Sea anemones (Actinaria, Cnidaria) are ancient sessile predators (Chen et al., 2002) that heavily depend on their venom for survival (Ruppert and Barnes, 1994). They immobilize their prey or deter their foe by using cells called nematocytes for stinging and delivery of venom. Analysis of the venom in many sea anemone species uncovered a rich repertoire of low molecular weight compounds such as serotonin and histamine (Beress, 1982), ~20 kDa pore-forming polypeptide toxins (Kem, 1988; Anderluh and Macek, 2002), 3.5–6.5 kDa polypeptide toxins active on voltage-gated potassium channels (Castaneda et al., 1995, Schweitz et al., 1995; Gendeh et al., 1997; Yeung et al., 2005) and 3–5 kDa polypeptide toxins active on voltage-gated sodium channels (Beress et al., 1975; Rathmayer et al., 1976; Honma and Shiomi, 2006). The combination combined effects of these compounds has apparently been successful over hundreds of millions of years as is evident by the success ability of sea anemones to colonize and thrive in a wide variety of ecological niches. despite their limiting physique. Moreover, the ever changing environment and appearance of new species had probably enforced diversification of toxins in sea anemones. Indeed, not only that a variety of toxin configurations can be found in their venom (Honma and Shiomi, 2006), but it was shown that each toxin is encoded by a gene family toxin is usually but one member of a gene family (Moran et al., 2008a).
The toxins active on voltage-gated sodium channels are abundant in all sea anemone venoms assayed to date, and are present even in rare and highly unique species (Ishida et al., 1997; Moran and Gurevitz, 2006). Their abundance of these toxins and the fact that these toxins they constitute a major fraction of the proteinaceous content of the venom (Beress et al., 1975) indicate that they have a point for their major role in predation and defense.
The voltage-gated sodium channel as a prime target for sea anemone toxins
Voltage-gated sodium channels (Navs) have a pivotal role in excitability of most animals as they are responsible for triggering enable the initiation and mediation propagation of action potentials. These channels are transmembrane complexes composed of a highly conserved pore-forming α-subunit and auxiliary subunits such as β-subunits in vertebrates (Catterall, 2000) and TipE subunits in insects (Feng et al., 1995). The ~260 kDa α-subunit is ~260 kDa protein is composed of four homologous domains (D1–D4), each composed of comprising six transmembrane segments (S1–S6; Fig. 1). Nine genes encoding the channel α-subunit exist in the human genome. These genes share >50% identity and in many instances their transcription expression is temporally and spatially regulated in tissues or cells, cell- and even membrane location within-cell specific (Goldin et al., 2002).
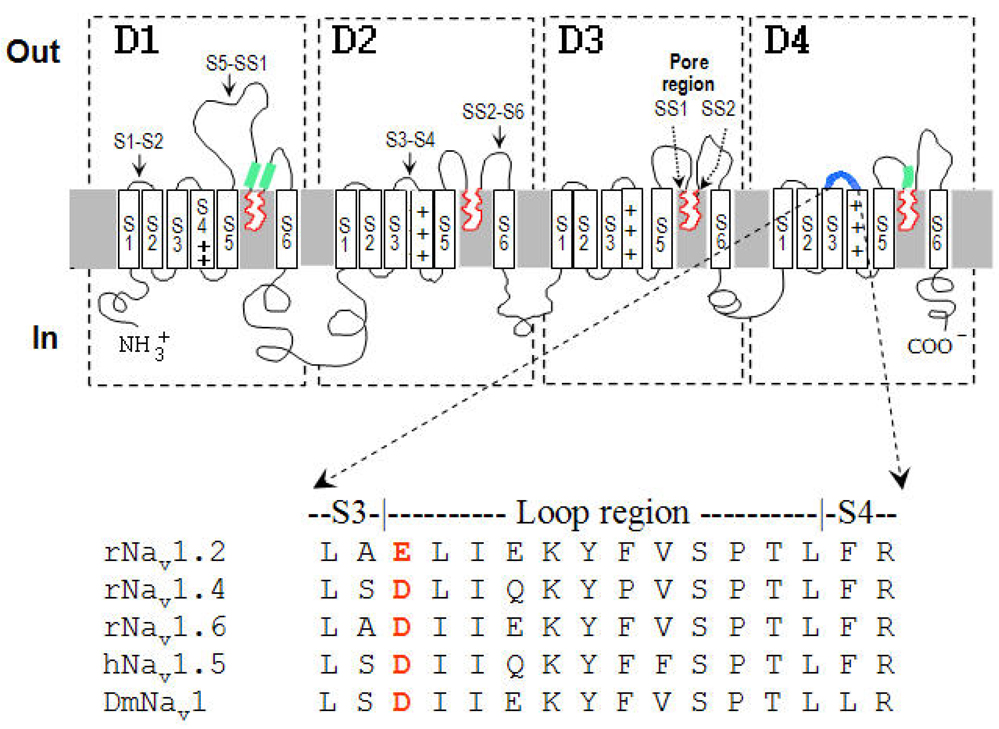
Fig. 1. Schematic illustration of the sodium channel α-subunit and regions that are putatively associated with receptor site-3.
Segments SS1 and SS2 form the pore region (in red), around which the four homologous domains (D1–D4) assemble. The main region identified as affecting site-3 binding is loop S3–S4 in D4 (Rogers et al., 1996; Benzinger et al., 1998) marked in blue. Channel regions (D1/S5-SS1, D1/SS2-S6, D4/S5-SS1) whose blocking by antibodies affected Lqq5 binding (Thomsen and Catterall, 1989) are marked in light green. Multiple sequence alignment of D4/S3-S4 from DmNav1 of Drosophila melanogaster and several mammalian sodium channel subtypes is presented at the lower panel. The negatively charged residue equivalent to Glu-1613 in rNav1.2a (Rogers et al., 1996) is in bold red.
Due to their crucial role in excitability Navs had become prime targets for a wide variety of toxins. Seven neurotoxin receptor sites have been elucidated thus far on Nav α-subunits (reviewed in Gordon et al., 2007; Blumenthal and Seibert, 2003). Four of these sites, formed by the extracellular loops that connect the transmembrane segments of the α-subunit, are receptor site-1, receptor site-3, receptor site-4 and receptor site-6, are recognized by water-soluble peptide toxins: and are likely composed of the extracellular loops that connect the transmembrane segments of the α-subunit. Receptor site-1 is targeted by μ-conotoxins from venomous cone snails (Terlau and Olivera, 2004) as well as by guanidinium toxins like tetrodotoxin and saxitoxin; Receptor site-3 is targeted by toxins from sea anemones, scorpions and spiders (Catterall and Beress, 1978; Gordon and Zlotkin, 1993; Rogers et al., 1996; Little et al., 1998); Receptor site-4 is targeted by toxins from scorpions and spiders (Cestele and Catterall, 2000; Corzo et al., 2005); and Receptor site-6 is targeted by δ-conotoxins from venomous cone snails (Fainzilber et al., 1994; Terlau and Olivera, 2004). Aside of μ-conotoxins, all these toxins affect the gating properties of the Nav, either activation (Receptor site 4; Cestele and Catterall, 2000) or inactivation (Receptor sites 3 and 6; Ulbricht, 2005), and therefore are called ‘gating modifiers’. The classification of toxins to pharmacological groups is based on their mode of action and ability to displace one another in binding competition assays (Jover et al., 1978; Catterall, 2000; Gordon, 1997).
The discovery of sea anemone toxins active on voltage-gated sodium channels
Substances Crude extracts from the tentacles of the sea anemone Anemonia sulcata (today named Anemonia viridis) were first isolated prepared in 1902 and shown to cause anaphylaxis in dogs (Richet, 1902). The paralytic and lethal mechanism of action activities of a sea anemone venom extract were investigated in more detail by Narahashi and co-workers (1969) who demonstrated that a toxic fraction from the Carribbean Condylactis gigantea delayed the inactivation of sodium currents in the crayfish giant axon. Six years later Beress and co-workers isolated toxins affecting sodium channels from Anemonia viridis and named them ATX I, ATX II and ATX III (named today Av1, Av2 and Av3, respectively; Beress et al., 1975). These three toxins were found to affect crayfish neuromuscular transmission and nerve action potentials (Rathmayer and Beress, 1976) by inhibiting the inactivation of sodium conductance at isolated crayfish neurons (Hartung and Rathmayer, 1985). Av2 induced similar effects in the node of Ranvier in frog nerve fibers (Bergman et al., 1976). The inhibition of inactivation results in prolonged action potentials that may lead to contractile paralysis and death. Sequence determination of these toxins revealed that Av1 and Av2 are 46 and 47 amino acid residue long, respectively, and that they share high sequence identity (Wunderer et al., 1976, 1978), while Av3 is only 27 amino acid residue long and its sequence is unrelated to those of Av1 and Av2 (Martinez and Kopeyan, 1977; Fig. 2). As sea anemone toxins and scorpion α-toxins induced similar effects on channel inactivation, they were examined in binding competition assays at neuroblastoma cells, which indicated that they share a common binding site on Navs (Catterall and Beress, 1978). This receptor binding site was later named ‘receptor site-3’ (Catterall, 1979). The competition for the same site was demonstrated later in numerous binding studies using mammalian and insect neuronal preparations and radio-iodinated scorpion or anemone toxins (e.g. Schweitz et al., 1981; Gordon and Zlotkin, 1993; Loret et al., 1994; Gordon et al., 1996; Moran et al., 2006, 2007).
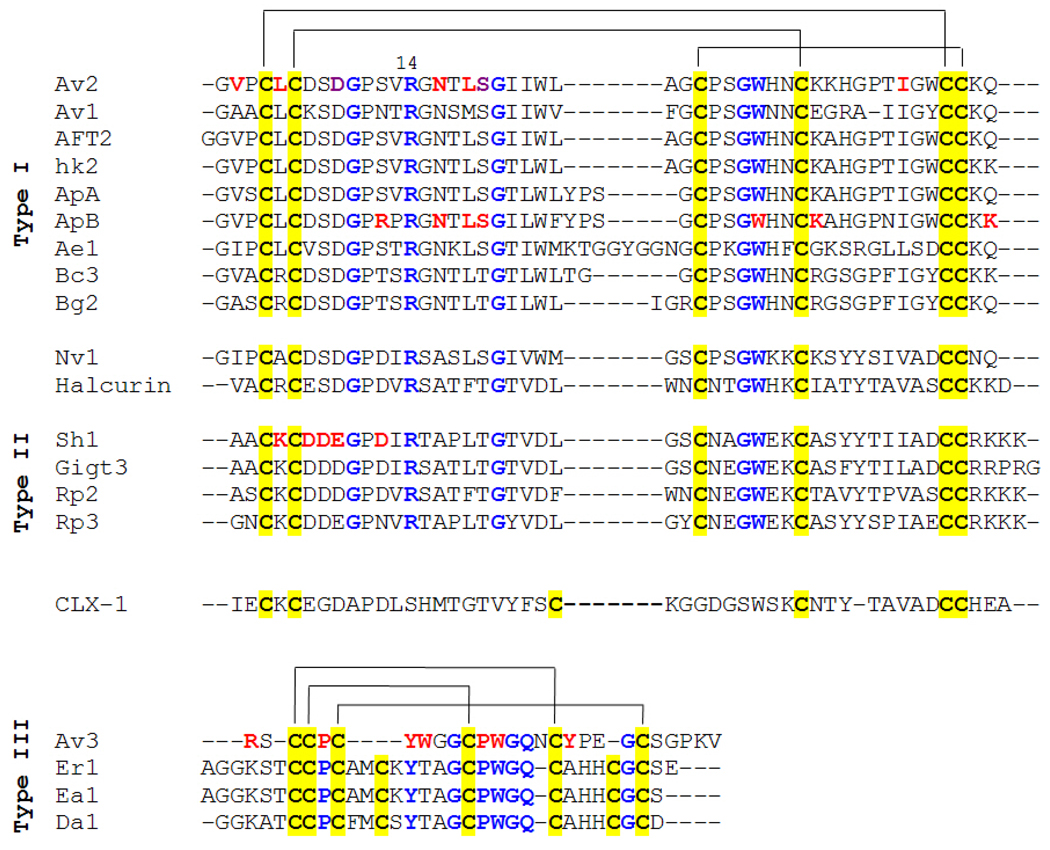
Fig. 2. Multiple sequence alignment of sea anemone toxins active on Navs.
Conserved cysteine residues are designated by a yellow background. Disulfide bridges are indicated on top. Highly conserved residues are in blue. Arg-14 of the conserved loop is numbered. Residues assigned to the putative bioactive surface are in red (Pennington et al., 1990; Blumenthal and Seibert, 2003; Moran et al., 2006, 2007). For most toxin references see Honma and Shiomi (2006) Bosmans and Tytgat (2007). Av1, Av2 and Av3 are from Anemonia viridis; AFT2 is from Anthopleura fuscoviridis; hk2 is from an uncharacterized species of the genus Anthopleura; ApA and ApB are from Anthopleura xanthogrammica; Ae1 is from Actinia equina; Bc3 is from Bunodosoma caissarum; Bg2 is from Bunodosoma granulifera; The Nv1 sequence was deduced from the genome sequence of Nematostella vectensis (Moran and Gurevitz, 2006; Putnam et al., 2007); Halcurin is from an uncharacterized species of the genus Halcurias; Sh1 is from Stichodactyla helianthus; Gigt3 (Gigantoxin III) is from Stichodactyla gigantea; Rp2 and Rp3 are from Heteractis paumotensis; CLX1 is from Calliactis parasitica; Er1 is from Entacmaea ramsayi; Ea1 (previously PaTx) is from Entacmaea actinostoloides; Da1 is from Dofleinia armata.
Classification of sea anemone toxins active on voltage-gated sodium channels
Comparison of toxins Av1, Av2 and Av3 revealed that they belong to at least two protein families. Over the years, numerous toxins that resemble Av1 and Av2 were found in various sea anemone species (Fig. 2). Schweitz and co-workers (1985) isolated four toxins (Rp1, Rp2, Rp3 and Rp4) from the species Heteractis paumotensis (previously called Radianthus paumotensis) and categorized them as a new class of sea anemone toxins because antibodies raised against Rp3 recognized other R. paumotensis toxins and did not recognize the A. viridis toxins. The long (46–51 aa) sea anemone toxins were subsequently classified into as ‘Type I sea anemone toxins’ (Av2-like toxins) and ‘Type II sea anemone toxins’ (Rp3-like toxins). However, since the toxins of both types share up to 50% sequence identity and identical the same disulfide bridging, and due to the finding of toxins of Halcurias sp. and Nematostella vectensis that resemble both Type I and Type II sequences (Ishida et al., 1997; Moran and Gurevitz, 2006), it seems that this classification should be reevaluated. it seems that this “binary”classification largely focused on sequence differences between the toxins of just the first two sea anemone families investigated. The NMR structure of all Type I and Type II sea anemone toxins exhibits an anti-parallel β-sheet composed of four β-strands and a highly flexible loop, named ‘Arg-14 loop’, after its most conserved residue (Widmer et al., 1989; Fogh et al., 1990; Wilcox et al., 1993; Pallaghy et al., 1995; Monks et al., 1995; Salceda et al., 2007).
The characterization of Av3 has established the Type III class of sea anemone toxins (Norton et al., 1991; Beress et al., 1975). Unlike Type I and Type II, which are common in the venom of various species, Type III toxins were identified only in a few species. The other known Type III toxins are Ea1 (previously PaTx) from Entacmaea actinostoloides (previously Parasicyonis actinostoloides) and its close homologues from Dofleinia armata and Entacmaea ramsayi (Honma et al., 2003; Fig. 2). NMR analysis of Av3 revealed an unusual compact structure containing only β and γ-turns (Manoleras and Norton, 1994). As Av3 lacks the fourth disulfide bridge found in Ea1 and its homologues, it is unclear to what extent the structure of Type III toxins is conserved.
Genomic organization and evolution of sea anemone toxin genes
The data regarding the genes and transcripts encoding sea anemone toxins is scarce. Transcripts for sea anemone toxins were isolated from several species usually mostly via PCR reactions based on and degenerate primers (Spagnuolo et al., 1994; Kelso and Blumenthal, 1998; Anderluh et al., 2000; Honma et al., 2005). As shown for other nematocyst proteins, sea anemone These toxins seem to be translated as precursors, carrying a leader peptide and a propart region, which have been suggested to have a role in intracellular sorting and delivery to the nematocyst (Anderluh et al., 2000). Until recently, the only available genes encoding sea anemone toxins affecting Navs were clx-1 and clx-2 of Calliactis parasitica. Yet, aside from their conserved cysteines, their toxic products exhibit very low sequence similarity to Type I and Type II toxins (Cariello et al., 1989; Spagnuolo et al., 1994). The two genes include an intron within the 5’ UTR and another intron within the region encoding the leader peptide (Fig. 3). Interestingly, this structure organization is also found in genes encoding unrelated sea anemone potassium channel blockers (Gendeh et al., 1997) and scorpion toxins (Zhu and Gao, 2006). The similar gene structures organization can be attributed to convergent evolution and may suggest that these introns have a selective value, which is corroborated by examples of critical roles of introns in gene expression (reviewed by Maniatis and Reed, 2002; Fedorova and Fedorov, 2003).
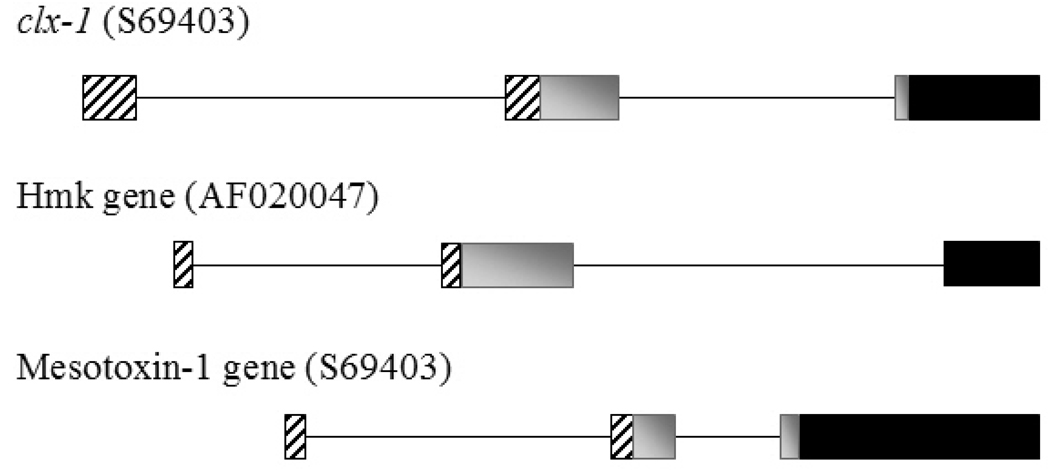
Fig. 3. The structure of the genes encoding toxins affecting voltage-gated ion channel.
A graphic scheme of three genes encoding voltage-gated ion toxins. clx-1 encodes the Nav modifier CLX-1 (also known as Calitoxin I) from the sea anemone Calliactis parasitica (Spagnuolo et al., 1994). The genes encoding the Nav modifier Mesotoxin-1 and the potassium channel blocker HmK are from the scorpion Mesobuthus martensii (Zhu and Gao, 2006) and the sea anemone Heteractis magnifica respectively. The intron is illustrated as a thin line and the exons as filled boxes. The exon region that encodes the 5’ untranslated region is represented by striped boxes. Gray boxes represent exon regions which encode the leader part, and the black boxes represent exon regions encoding the mature toxin.
The initial release of the genome sequence draft of the starlet anemone, N. vectensis (Sullivan et al., 2006) provided the first opportunity to explore the toxin-related genomic landscape of a cnidarian. Fourteen putative genes were identified by homology searches, but unexpectedly they were all found to encode the same mature toxin, Nv1, which highly resembles Type I and Type II sea anemone toxins (Moran and Gurevitz, 2006). The Nv1 gene structure is similar to that of the C. parasitica toxin genes, with two exons separated by an intron. Astoundingly, the gene region that encodes the mature toxin is devoid of any synonymous substitutions (codon substitutions that do not change the amino acid sequence) and the genes in general are strikingly similar (identity >97.5%). This is in sharp contrast to the previously described heterogeneity of animal peptide neurotoxins (Nakashima et al., 1993; Duda and Palumbi, 1999; Froy et al., 1999).
The official release of the more complete genome sequence of N. vectensis (Putnam et al., 2007) was useful in exploring the genomic organization of the Nv1 genes. At least eight Nv1 genes are located in a narrow genomic region of ~30,000 bp at the same transcriptional orientation (Moran et al., 2008a; Fig. 4A). The adjacent genomic regions of all genes are also highly similar (Fig. 4B; Identity >92.5% for at least 1000 bp). This unusual nucleotide conservation is reminiscent of the genes encoding ribosomal RNA (rDNA). The rDNA genes were suggested to evolve in a rare evolutionary mode known as ‘concerted evolution’ (Brown et al., 1972). This suggestion has been proven in yeast to be based on unequal crossover (Szostak and Wu, 1980). Due to their concerted evolution the rDNA copies and their surrounding regions in one species are all more similar to one another than to rDNA genes of other species. Indeed, when the transcripts and genes of Type I toxins from A. viridis and Actinia equina were amplified, the deduced toxins of each species were highly similar to one another, but less similar to those of other species (Fig. 5). A remarkable example was found in A. viridis, where six at least seven genes encode Av2, with noticeable insertions/deletions (indels) in their introns, but still with highly similar (>95%) exons (Moran et al., 2008a).
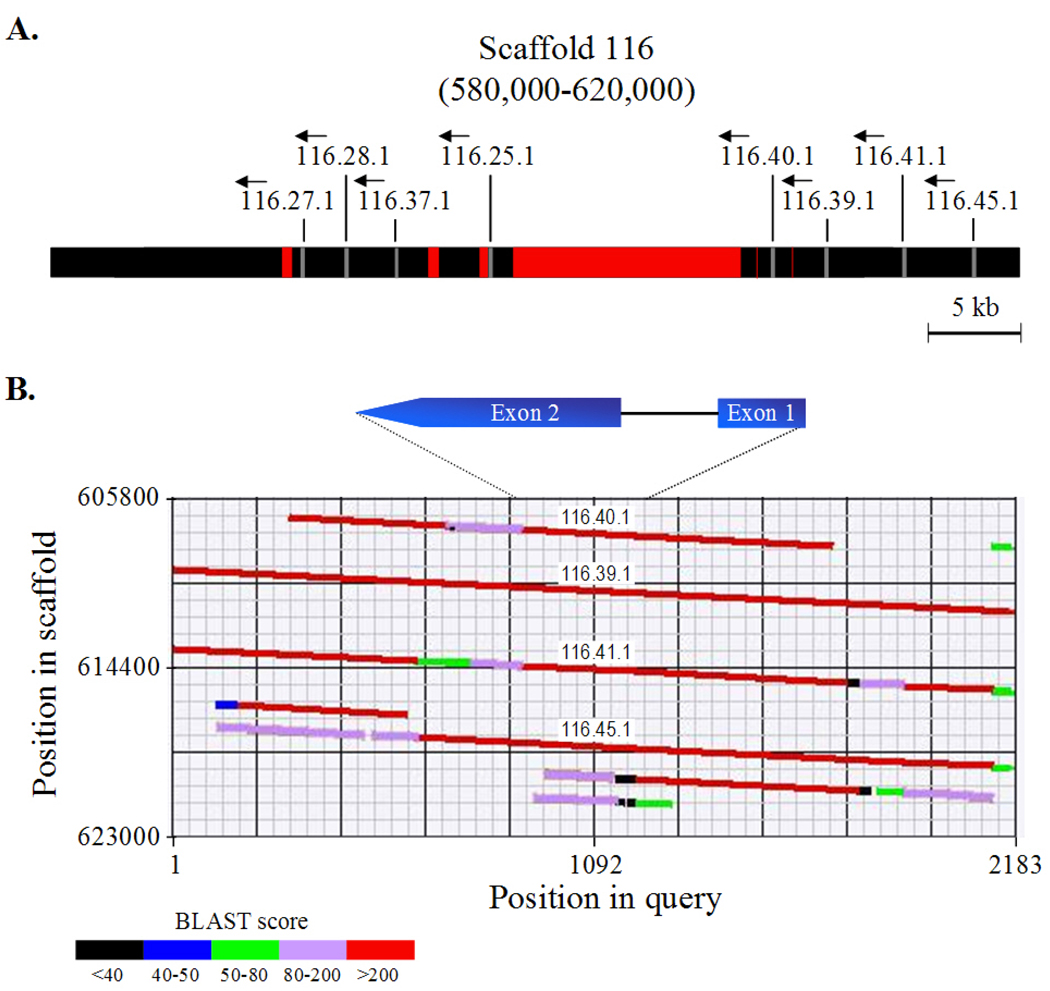
Fig. 4. The genomic region containing Nv1 neurotoxin genes.
A, Graphic map of the region on scaffold 116 in the N. vectensis genome draft, which contains eight Nv1 genes (positions 580,000–620,000). The genes colored in gray and indicated by vertical lines are named according to the JGI website nomenclature (http://genome.jgipsf.org/Nemve1/Nemve1.home.html) and their transcriptional orientations are indicated by arrows. Sequencing gaps are colored in red. B, Enhanced graphic output of a BLASTN query on part of the Nv1 region on scaffold 116 (positions 605,800–623,000) using the 116.39.1 gene and its adjacent sequences (1000 bp upstream and 1000 bp downstream). Gene names are indicated above their location on the scaffold. The conserved gene structure of Nv1 and its relative share of the query appear above the BLAST diagram. The two exons appear in blue and the intron appears as a black line.
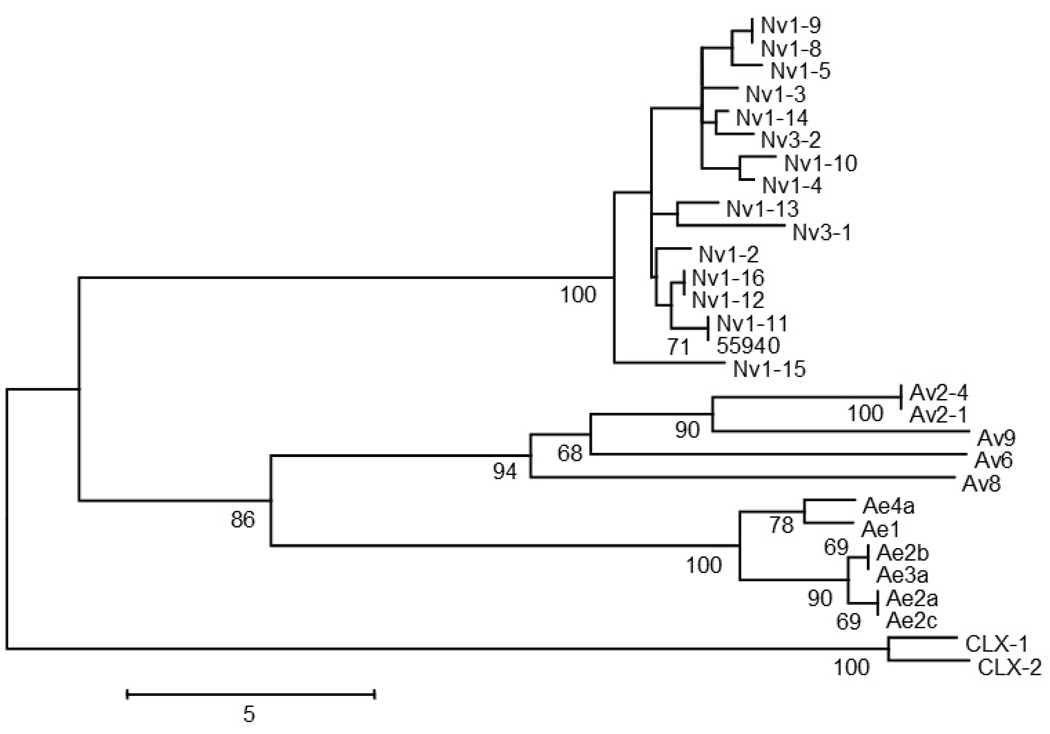
Fig. 5. Phylogenetic tree of sea anemone toxin sequences.
Complete-deletion and number of differences based distance were used in constructing the tree. Bootstrap values are based on 1,000 replications, and only those greater than 50% are shown. All GenBank accession numbers appear in Moran et al. (2008a) except for CLX-1 and CLX-2 (accession numbers AAB30193 and AAD14039, respectively) that form an outgroup.
The selective advantage of concerted evolution of toxin genes may relate to a 'dosage' effect of gene expression. This suggestion is supported by a strong statistical positive correlation found between gene expression level and concerted evolution of duplicated genes in yeast (Sugino and Innan, 2006). Unlike many other poisonous animals, sea anemones lack a venom gland or a stinging organ. Instead they use for venom delivery and use instead nematocytes, spread all over their body, distributed throughout the integumentary tissues (Kass-Simon and Scappaticci, 2002). Since these cells and since these cells are replaced after discharge are able to discharge toxins (via their discharging nematocysts; see Fautin paper in this issue) into a victim only once, and must be replaced, swift production of copious toxin in young nematocytes is seemingly advantageous. and the Such production of toxin may be accelerated by the presence of multiple copies of the same toxin gene. While this strategy for large-scale toxin production may appear primitive or ineffective compared to the acquisition of very strong promoters and enhancers, concerted evolution may also be useful for the rapid “transmission” of advantageous mutations from a single toxin gene locus to the other loci or in preventing the loss of a highly effective toxin.
In addition to the multiple genes encoding highly similar toxins in A. viridis and A. equina, genes encoding novel putative toxins were also identified in these species (Moran et al., 2008a). These genes evolved in an adaptive manner under positive Darwinian selection (‘accelerated evolution’) as shown for toxin genes of other poisonous animals (Nakashima et al., 1993, Duda and Palumbi, 1999; Zhu et al., 2004; Moran et al., 2008a). This mode of evolution can be highly advantageous as the evolution of pharmacological targets of neurotoxins might have been enhanced under the selective pressure exercised by the neurotoxins, resulting in an evolutionary “arms race”. Accelerated evolution driven by positive Darwinian selection and gene variability seems to contradict the proposed mechanism of concerted evolution. Still, variability is also found in rDNA, and several cases of rDNA that “escaped” concerted evolution were documented (Carranza et al., 1999; Keller et al., 2006). Accordingly, in A. viridis and A. equina it is possible that some toxin genes "escaped" the concerted process, diverged rapidly and were fixed depending on the selective value and neutral drift of these loci.
A better insight into cnidarian toxin genomics is still limited due to the lack of nucleotide data. The release of the Nematostella genome, and the highly anticipated future release of the Hydra and possibly Acropora genome drafts (Miller and Ball, 2008), will certainly shed more light in this field. However, as far as we are aware, homologues of sea anemone toxins active on Navs do not exist in the genomes of non-actinarian cnidarians, and therefore, the research of genes encoding these toxins will still heavily depend on isolation and amplification of single genes from genomic samples.
Expression of neurotoxins across the life cycle of sea anemones
Sea anemones begin their life as free swimming planulae that settle and metamorphose into adult polyps (Ruppert and Barnes, 1994). A thorough survey of the literature reveals that all sea anemone toxins reported thus far were isolated from polyps. Nevertheless, it is well documented that nematocytes are common in planulae and that the precursor cells of nematocytes, the nematoblasts, appear in cnidarian embryos as early as 12 hours after fertilization (reviewed by Kass-Simon and Scappaticci, 2002), raising the question of whether the embryonic and larval stages already produce toxins. Recently, an RT-PCR analysis indicated that Nv1 is transcribed in all life stages of N. vectensis, but the transcripts are spliced only in the polyp (Moran et al., 2008b). Since the intron introduces a stop codon prior to the region encoding the mature toxin, it is evident that Nv1 is not produced in the larval stages (Moran et al. 2008b). Immuno-staining assays on N. vectensis samples with an anti-Nv1 antibody indicated that toxin production begins at the four tentacle buds of primary polyps, the first developmental stage which captures prey, supporting the RT-PCR results (unpublished results). The rare mode of alternative splicing of the Nv1 transcript is called 'Intron retention', and it comprises approximately 3% of the documented cases of alternative splicing in animals (Ast, 2004). The age-dependent post-transcriptional mechanism that controls the alternative splicing of the neurotoxin transcripts is intriguing and raises new questions in the study of N. vectensis development. In contrast to the embryonic Nv1 transcripts, Av2 transcripts, found in A. viridis oocytes but not in sperm, are spliced (unpublished data). Furthermore, a transcript encoding a pore forming toxin from Hydra is abundant in embryos (Genikhovich et al., 2006). Thus it appears that various cnidarian toxins are expressed in embryonic and larval stages, at least at the nucleotide level, which deserves further examination as to their role.
The selectivity of sea anemone toxins
Profound differences in selectivity of sea anemone toxins toward various animal groups have been documented. Av1 and Av3 are very active in crustaceans but and inactive in mice, while Av2 is highly active in both organisms (Schweitz et al., 1981). In contrast, Anthopleurin A (ApA) and Anthopleurin B (ApB), Type I sea anemone toxins of Anthopleura xanthogrammica, are more active on mammals than on crustaceans. Other studies using cell-lines that express specific mammalian Nav subtypes and recombinant ApA and ApB toxins have shown that ApA is active on cardiac Navs, while ApB modifies equally well neuronal and cardiac sodium channels Navs (Khera and Blumenthal, 1994; Gallagher and Blumenthal, 1994; Khera et al., 1995). However, these results were contradicted in later elecrophysiological studies showing preference of both ApA and ApB for cardiac channels (Khera et al., 1995). Attempts to ameliorate improve by site-directed mutagenesis ApB selectivity to the rat cardiac sodium channel rNav1.5, while retaining the original potency, had limited success (Khera and Blumenthal, 1996; Dias-Kadambi et al., 1996a; Dias-Kadambi et al., 1996b; Kelso et al., 1996). Characterization of Av2 effects on various heterologously expressed mammalian Navs revealed that it was five times more active at hNav1.5 (human cardiac channel subtype) than at rNav1.4 (rat skeletal muscle channel subtype) (Chahine et al., 1996). Oliveira and co-workers (2004) assayed the activity of Av2, AFT2 of Anthopleura fuscoviridis and Bc3 of Bunodosoma caissarum (Fig. 2) on six distinct human Nav subtypes (Nav1.1–Nav1.6) expressed in human embryonic kidney (HEK293) cells. These assays demonstrated profound differences in selectivity of the three toxins toward the human Nav subtypes. The most remarkable result was the high potency of Av2 at the neuronal channel subtypes Nav1.1 and Nav1.2, while the effect of the other two toxins was very weak. As Av2 and AFT2 differ in only two amino acid residues, this difference in selectivity is intriguing, but since these toxins were isolated from whole sea anemones, traces amounts of other native compounds could affect the activity measured. the purification process could lead to differences in activity.
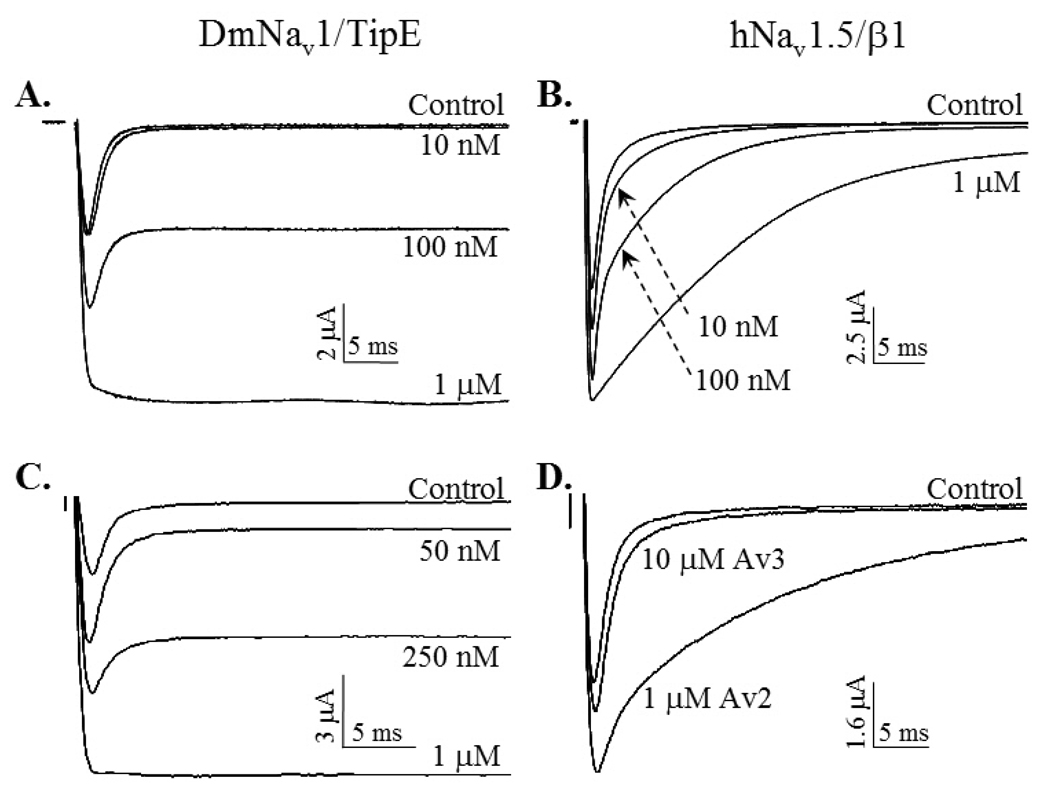
Comparison of Av2 effects at the Drosophila melanogaster sodium channel DmNav1 (Para) and rNav1.2a (rat brain channel subtype) expressed in Xenopus laevis oocytes has shown clear preference of Av2 for the insect sodium channel (Warmke et al., 1997). However, comparison of Av2 action on DmNav1 and rNav1.5 clearly indicated that this toxin is equally active at both channels (Fig. 6A, B). A sharp difference in selectivity was observed when the effects of Bg2, a toxin of Bunodosoma granulifera, were evaluated at insect versus mammalian Navs expressed in Xenopus oocytes (Bosmans et al., 2002). Bg2 affected DmNav1 in concentrations of 5 nM, but affected the mammalian Navs only in 50-fold higher concentrations. However, this reported insect-selectivity of Bg2 is in contrast to previous reports about anti-mammalian activity of this toxin when examined in binding competition assays against the scorpion α-toxin Aah2 (of Androctonus australis hector) at rat brain synaptosomes, and in electrophysiological assays performed on rat neurons (Loret et al., 1994; Salceda et al., 2002).
Fig. 6. Difference in Av2 and Av3 preferences for insect and cardiac Navs.
DmNav1 of Drosophila melanogaster and hNav1.5, the cardiac Nav subtype from human, were heterologously expressed together with their auxiliary subunits, TipE and β1 in Xenopus laevis oocytes. The oocytes were clamped at −80 mV and currents were elicited by step depolarization to either −20 mV (hNav1.5) or −10 mV (DmNav1). A and B, Av2 strongly inhibited the inactivation of the two channels with maximal effect obtained at 1 µM of toxin. C and D, Av3 is highly active at the insect channel (C) but practically inactive at the cardiac channel (D) compared with Av2.
The profound activity of sea anemone toxins on insects is puzzling considering the fact that the two groups of organisms live in separate habitats. Still, taken that insects and crustaceans are both arthropods suggests that their ion channels are similar. This assumption is corroborated at the bioinformatic level when the sequence encoding Nav derived from the initial genome draft of the crustacean Daphnia pulex (genome draft available at http://genome.jgi-psf.org/Dappu1/Dappu1.home.html) is compared to that of DmNav1 (GenBank accession P35500). Moreover, although most insects are terrestrial, in their larval stage some of them could encounter sea anemones in shallow water. Indeed, N. vectensis was reported to feed on insect larvae (Frank and Bleakney, 1978). At present, no Type I sea anemone toxin that is truly invertebrate selective has been reported, although recent studies have raised Av1 of A. viridis and Nv1 of N. vectensis as two promising candidates for invertebrate-selective Type I sea anemone toxins: Av1 of A. viridis and Nv1 of N. vectensis (Moran et al., 2008 and Unpublished data).
Although the study of Type II sea anemone toxins has not been as extensive as that of Type I toxins, some of them exhibit high preference for invertebrates. While Rp4 of H. paumotensis is more active on mice than on crabs, Rp2 from the same anemone is 280-fold more active on crabs than on mice (Schweitz et al., 1985). Electrophysiological analysis of the toxin Sh1 of Stychodactyla helianthus on crayfish and cockroach axons, as well as its injection to crabs, cockroaches and mice has shown no effect on the injected mice, a much stronger toxicity on crabs compared to cockroaches, and higher activity at the crayfish over cockroach axons (Salgado and Kem, 1992). In this study, Calliactis toxin was also shown to have an almost identical action on sodium channels. Evidently, The Type II toxins still await further characterization of toxin effects, especially on heterologously expressed channels, may contribute to the knowledge about selectivity of Type II sea anemone toxins.
The Type III sea anemone toxin, Av3, was found toxic to crabs but non-toxic to mice in intraperitoneal injection (Beress et al., 1975; Schweitz et al., 1981). In addition, Av3 competed very poorly with the anti-mammalian scorpion α-toxin Aah2 on binding at rat brain synaptosomes and had almost no effect on the sodium uptake of neuroblastoma cells (Schweitz et al., 1981). These results have clearly indicated that Av3 is inactive on mammalian Navs. Analysis of Av3 in its recombinant form on insect sodium channels revealed profound toxicity to blowfly larvae, high affinity for cockroach neuronal membranes in competition assays against the site-3 scorpion α-toxin LqhαIT, and salient preference for the insect channel DmNav1 over rNav1.2a, rNav1.4 and hNav1.5 mammalian channels (Moran et al., 2007; Fig. 6C, D). Since other Type III sea anemone toxins were rarely studied, it is unknown to what extent this group of toxins shares the invertebrate specificity of Av3.
The bioactive surfaces of sea anemone toxins
The initial attempt to uncover residues with a functional role in a sea anemone toxin was by chemical modifications of Av2 (Barhanin et al., 1981). This study raised the putative role in bioactivity of Asp-7, Asp-9, Arg-14 His-32, His-37, Lys-35, Lys-36, Lys-46 and the C-terminus. However, since (i) only charged residues were modified; (ii) in many instances a number of residues have been modified simultaneously; and (iii) the toxin fold might have been altered, the significance of these results is questionable. Indeed, mutagenesis of Av2 and other Type I toxins has provided a different picture (Blumenthal and Seibert, 2003; Moran et al., 2006).
The Type I sea anemone toxin that was most extensively studied during the last decade was ApB (Gallagher and Blumenthal, 1992). With the advent of its recombinant production using an Escherichia coli expression system, Blumenthal and his colleagues were able to modify the toxin residue by residue and to assess the effect of these substitutions on bioactivity. All ApB mutants were assayed either on cardiac rNav1.5 and brain rNav1.2a channels transiently expressed in HEK cells, or mammalian cell lines that constitutively express these channels among a minority of other channel subtypes. These studies have suggested a bioactive role for Arg-12, Asn-16, Leu-18, Ser-19, Trp-33, Lys-37 and Lys-49, of which most bioactive residues were associated with the Arg-14 loop and with the substitutions of Leu-18 having the strongest effect on ApB activity (Blumenthal and Seibert, 2003; Khera and Blumenthal, 1996; Dias-Kadambi et al., 1996a; Dias-Kadambi et al., 1996b; Kelso et al., 1996; Seibert et al., 2004; Fig. 2 and Fig. 7). In contrast to the results obtained by chemical modifications of Av2 (Barhanin et al., 1981), this mutagenic analysis has indicated that Arg-14, His-34 and His-39 have no bioactive role (Khera and Blumenthal, 1994, 1996).
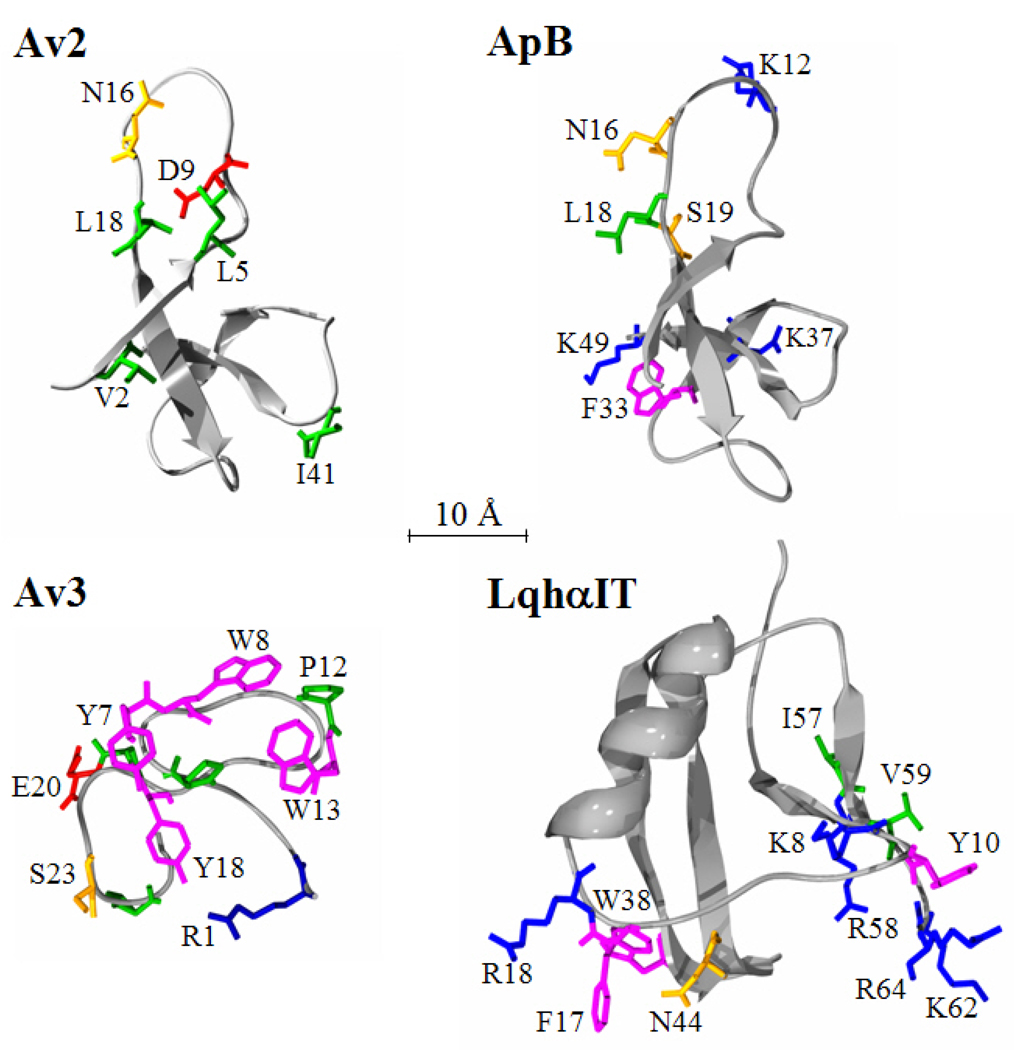
Fig. 7. Comparison of the bioactive surfaces of toxins that bind receptor site-3.
The residues of the bioactive surfaces of the scorpion α-toxin LqhαIT (Karbat et al., 2004), the Type I sea anemone toxin Av2 (Moran et al., 2006), the Type I sea anemone toxin ApB (Blumenthal and Seibert, 2003; Seibert et al., 2004) and the Type III sea anemone toxin Av3 (Moran et al., 2007) are presented as ‘sticks’ on a Cα ribbon structure. The PDB entries for the LqhαIT, ApB and Av3 structures are accessions 1LQI, 1APF and 1ANS, respectively. Amino acids carrying aromatic side chains are in magenta; those with aliphatic side chains are in green; those with polar side chains are in orange; positively-charged residues appear in blue and negatively-charged in red.
Recombinant expression of novel neurotoxin transcripts from Anthopleura of unknown species and activity assays using the contractile force of isolated rat atria, suggested that residues at sites 14, 22 and 25 might have a bioactive role (Wang et al., 2004). Nevertheless, these results await further validation using electrophysiological, binding and toxicity assays more common in the analysis of toxins (Blumenthal and Seibert, 2003; Moran et al., 2006).
Functional expression of Av2 enabled thorough alanine scanning and elucidation of its bioactive surface toward insects (Moran et al., 2006). Analysis of all mutants in toxicity to blowfly larvae and binding assays on cockroach neuronal membranes revealed a functional role for Val-2, Leu-5, Asn-16, Leu-18 and Ile-41 (Fig. 2 and Fig. 7). Further electrophysiological analysis of these mutants against at hNav1.5, indicated a bioactive surface toward the mammalian channel similar to that toward insect channels with the exception of Ser-19, whose substitution affected only the activity only at the cardiac channel. Av2 and ApB are 79% identical in sequence and excluding Arg-12 and Lys-49, all other residues shown to be important in Av2 appear also in ApB (Fig. 2). However, a major variation between both toxins is the functional role proposed for Trp-33 and Lys-37 in ApB, whereas substitution of their Trp-31 and Lys-35 equivalents in Av2 had no effect on the insecticidal activity, and affected only slightly the activity at Nav1.5 (Moran et al., 2006). Moreover, of the four functional aliphatic residues on the Av2 surface, only Leu-18 was ascribed to the anti-mammalian bioactive surface of ApB (Dias-Kadambi et al., 1996b). These glaring differences suggest that despite some commonality in the bioactive surface of Av2 and ApB, the two toxins may interact differently with receptor site-3 on insect and mammalian Navs. This assumption is corroborated by findings of Benzinger and co-workers (1997) that have suggested about differences in the binding sites of ApA and ApB on rat skeletal muscle and cardiac Navs.
Mutagenesis of the glycine residues spanning the flexible Arg-14 loop severely hampered the ability of ApB and Av2 to affect Navs (Seibert et al., 2003; Moran et al., 2006). On the basis of these results and the fact that glycines usually are found in protein sequences at positions that may enlarge structural flexibility, it was proposed that the freedom of the Arg-14 loop to tilt is an important feature for toxin interaction with the receptor site. Another residue whose substitution by alanine affects toxin action is the conserved Asp-9 (Khera and Blumenthal, 1996; Moran et al., 2006; Fig. 2). Because substitution D9N had only little effect on toxicity, the carboxyl group of Asp-9 is not critical for Av2 and ApB activity. As may be inferred from the NMR structures of ApA and ApB, a hydrogen bond exists between the carbonyl group at the side chain of Asp-9 and the amine of Cys-6 (Pallaghy et al., 1995; Monks et al., 1995). Theoretically this bond should be maintained when the aspartate is substituted by asparagine. Khera and Blumenthal (1996) reported that Asp-9 is critical for proper folding of recombinant ApB on the basis of the prominent decline in polypeptide yield when mutated at this position. However, the same phenomenon was not observed in Asp-9 mutants of Av2 (Moran et al., 2006), suggesting that the role of the hydrogen bond formed by the carbonyl of Asp-9 with Cys-6 is to limit the free tilting of the Arg-14 loop. Assuming that the binding of sea anemone toxins to receptor site-3 includes a step of induced fit (Koshland, 1958), which involves the Arg-14 loop, it is possible that its flexibility enables adoption of a shape necessary for binding. Yet, it cannot be ruled out that the carbonyl of Asp-9 simply interacts with the Nav receptor site.
Although Type II sea anemone toxins have been studied at the physiological level, none of them was analyzed using a molecular approach. their study at the molecular level was limited and focused on the charged residues in Sh-I of Stichodactyla helianthus (Pennington et al. 1990). It was shown that substitutions of Lys-4, Asp-6, Asp-7, Glu-8 and Asp-11 impaired severely the activity of Sh-I with no effect on its structure, suggesting that the bioactive surface of this toxin is highly charged. Although diverged from a putative common ancestor (Ishida et al., 1996; Moran and Gurevitz, 2006), sequence comparison of Type II toxins with Type I toxins reveals less than 50% identity, and the bioactive residues in both toxin types differ determined in Type I to be involved in bioactivity are not conserved in Type II toxins (Fig. 2). Despite the difference, both toxin types exert similar effects on a crustacean axon (Rathmayer et al., 1976; Salgado and Kem, 1992). Even more confusing is the fact that CLX-I (also known as CpI) of C. parasitica, which hardly shares any sequence similarity with other Type I and Type II sea anemone toxins, affects the crustacean axon in a very similar fashion (Cariello et al., 1989; Salgado and Kem, 1992). It seems therefore that the similar general structure, maintained by a conserved framework of 3 three disulfide cysteine bonds, and the flexibility of the Arg-14 loop, are of major importance in the ability of sea anemone toxins to bind at receptor site-3 of Navs. However, the question of how this receptor is recognized by a variety of toxin faces is still unclear.
Recombinant expression and mutagenesis of Av3 revealed that the bioactive surface of this toxin is clustered at one hemisphere of the molecule and consists of Arg-1 and a patch of hydrophobic residues, Pro-5, Tyr-7, Trp-8, Pro-12, Trp-13 and Tyr-18 (Fig. 7). This bioactive surface is much more condensed than those of the Type I sea anemone toxins ApB (Seibert et al., 2004) and Av2 (Moran et al., 2006). While the bioactive surface of Av2 lacks aromatic residues, the bioactive surface of Av3 heavily depends on them (Fig. 7). Moreover, some of the residues most critical for Av2 activity appear on the flexible Arg-14 loop, whereas such flexible determinant does not exist in Av3 (Manoleras and Norton, 1994). The bioactive surface of Av3 [245 Å2 (1 Å=0.1 nm)] is considerably smaller than those of scorpion α-toxins (e.g. 430 Å2 for LqhαIT), whose bioactive surfaces are divided into two major amino acid clusters (Karbat et al., 2004, 2007; Ye et al., 2005; Fig. 7). These prominent differences imply that the three toxin types interact differently with receptor site-3 on DmNav1, providing further support for the high heterogeneity of this site (Gordon et al., 1996, 2007; Moran et al., 2007).
The putative receptor for sea anemone toxins on voltage-gates sodium channels
Determination of receptor site-3 on the Nav requires identification of channel residues or determinants that constitute the face of interaction with toxin ligands defined pharmacologically as site-3 toxins. Since this receptor site is targeted by scorpion, spider, and sea anemone toxins that vary greatly in structure, the face of interaction between each toxin and the Nav evidently differs. Yet, considering that these three toxin types compete in binding (Catterall and Beress, 1978; Gordon and Zlotkin, 1993; Little et al., 1998; Gordon et al., 1996) suggests that their surfaces of interaction with the Nav overlap to some extent, which may explain the similar effect they induce on the Nav (Gordon et al., 2007). In addition, scorpion α-toxins and Type I and Type III sea anemone toxins exhibit similar synergism with site-4 toxins (Cohen et al., 2006; Moran et al. 2007). This implies that identification of channel residues involved in the surface of interaction of one of these toxins would provide initial information as to the channel extracellular surface, which that forms constitutes receptor site-3.
By using a variety of antibodies raised against synthetic peptides representing sequences of Nav extracellular loops, the binding of the scorpion α-toxin Lqq5 of Leiurus quinquestriatus quinquestriatus was inhibited by those recognizing the S5–S6 loops of domain 1 and domain 4 (Thomsen and Catterall, 1989; Fig. 1). Unfortunately, these antibodies were not used in the context of sea anemone toxin binding. As determination of the structure of a channel-toxin complex is still an unachievable task, attempts to expose channel regions or residues involved in such interaction focus nowadays on mutagenesis accompanied by analysis of the effect on toxin binding and action.
With the assumption that scorpion α-toxins and sea anemone toxins interact with the Nav via positively charged residues and on the basis of the results obtained with the antibodies (Thomsen and Catterall, 1989), Rogers and co-workers (1996) neutralized by mutagenesis acidic residues at the extracellular loops of domains 1 and 4 of rNav1.2a and examined the effects on Av2 and Lqq5 binding. These experiments highlighted a putative role for Glu-1613, at the loop connecting S3 and S4 of domain 4 (D4/S3-S4; Fig. 1). When the charge of Glu-1613 was inverted, the binding of both Av2 and Lqq5 was abolished. Substitutions E1616A, V1620A and L1624A also diminished the binding affinity of Av2, but had a minor effect on the binding of Lqq5 (Rogers et al., 1996). The difference in binding between the two toxins was further substantiated as substitutions L1614A and S1621A improved significantly the binding of only Av2. Thermodynamic mutant double cycle analysis of the K37A mutant of ApB against D1612N of rNav1.5 (Asp-1612 is the equivalent of Glu-1613 in rNav1.2a; Fig. 1) revealed coupling energy (ΔΔ G = 1.5 kcal/mol) between the two residues (Benzinger et al., 1998). This result implies that Lys-37 of the bound toxin is in close proximity to Asp-1612 of the channel, which therefore was suggested to be part of the receptor site of ApB on rNav1.5. Nevertheless, Lys-37 is not conserved in all Type I and Type II sea anemone toxins (Fig. 2) and therefore, despite the small effect on Av2 action when substituted (Moran et al., 2006), it could not be considered part of the bioactive surface of this sea anemone toxin. In light of the results obtained when the acidic residue at D4/S3-S4 was substituted in rNav1.2a (Rogers et al. 1996) and rNav1.5 (Benzinger et al., 1998), the equivalent residue in DmNav1, Asp-1701, was substituted to arginine and the activities of LqhαIT, Av2 and Av3 were tested on the mutated channel (Moran et al., 2007). While the scorpion and Type I sea anemone toxins lost their activity, the Av3 effect changed only slightly (Moran et al., 2007), indicating that its interaction with DmNav1 differs from that of LqhαIT and Av2. This result clearly indicated that Asp-1701 is not a common determinant for binding of all site-3 toxins. The data accumulated thus far question the extent by which the D4/S3-S4 loop is involved in receptor site-3. While it cannot be ruled out that for certain toxins, such as LqhαIT, ApB and Av2, this region is part of this receptor site, it might not be involved with the receptor site of Av3 (unpublished results). Another possibility is that D4/S3-S4 is in close proximity to receptor site-3 and therefore is involved indirectly in the binding of certain site-3 toxins. Despite the putative interaction of Lys-37 in ApB with Asp-1612 of rNav1.5 (Benzinger et al., 1998), further mutagenesis and thermodynamic mutant double cycle analysis performed in ApB and D4/S3-S4 of rNav1.5 failed to identify toxin-channel residue pairs that exhibit significant coupling energy (Siebert et al., 2004).
All these results suggest that receptor site-3 is a non-continuous epitop that may reside in close proximity to D4/S3-S4, yet identification of its constituents requires either swap of domains or loops between different Navs, or and extensive mutagenesis followed by thermodynamic mutant double cycle analysis, as was recently shown in the initial determination of receptor site-4 of scorpion β-toxins (Cestele et al., 1998, 2006; Leipold et al. 2006, Cohen et al., 2007). It has been shown by domain swapping that ApA and ApB bind to a channel chimera, composed of domain 4 of rNav1.5 and domains 1–3 of rNav1.4, in much higher affinity than to rNav1.4, but in lower affinity than to rNav1.5 (Benzinger et al., 1997). It should be noted that such an approach may highlight determinants that dictate toxin selectivity at different Navs rather than determinants that constitute the receptor site. However, this approach should be combined with a structural initiative, as it was recently suggested for LqhαIT and the D4/S3-S4 loop of DmNav1 (Schnur et al., 2008). Evidently, structural studies of a toxin-channel complex based either on X-ray crystallography or NMR techniques, as well as fluorescent-based biophysical studies could contribute to accurate mapping of this interaction. The recent success in determining the structure of a scorpion toxin bound to a Recently, since new crystallographic techniques have allowed structural determination of a voltage-gated potassium channel binding a scorpion toxin (Lange et al., 2006) suggests that it may not be long before a complex of a Nav channel with a site-3 ligand can be solved.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderluh G, Macek P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria) Toxicon. 2002;40:111–124. doi: 10.1016/s0041-0101(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Anderluh G, Podlesek Z, Macek P. A common motif in proparts of Cnidarian toxins and nematocyst collagens and its putative role. Biochem. Biophys. Acta. 2000;1476:372–376. doi: 10.1016/s0167-4838(99)00237-x. [DOI] [PubMed] [Google Scholar]
- Ast G. How did alternative splicing evolve? Nat. Rev. Genet. 2004;5:773–782. doi: 10.1038/nrg1451. [DOI] [PubMed] [Google Scholar]
- Barhanin J, Hugues M, Schweitz H, Vincent J-P, Lazdunski M. Structure-function relationships of sea anemone toxin II from Anemonia sulcata. J. Biol. Chem. 1981;256:5764–5769. [PubMed] [Google Scholar]
- Benzinger GR, Drum CL, Chen L-Q, Kallen RG, Hanck DA. Differences in the binding sites of two site-3 sodium channel toxins. Pflugers. Arch. 1997;424:742–749. doi: 10.1007/s004240050460. [DOI] [PubMed] [Google Scholar]
- Benzinger GR, Kyle JW, Blumenthal KM, Hanck DA. A specific interaction between the cardiac sodium channel and site-3 toxin Anthopleurin B. J. Biol. Chem. 1998;273:80–84. doi: 10.1074/jbc.273.1.80. [DOI] [PubMed] [Google Scholar]
- Beress L, Beress R, Wunderer G. Isolation and characterization of three polypeptides with neurotoxic activity from Anemonia sulcata. FEBS Lett. 1975;50:311–314. doi: 10.1016/0014-5793(75)80517-5. [DOI] [PubMed] [Google Scholar]
- Beress L. Biologically active compounds from coelenterates. Pure Appl. Chem. 1982;54:1981–1994. [Google Scholar]
- Bergman C, Dubois JM, Rojas E, Rathmayer W. Decreased rate of sodium conductance inactivation in the node of Ranvier induced by a polypeptide toxin from sea anemone. Biochim. Biophys. Acta. 1976;455:173–184. doi: 10.1016/0005-2736(76)90162-0. [DOI] [PubMed] [Google Scholar]
- Blumenthal KM, Seibert AL. Voltage-gated sodium channel toxins: poison, probes, and future promise. Cell. Biochem. Biophys. 2003;38:215–238. doi: 10.1385/CBB:38:2:215. [DOI] [PubMed] [Google Scholar]
- Bosmans F, Aneiros, Tytgat J. The sea anemone Bunodosoma granulifera contains surprisingly efficacious and potent insect-selective toxins. FEBS Lett. 2002;532:131–134. doi: 10.1016/s0014-5793(02)03653-0. [DOI] [PubMed] [Google Scholar]
- Brown DD, Wensink PC, Jordan E. A comparison of the ribosomal DNA’s of Xenpus Laevis and Xenopus Mulleri: the evolution of tandem genes. J. Mol. Biol. 1972;63:57–73. doi: 10.1016/0022-2836(72)90521-9. [DOI] [PubMed] [Google Scholar]
- Cariello L, de Santis A, Fiore F, Piccoli R, Spagnuolo A, Zanetti L, Parente A. Calitoxin, a neurotoxic peptide from the sea anemone Calliactis parasitica: amino acid sequence and electrophysiological properties. Biochemistry. 1989;28:2484–2489. doi: 10.1021/bi00432a020. [DOI] [PubMed] [Google Scholar]
- Carranza S, Baguna J, Riutort M. Origin and evolution of paralogous rRNA gene clusters within the flatworm family Dugesiidae (Platyhelminthes, Tricladida) J. Mol. Evol. 1999;49:250–259. doi: 10.1007/pl00006547. [DOI] [PubMed] [Google Scholar]
- Castaneda O, Sotolongo V, Amor AM, Stoklin R, Anderson AJ, Harvey AL, Engstrom A, Wernstedt C, Karlsson E. Characterization of a potassium channel toxin from the Caribbean sea anemone Stichodactyla helianthus. Toxicon. 1995;33:603–613. doi: 10.1016/0041-0101(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Neurotoxins as allosteric modifiers of voltage-sensitive sodium channels. Adv Cytopharmacol. 1979;3:305–316. [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Beress L. Sea anemone toxin and scorpion toxin share a common receptor site associated with the action potential sodium ionophore. J. Biol. Chem. 1978;253:7393–7396. [PubMed] [Google Scholar]
- Cestèle S, Qu Y, Rogers JC, Rochat H, Scheuer T, Catterall WA. Voltage sensor-trapping: enhanced activation of sodium channels by β-scorpion toxin bound to the S3–S4 loop in domain II. Neuron. 1998;21:919–930. doi: 10.1016/s0896-6273(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Cestèle S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- Cestèle S, Yarov-Yarovoy V, Qu Y, Sampieri F, Scheuer T, Catterall WA. Structure and function of the voltage sensor of sodium channels probed by a β-scorpion toxin. J. Biol. Chem. 2006;281:21332–21344. doi: 10.1074/jbc.M603814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine M, Plante E, Kallen RG. Sea anemone toxin (ATX II) modulation of heart and skeletal muscle sodium channel alpha-subunits expressed in tsA201 cells. J. Memb. Biol. 1996;152:39–48. doi: 10.1007/s002329900083. [DOI] [PubMed] [Google Scholar]
- Chen JY, Oliveri P, Gao F, Dornbos SQ, Li CW, Bottjer DJ, Davidson EH. Precambrian animal life: probable developmental and adult cnidarian forms from southwest China. Dev. Biol. 2002;248:182–196. doi: 10.1006/dbio.2002.0714. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lipstein N, Gordon D. Allosteric interaction between scorpion toxin receptor sites on voltage-gated Na channels imply a novel role for weakly active components in arthropod venom. FASEB J. 2006;20:1933–1935. doi: 10.1096/fj.05-5545fje. [DOI] [PubMed] [Google Scholar]
- Cohen L, Ilan N, Gur M, Stuhmer W, Gordon D, Gurevitz M. Design of a specific activator for skeletal muscle sodium channels uncovers channel architecture. J. Biol. Chem. 2007;282:29424–29430. doi: 10.1074/jbc.M704651200. [DOI] [PubMed] [Google Scholar]
- Corzo G, Escoubas P, Villegas E, Karbat I, Gordon D, Gurevitz M, Nakajima T, Gilles N. A spider toxin that induces a typical effect of scorpion α-toxins but competes with β-toxins on binding to insect sodium channels. Biochemistry. 2005;44:1542–1549. doi: 10.1021/bi048434k. [DOI] [PubMed] [Google Scholar]
- Dias-Kadambi BL, Drum CL, Hanck DA, Blumenthal KM. Leucine 18, a hydrophobic residue essential for high affinity binding of Anthopleurin B to the voltage-sensitive sodium channel. J. Biol. Chem. 1996a;271:9422–9428. doi: 10.1074/jbc.271.16.9422. [DOI] [PubMed] [Google Scholar]
- Dias-Kadambi BL, Combs KA, Drum CL, Hanck DA, Blumenthal KM. The role of exposed tryptophan residues in the activity of the cardiotonic polypeptide Anthopleurin B. J. Biol. Chem. 1996b;271:23828–23835. doi: 10.1074/jbc.271.39.23828. [DOI] [PubMed] [Google Scholar]
- Duda TF, Jr, Palumbi SR. Molecular genetics of ecological diversification: duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6820–6823. doi: 10.1073/pnas.96.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainzilber M, Kofman O, Zlotkin E, Gordon D. A new neurotoxin receptor site on sodium channels is identified by a conotoxin that affects sodium channel inactivation in molluscs, and acts as an antagonist in rat brain. J. Biol. Chem. 1994;269:2574–2580. [PubMed] [Google Scholar]
- Fedorova L, Fedorov A. Introns in gene evolution. Genetica. 2003;118:123–131. [PubMed] [Google Scholar]
- Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE: a novel membrane protein which enhances Drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Fogh RH, Kem WR, Norton RS. Solution structure of neurotoxin I from the sea anemone Stichodactyla helianthus. A nuclear magnetic resonance, distance geometry, and restrained molecular dynamics study. J. Biol. Chem. 1990;265:13016–13028. doi: 10.2210/pdb2sh1/pdb. [DOI] [PubMed] [Google Scholar]
- Frank PG, Bleakney JS. Asexual reproduction, diet, and anomalies of the anemone Nematostella vectensis in Nova Scotia. Canadian Field Naturalist. 1978;92:259–263. [Google Scholar]
- Froy O, Sagiv T, Poreh M, Urbach D, Zilberberg N, Gurevitz M. Dynamic diversification from a putative common ancestor of scorpion toxins affecting sodium, potassium, and chloride channels. J. Mol. Evol. 1999;48:187–196. doi: 10.1007/pl00006457. [DOI] [PubMed] [Google Scholar]
- Gallagher MJ, Blumenthal KM. Cloning and expression of wild-type and mutant forms of the cardiotonic polypeptide Anthopleurin B. J. Biol. Chem. 1992;267:13958–13963. [PubMed] [Google Scholar]
- Gallagher MJ, Blumenthal KM. Importance of unique cationic residues arginine 12 and lysine 49 in the activity of the cardiotoxic polypeptide anthopleurin B. J. Biol. Chem. 1994;269:254–259. [PubMed] [Google Scholar]
- Gendeh GS, Chung MC, Jeyaseelan K. Genomic structure of a potassium channel toxin from Heteractis magnifica. FEBS Lett. 1997;418:183–188. doi: 10.1016/s0014-5793(97)01365-3. [DOI] [PubMed] [Google Scholar]
- Genikhovich G, Kürn U, Hemmrich G, Bosch TC. Discovery of genes expressed in Hydra embryogenesis. Dev. Biol. 2006;289:466–481. doi: 10.1016/j.ydbio.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Evolution of voltage-gated Na+ channels. J. Exp. Biol. 2002;205:575–584. doi: 10.1242/jeb.205.5.575. [DOI] [PubMed] [Google Scholar]
- Gordon D. Sodium channels as targets for neurotoxins: mode of action and interaction of neurotoxins with receptor sites on sodium channels. In: Lazarowici P, Gutman Y, editors. Toxins and Signal Transduction. Amsterdam: Harwood Press; 1997. pp. 119–149. [Google Scholar]
- Gordon D, Karbat I, Ilan N, Cohen L, Kahn R, Gilles N, Dong K, Stühmer W, Tytgat J, Gurevitz M. The differential preference of scorpion α-toxins for insect or mammalian sodium channels: Implications for improved insect control. Toxicon. 2007;49:452–472. doi: 10.1016/j.toxicon.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Gordon D, Martin-Eauclaire M-F, Cestele S, Kopeyan C, Carlier E, Ben Khalifa R, Pelhate M, Rochat H. Scorpion toxins affecting sodium current inactivation bind to distinct homologous receptor sites on rat brain and insect sodium channels. J. Biol. Chem. 1996;271:8034–8045. doi: 10.1074/jbc.271.14.8034. [DOI] [PubMed] [Google Scholar]
- Gordon D, Zlotkin E. Binding of alpha scorpion toxin to insect sodium channels is not dependent on membrane potential. FEBS Lett. 1993;315:125–128. doi: 10.1016/0014-5793(93)81147-r. [DOI] [PubMed] [Google Scholar]
- Hartung K, Rathmayer W. Anemonia sulcata toxins modify activation and inactivation of Na+ currents in a crayfish neurone. Pflugers Arch. 1985;404:119–125. doi: 10.1007/BF00585406. [DOI] [PubMed] [Google Scholar]
- Honma T, Iso T, Ishida M, Nagashima Y, Shiomi K. Occurrence of type 3 sodium channel peptide toxins in two species of sea anemones (Dofleinia armata and Entacmaea ramsayi) Toxicon. 2003;41:637–639. doi: 10.1016/s0041-0101(02)00368-9. [DOI] [PubMed] [Google Scholar]
- Honma T, Hasegawa Y, Ishida M, Nagai H, Nagashima Y, Shiomi K. Isolation and molecular cloning of novel peptide toxins from the sea anemone Antheopsis maculata. Toxicon. 2005;45:33–41. doi: 10.1016/j.toxicon.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Honma T, Shiomi K. Peptide toxins in sea anemones: structural and functional aspects. Mar. Biotechnol. (NY) 2006;8:1–10. doi: 10.1007/s10126-005-5093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida M, Yokoyama A, Shimakura K, Nagashima Y, Shiomi K. Halcurin, a polypeptide from the sea anemone Halcurias sp., with a structural resemblance to type 1 and 2 toxins. Toxicon. 1997;35:537–544. doi: 10.1016/s0041-0101(96)00143-2. [DOI] [PubMed] [Google Scholar]
- Jover E, Martin, Moutot N, Couraud F, Rochat H. Scorpion toxin: specific binding to rat synaptosomes. Biochem. Biophys. Res. Commun. 1978;85:377–382. doi: 10.1016/s0006-291x(78)80053-9. [DOI] [PubMed] [Google Scholar]
- Karbat I, Frolow F, Froy O, Gilles N, Cohen L, Turkov M, Gordon D, Gurevitz M. Molecular basis of the high insecticidal potency of scorpion α-toxins. J. Biol. Chem. 2004;279:31679–31686. doi: 10.1074/jbc.M402048200. [DOI] [PubMed] [Google Scholar]
- Karbat I, Kahn R, Cohen L, Ilan N, Gilles N, Corzo G, Froy O, Gur M, Albrecht G, Heinemann SH, et al. The unique pharmacology of the scorpion α-like toxin Lqh3 is associated with its flexible C-tail. FEBS J. 2007;274:1918–1931. doi: 10.1111/j.1742-4658.2007.05737.x. [DOI] [PubMed] [Google Scholar]
- Kass-Simon G, Scappaticci AA., Jr. The behavioral and developmental physiology of nematocysts. Can. J. Zool. 2002;80:1772–1794. [Google Scholar]
- Keller I, Chintauan-Marquier IC, Veltsos P, Nichols RA. Ribosomal DNA in the grasshopper Podisma pedestris: Escape from concerted evolution. Genetics. 2006;174:863–874. doi: 10.1534/genetics.106.061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso GJ, Blumenthal KM. Identification and characterization of novel sodium chanel toxins from the sea anemone Anthopleura xanthogrammica. Toxicon. 1998;36:41–51. doi: 10.1016/s0041-0101(97)00064-0. [DOI] [PubMed] [Google Scholar]
- Kelso GJ, Drum CL, Hanck DA, Blumenthal KM. Role for Pro-13 in directing high-affinity binding of anthopleurin B to the voltage-sensitive sodium channel. Biochemistry. 1996;35:14157–14164. doi: 10.1021/bi961584d. [DOI] [PubMed] [Google Scholar]
- Kem WR. Sea anemone toxins: structure and action. In: Hessinger DA, Lenhoff HM, editors. The Biology of Nematocysts. New York: Academic Press; 1988. pp. 375–405. [Google Scholar]
- Khera PK, Blumenthal KM. Role of the cationic residues arginine 14 and lysine 48 in the function of the cardiotonic polypeptide anthopleurin B. J. Biol. Chem. 1994;269:921–925. [PubMed] [Google Scholar]
- Khera PK, Benzinger GR, Lipkind G, Drum CL, Hanck DA, Blumenthal KM. Multiple cationic residues of anthopleurin B that determine high affinity and channel isoform discrimination. Biochemistry. 1995;34:8533–8541. doi: 10.1021/bi00027a003. [DOI] [PubMed] [Google Scholar]
- Khera PK, Blumenthal KM. Importance of highly conserved anionic residues and electrostatic interactions in the activity and structure of the cardiotonic polypeptide Anthopleurin B. Biochemistry. 1996;35:3503–3507. doi: 10.1021/bi9528457. [DOI] [PubMed] [Google Scholar]
- Koshland DE. Application of a theory of enzyme specificity to protein synthesis. Proc. Natl. Acad. Sci. U.S.A. 1958;44:98–104. doi: 10.1073/pnas.44.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A, Giller K, Hornig S, Martin-Eauclaire MF, Pongs O, Becker S, Baldus M. Toxin-induced conformational changes in a potassium channel revealed by solid-state NMR. Nature. 2006;440:959–962. doi: 10.1038/nature04649. [DOI] [PubMed] [Google Scholar]
- Leipold E, Hansel A, Borges A, Heinemann SH. Subtype specificity of scorpion b-toxin Tz1 interaction with voltage-gated sodium channels is determined by the pore loop of domain 3. Mol. Pharmacol. 2006;70:340–347. doi: 10.1124/mol.106.024034. [DOI] [PubMed] [Google Scholar]
- Little MJ, Wilson H, Zappia C, Cestèle S, Tyler MI, Martin-Eauclaire MF, Gordon D, Nicholson GM. δ-Atracotoxins from Australian funnel-web spiders compete with scorpion α-toxin binding on both rat brain and insect sodium channels. FEBS Lett. 1998;439:246–252. doi: 10.1016/s0014-5793(98)01378-7. [DOI] [PubMed] [Google Scholar]
- Loret EP, Menendez R, Mansuelle P, Sampieri F, Rochat H. Positively charged amino acid residues located similarly in sea anemone and scorpion toxins. J. Biol. Chem. 1994;269:16785–16788. [PubMed] [Google Scholar]
- Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- Manoleras N, Norton RS. Three-dimensional structure in solution of neurotoxin III from the sea anemone Anemonia sulcata. Biochemistry. 1994;33:11051–11061. doi: 10.1021/bi00203a001. [DOI] [PubMed] [Google Scholar]
- Martinez G, Kopeyan C. Toxin III from Anemonia sulcata: primary structure. FEBS Lett. 1977;84:247–252. doi: 10.1016/0014-5793(77)80699-6. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Ball EE. Cryptic complexity captured: the Nematostella genome reveals its secrets. Trends Genet. 2008;24:1–4. doi: 10.1016/j.tig.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Monks SA, Pallaghy PK, Scanlon MJ, Norton RS. Solution structure of the cardiostimulant polypeptide Anthopleurin-B and comparison with Anthopleurin-A. Structure. 1995;15:791–803. doi: 10.1016/s0969-2126(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Moran Y, Cohen L, Kahn R, Karbat I, Gordon D, Gurevitz M. Expression and mutagenesis of the sea anemone toxin Av2 reveals key amino acid residues important for activity on voltage-gated sodium channels. Biochemistry. 2006;45:8864–8873. doi: 10.1021/bi060386b. [DOI] [PubMed] [Google Scholar]
- Moran Y, Gurevitz M. When positive selection of neurotoxin genes is missing. The riddle of the sea anemone Nematostella vectensis. FEBS J. 2006;273:3886–3892. doi: 10.1111/j.1742-4658.2006.05397.x. [DOI] [PubMed] [Google Scholar]
- Moran Y, Kahn R, Cohen L, Gur M, Karbat I, Gordon D, Gurevitz M. Molecular analysis of the sea anemone toxin Av3 reveals selectivity to insects and demonstrates the heterogeneity of receptor site-3 on voltage-gated Na-channels. Biochem. J. 2007;406:41–48. doi: 10.1042/BJ20070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Y, Weinberger H, Sullivan JC, Reitzel AM, Finnerty JR, Gurevitz M. Concerted Evolution of sea anemone neurotoxin genes is revealed through analysis of the Nematostella vectensis genome. Mol. Biol. Evol. 2008a;25:737–747. doi: 10.1093/molbev/msn021. [DOI] [PubMed] [Google Scholar]
- Moran Y, Weinberger H, Reitzel AM, Sullivan JC, Kahn R, Gordon D, Finnerty JR, Gurevitz M. Intron retention as a posttranscriptional regulatory mechanism of neurotoxin expression at early life stages of the starlet anemone Nematostella vectensis. J. Mol. Biol. 2008b;380:437–443. doi: 10.1016/j.jmb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Ogawa T, Oda N, Hattori M, Sakaki Y, Kihara H, Ohno M. Accelerated evolution of Trimeresurus flavoviridis venom gland phospholipase A2 isozymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5964–5968. doi: 10.1073/pnas.90.13.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Moore JW, Shapiro BI. Condylactis toxin: interaction with nerve membrane ionic conductance. Science. 1969;163:680–681. doi: 10.1126/science.163.3868.680. [DOI] [PubMed] [Google Scholar]
- Norton RS. Structure and structure-function relationships of sea anemone proteins that interact with the sodium channel. Toxicon. 1991;29:1051–1084. doi: 10.1016/0041-0101(91)90205-6. [DOI] [PubMed] [Google Scholar]
- Oliveira JS, Radaelli E, Zaharenko AJ, Cassulini RR, Konno K, Pimenta DC, Freitas JC, Clare JJ, Wanke E. Binding specificity of sea anemone toxins to Nav 1.1–1.6 sodium channels: Unexpected contributions from differences in the IV/S3-S4 outer loop. J. Biol. Chem. 2004;279:33323–33335. doi: 10.1074/jbc.M404344200. [DOI] [PubMed] [Google Scholar]
- Pallaghy PK, Scanlon MJ, Norton RS. Three-dimensional structure in solution of the polypeptide cardiac stimulant Anthopleurin-A. Biochemistry. 1995;34:3782–3794. doi: 10.1021/bi00011a036. [DOI] [PubMed] [Google Scholar]
- Pennington MW, Kem WR, Dunn BM. Synthesis and biological activity of six monosubstituted analogs of a sea anemone polypeptide neurotoxin. Pept. Res. 1990;3:228–232. [PubMed] [Google Scholar]
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- Rathmayer W, Beress L. The effect of toxins from Anemonia sulcata (Coelenterata) on neuromuscular transmission and nerve action potentials in crayfish (Astacus leptodactilus) J. Comp. Physiol. 1976;109:373–382. [Google Scholar]
- Richet C. Des effects anaphylactiques de l’actino-toxine sur la pression aterielle. C.R. Seances. Soc. Biol. Paris. 1902;54:837–838. [Google Scholar]
- Rogers JC, Qu Y, Tanada TN, Scheuer T, Catterall WA. Molecular determinants of high affinity binding of α-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the sodium channel α subunit. J. Biol. Chem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- Ruppert EE, Barnes RD. The cnidarians. In: Invertebrate Zoology, 6th edition. Saunders College Publishing, Philadelphia. 1994:103–154. [Google Scholar]
- Salceda E, Perez-Castells J, Lopez-Mendez B, Garateix A, Salazar H, Lopez O, Aneiros A, Standker L, Beress L, Forssmann WG, et al. CgNa, a type I toxin from the giant Caribbean sea anemone Condylactis gigantea shows structural similarities to both type I and type II toxins, as well as distinctive structural and functional properties. Biochem. J. 2007;406:67–76. doi: 10.1042/BJ20070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado VL, Kem WR. Actions of three structurally distinct sea anemone toxins on crustacean and insect sodium channels. Toxicon. 1992;30:1365–1381. doi: 10.1016/0041-0101(92)90512-4. [DOI] [PubMed] [Google Scholar]
- Schnur E, Turkov M, Kahn R, Gordon D, Gurevitz M, Anglister J. NMR analysis of interaction of LqhαIT scorpion toxin with a peptide corresponding to the D4/S3-S4 loop of insect Para voltage-gated sodium channel. Biochemistry. 2008;15:2656–2668. doi: 10.1021/bi701323k. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Bidard JN, Frelin C, Pauron D, Vijverberg HP, Mahasneh DM, Lazdunski M, Vilbois F, Tsugita A. Purification, sequence and pharmacological properties of sea anemone toxins from Radianthus paumotensis. A new class of sea anemone toxins acting on the sodium channel. Biochemistry. 1985;24:3554–3561. doi: 10.1021/bi00335a025. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Bruhn T, Guillemare E, Moinier D, Lancelin J-M, Beress L, Lazdunski M. Kalicludines and kaliseptine. Two different classes of sea anemone toxins for voltage-sensitive K+ channels. J. Biol. Chem. 1995;270:25121–25126. doi: 10.1074/jbc.270.42.25121. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Vincent JP, Barhanin J, Frelin C, Linden G, Hugues M, Lazdunski M. Purification and pharmacological properties of eight sea anemone toxins from Anemonia sulcata, Anthopleura xanthogrammica, Stoichactis giganteus, and Actinodendron plumosum. Biochemistry. 1981;20:5245–5252. doi: 10.1021/bi00521a023. [DOI] [PubMed] [Google Scholar]
- Seibert AL, Liu J, Hanck DA, Blumenthal KM. Arg-14 loop of site 3 anemone toxins: effects of glycine replacement on toxin affinity. Biochemistry. 2003;42:14515–14521. doi: 10.1021/bi035291d. [DOI] [PubMed] [Google Scholar]
- Seibert AL, Liu J, Hanck DA, Blumenthal KM. Role of Asn-16 and Ser-19 in Anthopleurin B binding. Implication for the electrostatic nature of Nav site 3. Biochemistry. 2004;43:7082–7089. doi: 10.1021/bi0496135. [DOI] [PubMed] [Google Scholar]
- Spagnuolo A, Zanetti L, Cariello L, Piccoli R. Isolation and characterization of two genes encoding calitoxins, neurotoxic peptides from Calliactis parasitica (Cnidaria) Gene. 1994;138:187–189. doi: 10.1016/0378-1119(94)90805-2. [DOI] [PubMed] [Google Scholar]
- Sugino RP, Innan H. Selection for more of the same product as a force to enhance concerted evolution of duplicated genes. Trends Genet. 2006;22:642–644. doi: 10.1016/j.tig.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Sullivan JC, Ryan JF, Watson JA, Webb J, Mullikin JC, Rokhsar D, Finnerty JR. Stellabase: the Nematostella vectensis genomic database. Nucleic. Acids Res. 2006;34:D495–D499. doi: 10.1093/nar/gkj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak JW, Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980;284:426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Catterall WA. Localization of the receptor site for α-scorpion toxins by antibody mapping: implications for sodium channel topology. Proc. Natl. Acad. Sci. U.S.A. 1989;86:10161–10165. doi: 10.1073/pnas.86.24.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W. Sodium channel inactivation: molecular determinants and modulation. Physiol. Rev. 2005;85:1271–1301. doi: 10.1152/physrev.00024.2004. [DOI] [PubMed] [Google Scholar]
- Wang L, Ou J, Peng L, Zhong X, Du J, Liu Y, Zhang Y, Dong M, Xu AL. functional expression and characterization of four novel neurotoxins from sea anemone Anthopleura sp. Biochem. Biophys. Res. Commun. 2004;313:163–170. doi: 10.1016/j.bbrc.2003.11.102. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Reenan AGR, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Van der Ploeg LHT, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels. Modulation by membrane protein TipE and toxin pharmacology. J. Gen. Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer H, Billeter M, Wuthrich K. Three-dimensional structure of the neurotoxin ATX Ia from Anemonia sulcata in aqueous solution determined by nuclear magnetic resonance spectroscopy. Proteins. 1989;6:357–371. doi: 10.1002/prot.340060403. [DOI] [PubMed] [Google Scholar]
- Wilcox GR, Fogh RH, Norton RS. Refinement of the solution structure of the sea anemone neurotoxin Sh I. J. Biol. Chem. 1993;268:24707–24719. [PubMed] [Google Scholar]
- Wunderer G, Eulitz M. Amino-acid sequence of toxin I from Anemonia sulcata. Eur. J. Biochem. 1978;89:11–17. doi: 10.1111/j.1432-1033.1978.tb20890.x. [DOI] [PubMed] [Google Scholar]
- Wunderer G, Fritz H, Wachter E, Machleidt W. Amino-acid sequence of a coelenterate toxin: toxin II from Anemonia sulcata. Eur. J. Biochem. 1976;68:193–198. doi: 10.1111/j.1432-1033.1976.tb10778.x. [DOI] [PubMed] [Google Scholar]
- Ye X, Bosmans F, Li C, Zhang Y, Wang DC, Tytgat J. Structural basis for the voltage-gated Na+ channel selectivity of the scorpion alpha-like toxin BmK M1. J. Mol. Biol. 2005;353:788–803. doi: 10.1016/j.jmb.2005.08.068. [DOI] [PubMed] [Google Scholar]
- Yeung SY, Thompson D, Wang Z, Fedida D, Robertson B. Modulation of Kv3 subfamily potassium currents by the sea anemone toxin BDS: significance for CNS and biophysical studies. J. Neurosci. 2005;25:8735–8745. doi: 10.1523/JNEUROSCI.2119-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Bosmans F, Tytgat J. Adaptive evolution of scorpion sodium channel toxins. J. Mol. Evol. 2004;58:145–153. doi: 10.1007/s00239-003-2534-2. [DOI] [PubMed] [Google Scholar]
- Zhu S, Gao B. Molecular characterization of a possible progenitor sodium channel toxin from the Old World scorpion Mesobuthus martensii. FEBS Lett. 2006;580:5979–5987. doi: 10.1016/j.febslet.2006.09.071. [DOI] [PubMed] [Google Scholar]