redundantly to regulate the cytotoxic effector function of these cells (8–10). Although studies of T-bet-deficient mice have helped elucidate numerous functions of T-bet, the precise role of Eomes in effector and memory CD8+ T cell differentiation has yet to be resolved because unconditional genetic ablation of Eomes results in early embryonic lethality (11).
In order to examine the function of Eomes in T cells, we used homologous recombination to generate mice carrying a loxP-flanked allele of Eomes (12). We bred this strain to mice expressing Cre recombinase under control of CD4 promoter/enhancer elements, thereby allowing selective deletion of Eomes in T cells. Mice with homozygous T cell-specific deletion of Eomes (herein referred to as “Eomes KO”) were, in turn, crossed to mice with germline deletion of Tbx21 (“T-bet KO”), creating mice with combined deficiency for both T-bet and Eomes in the T cell compartment (“Double KO”). T-bet KO, Eomes KO, and Double KO mice exhibited no gross developmental abnormalities or disease phenotypes. Conventional CD4+ and CD8+ T cell development in T-bet KO, Eomes KO, and Double KO was unaffected, with numbers and ratios of thymocyte subsets that were similar to wild-type mice (12). We observed relatively normal CD4+ to CD8+ T cell ratios in the spleen and lymph nodes of T-bet KO, Eomes KO, and Double KO mice (12), although Eomes KO and Double KO mice exhibited a deficiency in memory-phenotype (CD44hiCD122hi) CD8+ T cells, as predicted by previous findings (9).
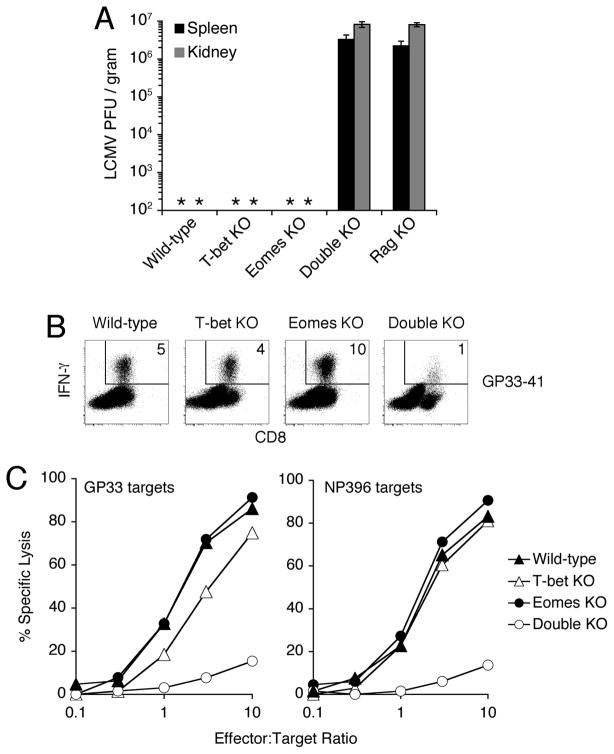
Infection with lymphocytic choriomeningitis virus (LCMV) induces robust proliferation and cytotoxic differentiation of CD8+ T cells, which are essential to eliminate virus from the host (13). To test the functional capacity of CD8+ T cells deficient for T-bet and/or Eomes, we infected wild-type, T-bet KO, Eomes KO, and Double KO mice with LCMV and assessed their ability to control the viral infection. Virus was efficiently cleared from the spleens and kidneys of wild-type, T-bet KO, and Eomes KO mice (Fig. 1A). In contrast, Double KO mice harbored high titers of persistent virus, comparable to lymphocyte-deficient Rag1−/− (“Rag KO”) mice (Fig. 1A).
Fig. 1.
Mice with T cells lacking both T-bet and Eomes fail to control LCMV infection. (A) Quantification of LCMV virus in spleen and kidney from mice of the indicated genotype 23d after infection. Detection threshold of limiting dilution assay is approximately 2×102 PFU/gram; * indicates undetectable. Values represent mean ±SEM of at least 3 mice per genotype. (B, C) Defective IFN-γ expression and cytolysis of target cells by CD8+ T cells lacking T-bet and Eomes. Splenocytes from mice of the indicated genotypes were isolated 8d after infection with LCMV. (B) Flow cytometry of IFN-γ production by CD8+ T cells in response to stimulation with LCMV-derived peptide GP33–41. % of live cells expressing IFN-γ is indicated. (C) Cytolytic activity of virus-specific CD8+ T cells. Equalized numbers of GP33- or NP396-specific CD8+ T cells were added to target cells pulsed with LCMV-derived peptides GP33–41 or NP396–404. % specific lysis was determined after 24h (12). Virus-specific CD8+ T cells from Double KO mice also exhibited impaired accumulation and abnormal expression of numerous surface receptors, reflecting both persistent antigen exposure and abnormal differentiation (12). Results are representative of at least 3 independent experiments.
Consistent with the inability of Double KO mice to control viral replication, LCMV-specific CD8+ T cells from Double KO mice exhibited a substantial defect in IFN-γ expression (Fig. 1B) and target cell cytotoxicity (Fig. 1C) in response to stimulation with viral peptides. Double KO cells also exhibited marked impairment in the induction of granzyme B protein and perforin mRNA (12). In addition to impaired cytotoxic differentiation, virus-specific Double KO CD8+ T cells exhibited defective accumulation (4.5-fold reduced), likely owing to impaired proliferation and survival (12). While the reduced frequency of antigen-specific cells may contribute to impaired clearance of virus, the severe defect in cytotoxic gene expression (12) and function (Fig. 1C) of Double KO cells is wholly evident when cellularity is normalized. Antiviral CD8+ T cell effector differentiation can, thus, withstand the loss of either T-bet or Eomes, but the loss of both factors is incompatible with appropriate anti-viral host defense.
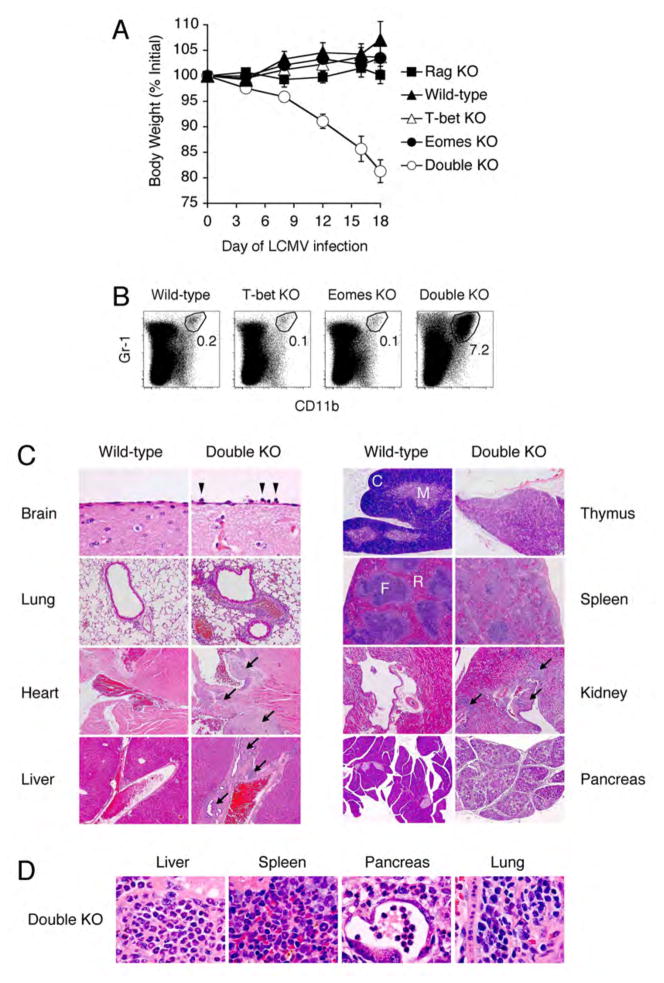
During the first week of LCMV infection, there were no apparent differences in the health of mice of all four genotypes. At approximately one week after infection, however, Double KO mice began to lose weight, which steadily progressed over the course of several weeks (Fig. 2A) and invariably necessitated euthanasia. In addition to the virus-induced weight loss observed in Double KO mice, we found evidence of extensive organ pathology. Examination of the spleens of LCMV-infected Double KO mice, revealed an excessive neutrophil response (Fig. 2B), a pattern typically associated with bacterial or fungal rather than viral infection (14–16). Histologic examination revealed widespread immunopathology, characterized by heavy neutrophil infiltration and architectural destruction of numerous organs (Fig. 2C and D). Immunodeficient Rag KO mice did not lose weight (Fig. 2A) nor develop destructive organ pathology despite comparable viral burdens (Fig. 1A), suggesting that the immunopathology of Double KO mice was lymphocyte-dependent.
Fig. 2.
Virus-induced wasting disease in mice with T cells lacking both T-bet and Eomes. (A) Progressive weight loss of Double KO mice after LCMV infection. Percent of weight before infection is depicted graphically over time. Values represent mean ±SEM of at least 3 mice per genotype. (B) Virus-induced neutrophilia in Double KO mice. Values represent % neutrophils in spleen 23d after infection with LCMV. (C) Virus-induced pathology in multiple organs (tissue, magnification) of Double KO mice. H&E staining of Brain (meninges), 60x; Lung (airway with associated vessel), 10x; Heart (base), 5x; Liver (portal vessel), 5x; Thymus (C = cortex, M = medulla), 5x. Spleen (F = follicle, R = red pulp), 5x; Kidney (hilar vessel near calyx), 5x; Pancreas, 5x. Arrowheads indicate neutrophils; arrows indicate immune cell infiltration. All organs from d28 post-infection, except for kidney and pancreas (d23). (D) High power magnification (100x) demonstrating neutrophilic infiltration of Liver, Spleen, and Pancreas, and mixed monocytic/neutrophilic infiltration of Lung. Results are representative of 2 independent experiments with >6 mice per group.
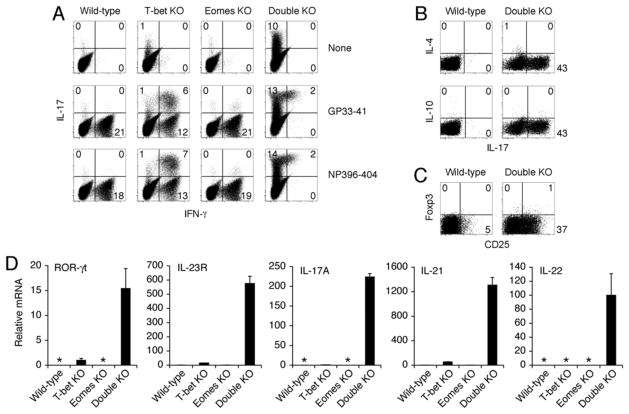
IL-17-secreting CD4+ helper T cells are thought to orchestrate beneficial and pathological immune responses by the recruitment and activation of neutrophils (14–19). The presence of widespread neutrophilic pathology in Double KO mice prompted us to re-evaluate the lineage markers of virus-specific CD8+ T cells. The cytokine profile of CD8+ T cells from Double KO mice revealed substantial expression of IL-17 in response to stimulation with virus-derived peptides (Fig. 3A), with little evidence of IFN-γ (Fig. 3A), IL-4, IL-10 or Foxp3 expression (Fig. 3B), indicating that virus-specific CD8+ T cells from Double KO mice were undergoing skewed differentiation exclusively towards the Type 17 fate. Indeed the transcriptional profile of virus-specific CD8+ T cells from Double KO mice was consistent with bona fide Type 17 differentiation, with substantial induction of the lineage-specifying transcription factor (RORγt) signature cytokines (IL-17A, IL-21, and IL-22), and characteristic cytokine receptor (IL-23R) of the Type 17 fate [Fig. 3C and (20–24)]. Although virus-specific CD8+ T cells from wild-type and Eomes KO mice did not express IL-17, we found some evidence of de-repressed Type 17 markers in the CD8+ T cells from T-bet KO mice (Fig. 3A and C). In separate experiments, we determined that IL-17 expression by CD8+ T cells was not contingent on T-bet deficiency in CD4+ T cells or cells of the innate immune system (12). It is yet unclear whether T-bet KO mice are spared from immunopathology because of the decreased intensity of the Type 17 response, the lack of persistent antigen stimulation, or a combination of these factors.
Fig. 3.
Type 17 effector differentiation of CD8+ T cells lacking both T-bet and Eomes. (A) IFN-γ and IL-17 production by LCMV-specific CD8+ T cells from Double KO mice. Splenocytes from mice of the indicated genotypes were isolated 8d after LCMV infection and given no stimulation (top row) or stimulated with LCMV-derived peptides GP33–41 (middle row) or NP396–404 (bottom row); cytokine production was assessed by intracellular staining; plots show CD8+ events; numbers indicate % of CD8+ T cells expressing the indicated cytokine. Expression of IL-17 by Double KO CD8+ T cells in the absence of re-stimulation (upper right plot) could be due to persistent virus in vivo (Fig. 1A) and/or constitutive expression of this cytokine (12). In addition to the abnormal CD8+ T cell response, CD4+ T cells from both T-bet KO and Double KO expressed IL-17 in response to stimulation with LCMV-derived peptide GP61–80 (12). Additional controls were performed to implicate the CD8+ T cell-intrinsic loss of T-box factors as the cause of anomalous differentiation: Analysis of IFN-γ- and perforin-deficient mice suggested that IL-17 production by CD8+ T cells from Double KO mice was not solely attributable to impaired cytotoxic effector differentiation (12). In separate experiments, we determined that IL-17 expression by CD8+ T cells was not contingent on T-bet deficiency in CD4+ T cells or cells of the innate immune system (12). (B, C) Lack of IL-4, IL-10, or Foxp3 expression by CD8+ T cells from Double KO mice. Splenocytes from indicated genotypes were isolated 8d after LCMV infection. Cells were either stimulated with anti-CD3 (B) or examined directly (C). Cytokine production (B) or Foxp3 expression (C) were assessed by intracellular staining. Numbers indicate % of CD8+ T cells expressing the indicated cytokine or lineage marker. Despite their defective cytotoxocity and IFN-γ expression, virus-specific CD8+ T cells from Double KO mice were capable of producing TNF-α and IL-2 (12). (D) Type 17 transcriptional profile of LCMV-specific CD8+ T cells from Double KO mice. GP33-specific CD8+ T cells were isolated using peptide-MHC tetramers from indicated genotypes 8d after LCMV infection. mRNA expression was assessed by quantitative real-time PCR. * indicates undetectable signal. Lowest detectable sample was arbitrarily set at a value of 1, except for IL-22, where only Double KO had detectable signal. Values represent mean ±SEM of triplicate determinations normalized to HPRT. Results are representative of at least 2 independent experiments.
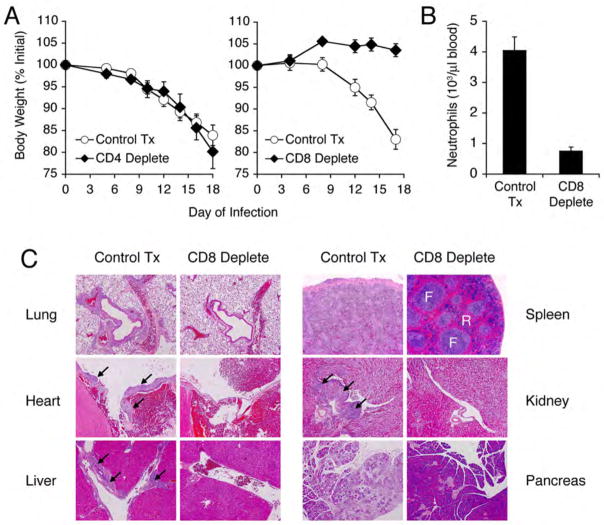
To determine whether the anomalous T cell response was required for immunopathology observed in Double KO mice, we performed antibody-mediated depletion of either CD4+ or CD8+ T cells during LCMV infection. Despite the finding that CD4+ T cells from Double KO mice also express IL-17 (12), depletion of CD4+ T cells did not alter the course of the wasting disease (Fig. 4A) or ameliorate the pathological findings. Depletion of CD8+ T cells, however, prevented the virus-induced wasting syndrome (Fig. 4A), blood neutrophilia (Fig. 4B), and organ pathology (Fig. 4C), demonstrating that the anomalous CD8+ T cell subset is indeed is required to mediate the virus-induced wasting disease. Because CD8 is also expressed on a subset of dendritic cells, we cannot exclude the possibility that reversal of the lymphocyte-dependent wasting syndrome and immunopathology could be due to the combined loss of Double KO CD8+ T cells and CD8+ DCs. Despite the defective accumulation of antigen-specific CD8+ T cells, the virus-induced pathology of Double KO mice is distinct from the functional ignorance of virus and the absence of pathology in Rag KO mice.
Fig. 4.
CD8+ T cell depletion prevents virus-induced wasting disease in Double KO mice. (A) Double KO mice were treated with PBS (“Control Tx”), anti-CD4 (“CD4 deplete”, mAb GK1.5, 0.5 mg i.p., left), or anti-CD8 (“CD8 deplete”, mAb 2.43, 0.5 mg i.p., right) 1d before, 1d after, and every 3d following LCMV infection. Weight at indicated day post-infection is displayed as a percentage of weight before infection. (B) Neutrophils were quantified in the blood of control or anti-CD8 treated mice by automated complete blood count with differential (right). Number of neutrophils per microliter of blood is depicted graphically. (C) H&E staining of organs from control-treated or CD8+ T cell-depleted Double KO mice at d18 post-infection. Lung (airway with associated vessel), 10x; Heart (base), 5x; Liver (portal vessel), 5x; Spleen (F = follicle, R = red pulp), 5x; Kidney (hilar vessel near calyx), 5x; Pancreas, 5x. Arrows indicate immune cell infiltration. Values for (A) and (B) represent mean ±SEM of 3 individual mice per treatment group. Results are representative of 2 independent experiments.
When a pathogen invades a mammalian host, CD4+ T cells differentiate into various fates, which mobilize the most appropriate intracellular or extracellular defense mechanisms befitting the nature of pathogen. In contrast, CD8+ T cells characteristically provide defense against intracellular infections by differentiating into cytotoxic killers. Based on the incomplete loss of effector function in T-bet KO CD8+ T cells (4) and the surprisingly dense defect imposed on this lineage by a dominant-interfering form of T-bet, we proposed that a second T-box factor expressed in CD8+ T cells, Eomes, might account for the T-bet-independent effector function of CD8+ T cells (10). The present findings offer genetic evidence to suggest that T-bet and Eomes indeed redundantly activate a transcriptional network required for CD8+ T cell participation in defense against intracellular pathogens, while simultaneously repressing the Type 17 genetic program involved in defense against extracellular bacteria and fungi.
It remains to be determined what non-overlapping functions are mediated by these two T-box transcription factors. One provisional model suggests that they may play complementary roles in adjusting the balance between terminally differentiated effector cells and self-renewing memory CD8+ T cells (25). Future studies will be directed toward understanding whether Type 17 CD8+ T cells play a protective role in host defense against any classes of pathogens, or whether this represents a vestigial and pathological response for the CD8+ T cell that has primarily evolved for intracellular defense.
Supplementary Material
References and Notes
- 1.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Nat Rev Immunol. 2004;4:900. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 2.Glimcher LH. Nat Immunol. 2007;8:448. doi: 10.1038/ni0507-448. [DOI] [PubMed] [Google Scholar]
- 3.Szabo SJ, et al. Cell. 2000;100:655. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 4.Szabo SJ, et al. Science. 2002;295:338. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 5.Mullen AC, et al. Science. 2001;292:1907. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 6.Mullen AC, et al. Nat Immunol. 2002;3:652. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 7.Afkarian M, et al. Nat Immunol. 2002;3:549. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 8.Townsend MJ, et al. Immunity. 2004;20:477. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 9.Intlekofer AM, et al. Nat Immunol. 2005;6:1236. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 10.Pearce EL, et al. Science. 2003;302:1041. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 11.Russ AP, et al. Nature. 2000;404:95. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- 12.Supporting online material is available on Science online.
- 13.Williams MA, Bevan MJ. Annu Rev Immunol. 2007;25:171. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R. Nature. 2007;449:819. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 15.Reiner SL. Cell. 2007;129:33. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Weaver CT, Hatton RD, Mangan PR, Harrington LE. Annu Rev Immunol. 2007;25:821. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 17.Park H, et al. Nat Immunol. 2005;6:1133. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington LE, et al. Nat Immunol. 2005;6:1123. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 19.Mangan PR, et al. Nature. 2006;441:231. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov, et al. Cell. 2006;126:1121. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, et al. Nat Immunol. 2007;8:967. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, et al. Nature. 2007;445:648. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 23.Nurieva R, et al. Nature. 2007;448:480. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 24.Korn T, et al. Nature. 2007;448:484. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Intlekofer AM, et al. J Exp Med. 2007;204:2015. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.We are grateful to E. Allenspach, S. Blackburn, J. Chang, D. Garalde-Intlekofer, I. Kinjyo, P. Mangan, V. Palanivel, F. Schambach, H. Yu and Q.C. Yu for assistance and discussion. These studies were supported by National Institutes of Health grants (AI042370, AI061699, AI076458, AI071309, AI042334, and AI007532) and the Abramson Family Cancer Research Institute.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.