Abstract
Genetic studies in yeast, plants, insects and mammals have identified four universally conserved proteins, together called Vps Class C, that are essential for late endosome and lysosome assembly and for numerous endolysosomal trafficking pathways, including the terminal stages of autophagy. Two Vps-C complexes, HOPS and CORVET, incorporate diverse biochemical functions: they tether membranes, stimulate Rab nucleotide exchange, guide SNARE assembly to drive membrane fusion, and possibly act as ubiquitin ligases. Recent studies offer new insight into the complex relationships between Vps-C complexes and their cognate Rab small GTP-binding (G-)proteins at endosomes and lysosomes. Accumulating evidence supports the view that Vps-C complexes implement a regulatory logic that governs endomembrane identity and dynamics.
INTRODUCTION
The Vps-C complexes CORVET (class C core vacuole/endosome tethering) and HOPS (homotypic fusion and protein sorting) are found on endosomes and lysosomes, where they control membrane traffic by keeping a close eye on the membrane fusion machinery. HOPS and CORVET are essential for the maturation, integrity, and inheritance of late endosomes and vacuolar lysosomes, and appear to control all traffic passing into and through these organelles (Figure 1). Hence, Vps-C complexes are likely central regulators of a multitude of cellular and physiological processes associated with late endocytic organelles, including downregulation of growth factor receptors and nutrient transporters [1,2], disposal and recycling of cytoplasmic components through autophagy [3-5], cholesterol and lipid metabolism [6,7], and antigen processing and presentation [8,9]. In this review we focus on the yeast Vps-C complexes because they have been subjected to the closest scrutiny. However, conserved complexes appear to carry out similar functions in all eukaryotes[10-13].
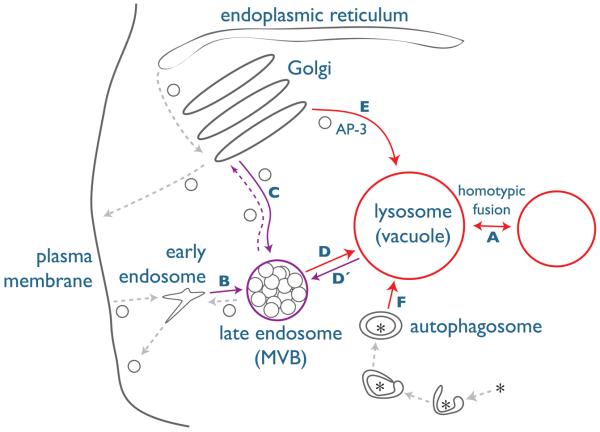
Figure 1. Cellular trafficking pathways governed by Vps-C complexes.
The vacuole is the terminal organelle in the yeast endocytic pathway, equivalent to the mammalian lysosome. Vps-C complexes regulate several transport routes to and from the vacuole: (A) vacuoles fuse homotypically in response to environmental signals and during cell division and cell fusion; (B) endocytic vesicles and early endosomes fuse homotypically as they mature; (C) transport vesicles derived from the TGN fuse with endosomes to deliver biosynthetic cargos; (D) late endosome cargo is delivered to the vacuole via heterotypic fusion; (D′) Retrograde traffic from the vacuole passes through the endosome; (E) some vacuole-resident proteins are transported from TGN to vacuole in an AP-3-dependent pathway that bypasses endosomes; and (F) soluble cargos and defective organelles are transported from the cytosol into the vacuole lumen via the CVT and autophagy pathways. Solid arrows depict transport steps thought to require Vps-C complexes. Endosomes and endosomal trafficking events requiring CORVET are depicted in purple, while vacuoles and vacuolar trafficking events requiring HOPS are depicted in red. While this model depicts traffic in yeast, endolysosomal traffic in plants, invertebrates and mammals is broadly similar. AP-3, adaptor protein-3 complex; TGN, trans-Golgi network; CVT, cytoplasm to vacuole targeting; HOPS, fusion and vacuole protein sorting; CORVET, class C core vacuole/endosome tethering complex.
VPS-C COMPLEXES: MOLECULAR ORGANIZATION
Classical genetic screens in Saccharomyces cerevisiae identified 41 VPS (vacuole protein sorting) genes [14], and over a dozen PEP (peptidase sorting) genes [15], involved in delivery of cargo to the yeast vacuole. Mutations in four of these caused the severe Class C phenotype, with mutant cells lacking an identifiable vacuolar lysosome. The proteins encoded by these genes, Vps11 (Pep5), Vps16, Vps18 (Pep3), and Vps33, associate to form the stable Vps-C core complex [16,17]. The VPS family today includes about 80 genes, but deletion of no others specifically controlling endocytic traffic produce stronger growth, trafficking and membrane morphology defects than those seen in Class C vps mutants, reflecting the pivotal roles played by these proteins in multiple endolysosomal trafficking events (Figure 1).
HOPS
Two accessory subunits, Vps39 and Vps41, associate with the Vps-C core to form the ~633 kDa HOPS (homotypic fusion and protein sorting) complex (Table I). HOPS controls all membrane fusion at the vacuolar lysosome. Consistent with this role, vps39 and vps41 null mutants contain highly fragmented vacuoles characteristic of the class B VPS phenotype [14]. Vps39 functions in yeast as a guanosine exchange factor (GEF) that activates the vacuolar Rab Ypt7 [18], while Vps41 binds Ypt7 as a direct effector, physically linking the HOPS holocomplex to the activated, GTP-bound Rab [19]. HOPS also associates with acidic phospholipids and SNAREs [20,21]
Table I.
Rab GTPase and Vps-C complex components governing endolysosomal membrane fusion
| Factor | Yeast | Human | Function(s) |
|---|---|---|---|
| Rab GTPase | Vps21 | Rab5 | Early endosome membrane identity marker and fusion regulation |
| Ypt7 | Rab7 | Late endosome/lysosome membrane identity marker and fusion regulator | |
| Vps-C core | Vps11/Pep5 | hVps11 | Elongated, coiled-coil proteins that function in membrane tethering and SNARE chaperone/proofreading |
| Vps16 | hVps16 | ||
| Vps18/Pep3 | hVps18 | Human subunit possesses ubiquitin-ligase activity in vitro | |
| Vps33 | hVps33A, B | Sec1/Munc18 (SM) family, putative SNARE interactor | |
| HOPS | Vps39/Vam6 | hVps39/hVam6* | Putative GEF for Ypt7* |
| Vps41/Vam2 | hVps41 | Ypt7 effector protein, possibly involved in AP-3 vesicle biogenesis | |
| CORVET | Vps3 | hVps3 | Putative GEF for Vps21 |
| Vps8 | hVps8 | Vps21 effector protein | |
| Other | Yck3 | Casein kinase I | Protein kinase regulated by Ypt7, negatively regulates fusion by phosphorylating Vps41 and Vam3 |
| Gyp7 | GAP for Ypt7 | ||
| Gdi1/Sec19 | GDI | GDI, universal Rab chaperone | |
| Sec18 | NSF | AAA ATPase for SNARE complex disassembly | |
| Sec17 | α-SNAP | SNARE-complex adaptor for Sec18/NSF |
Rab, monomeric G protein of the Ras GTPase superfamily; GTPase, guanosine triphosphate phosphatase; Vps, vacuole protein sorting; Ypt, yeast protein targeting; Pep, carboxypeptidase Y-deficient; Vam, vacuolar morphogenesis; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; SM, Sec1/Munc18 family; HOPS, homotypic fusion and vacuole protein sorting; GEF, guanine nucleotide exchange factor; CORVET, class C core vacuole/endosome tethering complex; Yck, yeast casein kinase; GAP, GTPase activating protein; Gyp, GTPase-activating protein for Ypt; GDI, guanosine nucleotide dissociation inhibitor; Sec, secretory; AAA, ATPase associated with various cellular activities; NSF, N-ethylmaleimide-sensitive factor; SNAP, N-ethylmaleimide-sensitive factor attachment protein.
Metazoan Vps39 shares sequence homology with yeast Vps3 and Vps39.
Many Rab effectors are big proteins with open, elongated structures hypothesized to facilitate long-distance membrane tethering. Most subunits of the HOPS complex, with the exception of the Sec1/Munc18 (SM) family subunit Vps33, appear to fit this description, while at the same time displaying a domain architecture common to membrane coat proteins (Figure 2). Computational analyses predict that all Vps-C subunits (except Vps33) possess an amino-terminal β-propellor and an α-solenoid that extends to the carboxy-terminus. This architecture is strikingly similar to common building blocks of other membrane-shaping, multi-subunit complexes, including COPI, COPII, clathrin and subunits of the nuclear pore [22]. Thus, it is no surprise that HOPS might possess similar capacities to self-assemble into higher-order structures on membranes [23-26], with the Vps41 subunit perhaps functioning as a coat protein for AP-3 vesicles [25]. The prospect that HOPS senses or generates membrane curvature and functions as a long-distance tethering apparatus finds analogy in the recently demonstrated capacity of a Golgin, GMAP-210, to tether liposomes with selectivity for degrees of membrane curvature [27].
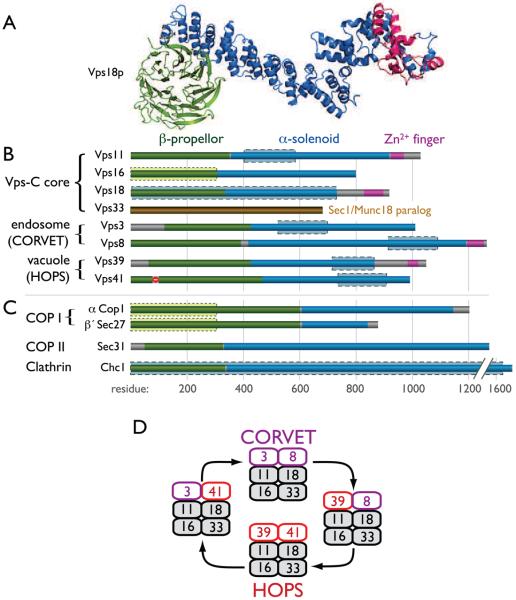
Figure 2. Composition and domain architecture of Vps-C complexes.
Predicted folding structures for the Vps-C core and accessory subunits were generated using the tertiary-fold prediction algorithm Rosetta [74]. (A) Ribbon diagram depicting folding model for Vps18. (B and C) Summary of tertiary structure predictions indicated by color coding for Vps-C components (B) and vesicle coat outer shell subunits (C). Primary sequence homologies (PSI-BLAST) between Vps-C subunits and clathrin heavy chain are indicated by blue boxes with dashed boundaries. Homology between the Vps16 amino-terminus and COPI subunits is denoted by green boxes with dotted boundaries. Structural predictions for the yeast Vps-C components are shown; nearly identical predictions were obtained for their mammalian orthologs. (D) Working schema for Vps-C complex function, in which interactions of CORVET and HOPS with endosomal and lysosomal Rabs regulate membrane fusion events at late endosomes and lysosomes, respectively. Hybrid complexes containing both HOPS and CORVET subunits have been detected [28], but their associations with anterograde or retrograde traffic are speculative. We also note that hVps39 interacts with both GDP- and GTP-bound Rab5 [10], but has not been shown to interact with Rab7, indicating the model requires further testing to reconcile differences between the yeast and metazoan systems. COP/Cop, coat protein; Sec, secretory; Chc, clathrin heavy chain.
CORVET
Beyond its established role in HOPS, the Vps-C core was recently shown to associate with a pair of alternative accessory subunits, Vps3 [28] and Vps8 [28,29] which share homology with the HOPS subunitsVps39 and Vps41 (Table I) [28]. This second Vps-C complex, CORVET (class C core vacuole/endosome tethering), associates with the early endosome Rab5 ortholog Vps21 [28]. Despite strong sequence homology between the carboxy-terminal domains of Vps3 and Vps39, the more poorly-conserved amino-terminal domain of Vps39 harbors its Rab-binding site [11,12,18], and there is at this writing no evidence that Vps3 catalyzes nucleotide exchange. The only confirmed GEF for Vps21 is Vps9 [30]; vps9 null mutants phenocopy trafficking defects found in vps3, vps8 and vps21 mutants, all of which have defects in traffic through the late endosomal prevacuolar compartment and exhibit Class D vps swollen vacuoles [14,28,31].
The discovery of CORVET clarifies the role of Vps33, the SM subunit of the Vps-C core, on endosomes [29]: Vps33 localizes to both endosomes and vacuoles due to its participation in both CORVET and HOPS holocomplexes. The presence of the same SM protein at both endosomes and vacuoles, meanwhile, may explain the partial functional redundancy of the endosomal and vacuolar Qa-SNARE proteins Pep12 and Vam3 [32-35], both reported to interact with Vps33 [17,29]. Pep12, however, also interacts with a second endosomal SM protein, Vps45 [36]. It will be important to understand the division of labor between Vps33 and Vps45.
The discovery of CORVET raises a host of new questions. While structural homologies between HOPS and CORVET (Figure 2) might tempt us to speculate that CORVET functions in early or late endosome tethering and fusion, many phenotypes resulting from loss of Vps21, Vps3 or Vps8 are consistent with defects in retrograde traffic from endolysosomes. Class D vps mutants have swollen vacuoles, indicative of a failure in membrane cycling away from the vacuole (we call this the Roach Motel phenotype: ‘membrane checks in, but it doesn't check out’). The Class D defect could in principle arise from a failure of retrograde vesicle formation at the vacuole, a failure of retrograde fusion at the endosome, or both [37]. Deletion of VPS8 in yeast lacking the vacuole Qa-SNARE Vam3 substantially rescues the vam3 fragmented vacuole phenotype [28], suggesting defects in membrane consolidation and divestiture are offsetting and compensatory. Overexpression of the CORVET subunit Vps8 causes clustering of Vps21-positive endosomes, suggestive of a direct tethering function [28]. As this phenotype results from superstoichiometric expression of Vps8, it might reflect an autonomous Vps8 function not requiring the Vps-C core. Overexpression of Vps3 also produces clustering of Vps21-positive endosomes, but is accompanied by vacuolar fragmentation, perhaps due to the capacity of Vps3 to compete with the Ypt7 GEF, Vps39, for assembly on the Vps-C core. These findings, and the aberrant colocalization of endosomal and vacuolar Rabs that occurs in a vps8 mutant [28], together indicate that the balance between different Vps-C complexes is a key parameter that governs traffic between late endosomes and vacuoles/lysosomes. How these complexes function during normal endolysosomal tranport processes, however, is at present understood only superficially.
Vps-C complexes and ubiquitin
Covalent attachment of single or short chains of ubiquitin to both transmembrane cargo proteins and sorting receptors is a widespread regulatory signal in endolysosomal cargo sorting and membrane dynamics [38]. While ubiquitin-mediated regulation of Vps-C complexes is not firmly established, the conspicuous recurrence of Zn2+ finger RING motifs (common to many E3 ubiquitin ligases) within Vps-C core and accessory subunits (Figure 2) is suggestive. The RING domains of Vps18 and Vps8, at least, are required for efficient Vps-C function [31,39], as is that of Vps11 (our unpublished results). Moreover, the RING domain of mammalian Vps18 was reported to exhibit E3 ligase activity toward the GGA3 adaptor protein, at least in vitro [40,41]. However, neither ubiquitination mediated by Vps-C RING domains nor ubiquitination of Vps-C subunits has been demonstrated in vivo, and despite evidence that the HOPS-associated Rab Ypt7 is ubiquitinated [42], no study has clearly linked a specific ubiquitination reaction to in vivo functions of Vps-C complexes.
CELLULAR FUNCTIONS AND REGULATION
Endosome maturation and Rab/Vps-C complex conversion
Nearly 20 years ago it was recognized that mammalian early endosomes are decorated with Rab5, while late endosomes and lysosomes bear Rab7 [43]. The discovery that CORVET and HOPS complexes specifically interact with endosomal and lysosomal Rabs, and the identification of intermediate complexes containing both CORVET and HOPS accessory subunits [10,28,31], suggests a range of plausible models for compartmental maturation during endolysosomal transport. Through exchange of Vps3 for Vps39, the Ypt7 GEF, endosomes might encourage the recruitment and activation of the downstream lysosomal Rab. An exchange of Rab5 for Rab7 has been observed on mammalian endosomes [10], and while there is yet no study characterizing possible CORVET-HOPS dynamics in this system, Rab5 (ortholog of Vps21) captures a protein complex containing Vps-C core components and the putative HOPS ortholog hVps39, suggesting that Rab5 might similarly promote recruitment of Rab7 through an interaction with the Rab7 GEF [10]. While this model is both appealing and plausible it is far from established, and key issues are unresolved. In yeast, the physiological relevance of intermediate complexes containing both HOPS and CORVET accessory subunits (i.e., Vps8 and 39, or Vps3 and 41; Figure 2D) has yet to be established. The mammalian Vps8 ortholog is unstudied. The major mammalian Vps3/39 ortholog when overexpressed caused clustering and fusion of late endosomes and lysosomes [11,12], but it is unclear whether this protein is a functional homolog of yeast Vps3 or Vps39, or whether it subsumes functions of both. Mammalian cells also contain several largely uncharacterized proteins with homology to the carboxy-terminal domain of Vps39. No mammalian protein has yet been demonstrated to harbor any GEF activity toward Rab7.
The available data from yeast are consistent both with dynamic subunit exchange models in which CORVET and HOPS accessory subunits sequentially dissociate from and associate with the Vps-C core, and with models in which CORVET and HOPS exist predominantly as stable, discrete complexes. In the first case, endolysosomal transport would be accompanied by dynamic remodeling of complex composition. In the second case, association and dissociation of entire complexes would accompany transport. Because HOPS and CORVET are biochemically stable complexes, dynamic subunit remodeling would imply the existence of an as-yet unidentified chaperone or degradation system; the requirement for such a system could conceivably provide the missing link between Vps-C biochemistry and ubiquitin signaling.
There is also the open question of whether the acquisition of Rab7 by Rab5 endosomes results in maturation of the entire organelle [10] or whether discrete Rab5-and Rab7-enriched microdomains emerge to form separate endosome- and lysosome-targeted transport vesicles [44]. The rapid tempo of improvements to live-cell imaging systems and the development of improved fluorescent probes should provide more definitive answers.
SNARE selectivity and chaperone activity by HOPS
HOPS, and perhaps CORVET as well, promote membrane fusion by interacting directly with the core fusion machinery. SNARE-mediated fusion invariably requires four complementary SNARE domains, identified as R, Qa, Qb, and Qc, depending on the presence of Arg or Gln within the central ‘0-layer’ of the SNARE domain. SNARE domains span a docking junction and ‘zipper’ together in trans, driving the apposed membranes together to trigger lipid mixing and fusion. Post-fusion cis-SNARE complexes are then disassembled by the ATP-dependent chaperone NSF and its α–SNAP cochaperone (Sec18 and Sec17 in yeast), freeing the SNAREs to catalyze subsequent rounds of fusion [45].
How does HOPS interact with SNAREs? Perhaps surprisingly, no study to date has addressed which HOPS subunits directly mediate specific SNARE contacts. While the SM protein Vps33 is an obvious candidate, studies reporting interactions between the HOPS holocomplex and the vacuole Qa-SNARE, Vam3 [17,46], or the Qc-SNARE, Vam7 [20], did not exclude participation by other members of the holocomplex. While Vam3 includes an amino-terminal regulatory motif that mediates interactions of other Qa-SNAREs with their cognate SM proteins, this domain was not required for Vam3-HOPS binding in pulldowns employing cell lysates [46]. HOPS apparently binds not the Vam7 SNARE domain, but the amino-terminal lipid-binding PX domain, a domain found in no other SNARE, including those found on mammalian endosomes and lysosomes [20]. Thus, while the HOPS-Vam7(PX) interaction may be functionally significant in fungi, it is not evolutionarily conserved. In any case, these somewhat unconventional SNARE-binding profiles raise the possibility that Vps33 is an unconventional SM protein, and may indicate that additional HOPS components participate in SNARE recognition. No study to date has specifically examined CORVET-SNARE interactions.
Recent studies affirm the importance of HOPS in trans-SNARE complex assembly using both purified organelles [47] and a fully reconstituted system [48]. But does this reflect a HOPS-mediated catalytic SNARE assembly activity or, instead, enhanced membrane tethering and increased local SNARE concentration? Altering the 0-layer amino acids of vacuolar SNAREs has provided important insights. Mutating the Qc-SNARE Vam7 to resemble an R-SNARE reduces its ability to mediate fusion, despite an apparent ability to form kinetically stable 2Q•2R SNARE complexes in a HOPS-dependent manner [49]. When provided with both wild-type and mutant Vam7, however, vacuoles overwhelmingly favor incorporation of wild-type Vam7 into trans-complexes [49], demonstrating a SNARE filtering function that likely resides in HOPS. As further evidence that HOPS enforces SNARE selectivity, ‘rotation’ of the 0-layer by compensatory mutation of the R-SNARE, Nyv1, restores the 3Q•1R ratio when mixed with R-mutant Vam7 and partially restores fusion capacity [49]. Addition of exogenous HOPS to fusion reactions inhibits fusion mediated by mismatched and rotated 0-layer SNAREs, but not wild-type SNAREs [50]. Sec17 can outcompete HOPS for binding to cis- [47] and trans-SNARE complexes [51], so HOPS is readily displaced to allow SNARE disassembly by Sec17-Sec18 [48]. Apparently a multilayered quality control system monitors each stage of SNARE-mediated fusion lest accidental lysis should occur [52].
The primary engine of SNARE disassembly is the ATPase Sec18/NSF, so how could SNARE proofreading and mismatch correction occur in the absence of ATP, a condition under which many of the above results were obtained? Insight has emerged from recent work using carboxy-terminal truncations of SNARE domains to limit the degree of SNARE zippering. While fully-zippered SNAREs are remarkably stable, with spontaneous off-rates much longer than biologically relevant time scales [53], formation of kinetically stable trans-SNARE complexes on vacuoles requires zippering three α-helical turns beyond the SNARE domain 0-layer [51], a zippering pivot point recently shown to correspond to an energetic barrier to further folding [54]. Taken together, these recent data raise the possibility that HOPS ensures SNARE-pairing fidelity by linking inspection of the trans-complex backbone conformation at the 0-layer to the completion of zippering and initiation of membrane fusion.
Rab-dependent activation and phosphoinhibition of HOPS
Yeast vacuoles undergo fusion and fission in response to hypo- and hypertonic stresses [55,56]. These rapid activations and deactivations of membrane tethering and fusion depend on the nucleotide-binding status of the master regulator Ypt7 and on the phosphorylation of the HOPS subunit Vps41 [56,57]. HOPS consolidates Rab GEF (Vps39) [18,19] and effector (Vps41) [19] functions into a stable complex. This relationship suggests a positive feedback loop similar to a loop described for Rab5 and its Rabex-5/Rabaptin GEF/effector complex [58]. The Ypt7 GAP (GTPase activating protein), Gyp7, inhibits HOPS activity by returning Ypt7 to its GDP-bound, inactive state, but even when heavily overexpressed, Gyp7 is insufficient to terminate vacuole fusion without the aid of a casein kinase I, Yck3 [19]. Yck3 is palmitoylated and traffics via the AP-3 pathway from Golgi to vacuole [59] where it directly phosphorylates the HOPS subunit Vps41 [21,57] and the Qa-SNARE Vam3 [19; our unpublished results] to inhibit vacuole fusion. Deletion of Yck3, or expression of constitutively ‘active’ Vps41 that evades phosphoregulation by Yck3, results in hyperaccumulation of Vps41 at the vacuole [60] and missorting of AP-3 cargoes [60,61]. The molecular basis of this sorting defect is unclear.
Yck3 activity toward Vps41 and Vam3 is opposed by active Ypt7 on the vacuole [19], demonstrating that Ypt7 positively regulates HOPS both directly through effector binding and indirectly by counteracting inhibitory phosphorylation. HOPS is anchored to the vacuole through interactions with Ypt7, SNARE proteins, and acidic phospholipids. Phosphorylation of Vps41 by Yck3 does not change the apparent affinity of Vps41 for Ypt7 [21,60], but it dramatically reduces the affinity of HOPS for acidic phospholipids and SNARES [21]. Consequently, in the presence of Yck3 HOPS association with the vacuole, and fusion, are dependent on the activation status of Ypt7. In experiments with proteoliposomes and purified components, HOPS –mediated fusion was Ypt7-dependent when Yck3 was present and active, but Ypt7-independent when Yck3 was absent [21]. However, experiments with native membranes suggest that this situation is somewhat nonphysiological. Both in vivo genetic experiments [19], and experiments with native vacuoles and antibodies raised against Ypt7 [57] indicate that in the absence of Yck3, residual Ypt7 is still essential for fusion. How can this discrepancy be explained? Wickner's group found that native vacuoles bearing surplus SNARES undergo fusion despite inhibition of Ypt7 [52] or, when supplemented with HOPS, in the absence of Ypt7 [48]. Thus, at superphysiological levels of SNAREs and HOPS, Ypt7 is not required for fusion at all. At physiological levels of SNAREs and HOPS, but in the absence of the Yck3 kinase, only residual levels of Ypt7 are needed, and the system is nearly or completely insensitive to the activation status of Ypt7; when Yck3 is present, fusion requires relatively high levels of active Ypt7. Thus, under in vivo conditions, Yck3 enforces a tight coupling between the activation status of Ypt7 and HOPS-mediated docking and fusion.
CONCLUDING REMARKS
Vps-C complexes control the locations, timing, specificity and integrity of fusion in the terminal branches of the endocytic system. Besides the fundamental concern of maintaining organelle identity, inappropriate delivery of cargo to the lysosome can result in improper proteolytic activation, as illustrated by mistaken lysosomal maturation of trypsinogen to trypsin, which leads to intracellular proteolysis and pancreatitis [62]. Fusion is an inherently dangerous business, as demonstrated by the lysis that accompanies fusion upon overexpression of vacuolar SNAREs [50,52]. Remarkably, HOPS not only stimulates fusion, but simultaneously reduces lysis. Lipid mixing as membrane bilayers progress from hemifusion to full fusion might produce transient holes adjacent to the fusion pore that require repair mechanisms coordinated with fusion machinery, a topic explored in an excellent recent review [63]. Moreover, HOPS and Ypt7 signaling respond to osmotic stresses [56] that, if uncontrolled, cause lysosomal rupture [64]. Understanding the coordinated mechanisms through which HOPS, SNAREs and specific membrane lipids effect fusion while restricting lysis presents a central challenge.
Recent work suggests possible connections between Vps-C complexes and other membrane trafficking processes. Microautophagy, or the internalization of cytoplasm through invagination of the vacuole limiting membrane, is thought to regulate the balance of membrane in the endolysosomal system through regulation by the EGO (exit from rapamycin-induced growth arrest) and TOR target of rapamycin) complexes at the vacuole [65]. Thus it is intriguing that mutations in the TOR and HOPS complexes are synthetically lethal [66], but the nature of the genetic interaction remains largely unexplored. The mechanism by which microautophagic tubules are released from the vacuole limiting membrane is also unknown, but it is not unreasonable to suppose HOPS and its associated fusion machinery could participate. Beyond microautophagy, the discovery in mammals that UVRAG recruits a Vps-C complex to autophagosomes to permit delivery of autophagosomes to endolysosomes provides a missing link between Vps-C function and autophagosome maturation [67].
The discovery of CORVET at endosomes introduces numerous possibilities for interactions with endosomal cargo sorting machineries. A recent report that human VpsC component hVps18 interacts with Hrs and TSG101 [68] hints at the possibility of cross-talk between Vps-C and ESCRTs (endosomal sorting complexes required for transport) that regulate cargo sorting into multivesicular bodies [69] — a clue that requires further exploration. Also, the apparent defects in retrograde endolysosomal traffic upon disruption of CORVET require study. The recently observed complementary roles of Rab5 and Rab7 in recruiting retromer components [70] brings us back to the idea that CORVET and HOPS might influence retrograde traffic through activation and exchange of Rabs, but this hypothesis is so far untested.
Vps-C complex components have proven resistant to biochemical and structural characterization in large part due to the difficulty of expressing them recombinantly. Even ten years after the discovery of Vps-C holocomplexes, our knowledge remains limited to computational structural predictions (Figure 1). A daunting but essential challenge going forward is to determine which biochemical functions reside within specific Vps-C subunits, and which are emergent properties of the larger complexes. Such information is vital not only to understand Vps-C functions in more than a cartoon outline, but also to extend the findings gleaned from studies of yeast (in which cells lacking entire Vps-C genes are viable) to multicellular organisms in which full-length gene deletions are almost always lethal. While key insights have been gleaned from studies of melanocytes [71], tissue-specific disruptions [72] and partial loss-of-function mutants such as the fly VPS18 allele deep orange [73] have generally proven most tractable to study in whole organisms. Furthermore, dissection of specific Vps-C complex functionalities will enable untangling of the pleiotropic defects seen upon disruption or loss of entire subunits. Given their rapid morphological responses to stress and highly volatile contents, endolysosomal organelles must be simultaneously plastic and indestructable. We are only beginning to appreciate the myriad Vps-C interactions that impart this dual character.
ACKNOWLEDGMENTS
We thank members of the Merz lab for critical reading of the manuscript. This work was supported by grant GM077349 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Edinger AL, Cinalli RM, Thompson CB. Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev Cell. 2003;5:571–582. doi: 10.1016/s1534-5807(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 2.Wilkin M, Tongngok P, Gensch N, Clemence S, Motoki M, Yamada K, Hori K, Taniguchi-Kanai M, Franklin E, Matsuno K, et al. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev Cell. 2008;15:762–772. doi: 10.1016/j.devcel.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 5.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 7.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 9.Stinchcombe J, Bossi G, Griffiths GM. Linking albinism and immunity: the secrets of secretory lysosomes. Science. 2004;305:55–59. doi: 10.1126/science.1095291. [DOI] [PubMed] [Google Scholar]
- 10.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 11.Caplan S, Hartnell LM, Aguilar RC, Naslavsky N, Bonifacino JS. Human Vam6p promotes lysosome clustering and fusion in vivo. J Cell Biol. 2001;154:109–122. doi: 10.1083/jcb.200102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson SC, Winistorfer SC, Poupon V, Luzio JP, Piper RC. Mammalian late vacuole protein sorting orthologues participate in early endosomal fusion and interact with the cytoskeleton. Mol Biol Cell. 2004;15:1197–1210. doi: 10.1091/mbc.E03-06-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu GD, Salazar G, Zlatic SA, Fiza B, Doucette MM, Heilman CJ, Levey AI, Faundez V, L'Hernault SW. SPE-39 family proteins interact with the HOPS complex and function in lysosomal delivery. Mol Biol Cell. 2009;20:1223–1240. doi: 10.1091/mbc.E08-07-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston RA, Reinagel PS, Jones EW. Genes required for vacuolar acidity in Saccharomyces cerevisiae. Genetics. 1992;131:551–558. doi: 10.1093/genetics/131.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci U S A. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato TK, Rehling P, Peterson MR, Emr SD. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- 18.Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151:551–562. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Brett CL, Plemel RL, Lobinger BT, Vignali M, Fields S, Merz AJ. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J Cell Biol. 2008;182:1141–1151. doi: 10.1083/jcb.200801001. This work demonstrates competing regulatory pathways for the activation and deactivation of HOPS-mediated vacuole membrane fusion. The dependence of the HOPS-inhibiting kinase, Yck3, on the nucleotide-binding state of Ypt7 reinforces the role of Ypt7 as the master fusion regulator at the vacuole. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickey CM, Stroupe C, Wickner W. The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J Biol Chem. 2009 doi: 10.1074/jbc.M109.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2004;2:e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura N, Hirata A, Ohsumi Y, Wada Y. Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membranes and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae. J Biol Chem. 1997;272:11344–11349. doi: 10.1074/jbc.272.17.11344. [DOI] [PubMed] [Google Scholar]
- 24.Price A, Seals D, Wickner W, Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol. 2000;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darsow T, Katzmann DJ, Cowles CR, Emr SD. Vps41p function in the alkaline phosphatase pathway requires homo-oligomerization and interaction with AP-3 through two distinct domains. Mol Biol Cell. 2001;12:37–51. doi: 10.1091/mbc.12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108:357–369. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 27•.Drin G, Morello V, Casella JF, Gounon P, Antonny B. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science. 2008;320:670–673. doi: 10.1126/science.1155821. A beautiful demonstration that a membrane tethering factor can select a suitable binding partner based not only on a membrane's molecular constituents, but also by scanning the geometry of the membrane, provides insight that might apply to many other tethers, including HOPS. [DOI] [PubMed] [Google Scholar]
- 28••.Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell. 2007;12:739–750. doi: 10.1016/j.devcel.2007.03.006. The discovery of an alternative Vps-C holocomplex, CORVET, that associates with the endosome Rab, Vps21, represents a tremendous advance. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian S, Woolford CA, Jones EW. The Sec1/Munc18 protein, Vps33p, functions at the endosome and the vacuole of Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:2593–2605. doi: 10.1091/mbc.E03-10-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hama H, Tall GG, Horazdovsky BF. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J Biol Chem. 1999;274:15284–15291. doi: 10.1074/jbc.274.21.15284. [DOI] [PubMed] [Google Scholar]
- 31.Horazdovsky BF, Cowles CR, Mustol P, Holmes M, Emr SD. A novel RING finger protein, Vps8p, functionally interacts with the small GTPase, Vps21p, to facilitate soluble vacuolar protein localization. J Biol Chem. 1996;271:33607–33615. doi: 10.1074/jbc.271.52.33607. [DOI] [PubMed] [Google Scholar]
- 32.Gotte M, Gallwitz D. High expression of the yeast syntaxin-related Vam3 protein suppresses the protein transport defects of a pep12 null mutant. FEBS Lett. 1997;411:48–52. doi: 10.1016/s0014-5793(97)00575-9. [DOI] [PubMed] [Google Scholar]
- 33.Darsow T, Burd CG, Emr SD. Acidic di-leucine motif essential for AP-3-dependent sorting and restriction of the functional specificity of the Vam3p vacuolar t-SNARE. J Cell Biol. 1998;142:913–922. doi: 10.1083/jcb.142.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerrard SR, Mecklem AB, Stevens TH. The yeast endosomal t-SNARE, Pep12p, functions in the absence of its transmembrane domain. Traffic. 2000;1:45–55. doi: 10.1034/j.1600-0854.2000.010108.x. [DOI] [PubMed] [Google Scholar]
- 35.Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burd CG, Peterson M, Cowles CR, Emr SD. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryant NJ, Piper RC, Weisman LS, Stevens TH. Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J Cell Biol. 1998;142:651–663. doi: 10.1083/jcb.142.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 39.Rieder SE, Emr SD. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yogosawa S, Hatakeyama S, Nakayama KI, Miyoshi H, Kohsaka S, Akazawa C. Ubiquitylation and degradation of serum-inducible kinase by hVPS18, a RING-H2 type ubiquitin ligase. J Biol Chem. 2005;280:41619–41627. doi: 10.1074/jbc.M508397200. [DOI] [PubMed] [Google Scholar]
- 41.Yogosawa S, Kawasaki M, Wakatsuki S, Kominami E, Shiba Y, Nakayama K, Kohsaka S, Akazawa C. Monoubiquitylation of GGA3 by hVPS18 regulates its ubiquitin-binding ability. Biochem Biophys Res Commun. 2006;350:82–90. doi: 10.1016/j.bbrc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Kleijnen MF, Kirkpatrick DS, Gygi SP. The ubiquitin-proteasome system regulates membrane fusion of yeast vacuoles. EMBO J. 2007;26:275–287. doi: 10.1038/sj.emboj.7601486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 44.Vonderheit A, Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 2005;3:e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 46.Dulubova I, Yamaguchi T, Wang Y, Sudhof TC, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol. 2001;8:258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- 47.Collins KM, Thorngren NL, Fratti RA, Wickner WT. Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion. EMBO J. 2005;24:1775–1786. doi: 10.1038/sj.emboj.7600658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 2008;27:2031–2042. doi: 10.1038/emboj.2008.139. An experimental tour de force, this study artifically reconstitutes a vacuole membrane fusion system. The low levels of liposome lysis observed in this reconstituted fusion system suggest that physiologic concentrations of SNAREs, HOPS, Sec17-Sec18 and fusion site lipids represents a sufficient machinery to both catalyze fusion and restrict lysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fratti RA, Collins KM, Hickey CM, Wickner W. Stringent 3Q.1R composition of the SNARE 0-layer can be bypassed for fusion by compensatory SNARE mutation or by lipid bilayer modification. J Biol Chem. 2007;282:14861–14867. doi: 10.1074/jbc.M700971200. [DOI] [PubMed] [Google Scholar]
- 50•.Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. Together, [49] and [50] provide the first strong evidence that HOPS directly guides the assembly of trans-SNARE complexes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz ML, Merz AJ. Capture and release of partially zipped trans-SNARE complexes on intact organelles. J Cell Biol. 2009;185:535–549. doi: 10.1083/jcb.200811082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starai VJ, Jun Y, Wickner W. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc Natl Acad Sci U S A. 2007;104:13551–13558. doi: 10.1073/pnas.0704741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fasshauer D, Antonin W, Subramaniam V, Jahn R. SNARE assembly and disassembly exhibit a pronounced hysteresis. Nat Struct Biol. 2002;9:144–151. doi: 10.1038/nsb750. [DOI] [PubMed] [Google Scholar]
- 54•.Li F, Pincet F, Perez E, Eng WS, Melia TJ, Rothman JE, Tareste D. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat Struct Mol Biol. 2007;14:890–896. doi: 10.1038/nsmb1310. [51] and [54] suggest that trans-SNARE zippering to the third α-helical turn beyond the central SNARE domain 0-layer is a critical junction in determining whether trans-SNAREs form kinetically stable complexes or abort. The observation that zippering beyond the third α-helical turn is required to initiate lipid-mixing at vacuoles suggests SNARE domain pairing between the 0-layer and third turn represents a critical checkpoint in regulating membrane fusion. [DOI] [PubMed] [Google Scholar]
- 55.Bone N, Millar JB, Toda T, Armstrong J. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr Biol. 1998;8:135–144. doi: 10.1016/s0960-9822(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 56.Brett CL, Merz AJ. Osmotic regulation of Rab-mediated organelle docking. Curr Biol. 2008;18:1072–1077. doi: 10.1016/j.cub.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaGrassa TJ, Ungermann C. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J Cell Biol. 2005;168:401–414. doi: 10.1083/jcb.200407141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M. Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell. 2001;12:2219–2228. doi: 10.1091/mbc.12.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun B, Chen L, Cao W, Roth AF, Davis NG. The yeast casein kinase Yck3p is palmitoylated, then sorted to the vacuolar membrane with AP-3-dependent recognition of a YXXPhi adaptin sorting signal. Mol Biol Cell. 2004;15:1397–1406. doi: 10.1091/mbc.E03-09-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cabrera M, Ostrowicz CW, Mari M, LaGrassa TJ, Reggiori F, Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell. 2009;20:1937–1948. doi: 10.1091/mbc.E08-09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anand VC, Daboussi L, Lorenz TC, Payne GS. Genome-wide analysis of AP-3-dependent protein transport in yeast. Mol Biol Cell. 2009;20:1592–1604. doi: 10.1091/mbc.E08-08-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hashimoto D, Ohmuraya M, Hirota M, Yamamoto A, Suyama K, Ida S, Okumura Y, Takahashi E, Kido H, Araki K, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engel A, Walter P. Membrane lysis during biological membrane fusion: collateral damage by misregulated fusion machines. J Cell Biol. 2008;183:181–186. doi: 10.1083/jcb.200805182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luke CJ, Pak SC, Askew YS, Naviglia TL, Askew DJ, Nobar SM, Vetica AC, Long OS, Watkins SC, Stolz DB, et al. An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell. 2007;130:1108–1119. doi: 10.1016/j.cell.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell. 2005;19:15–26. doi: 10.1016/j.molcel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 66.Zurita-Martinez SA, Puria R, Pan X, Boeke JD, Cardenas ME. Efficient Tor signaling requires a functional class C Vps protein complex in Saccharomyces cerevisiae. Genetics. 2007;176:2139–2150. doi: 10.1534/genetics.107.072835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, et al. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol. 2008;10:776–787. doi: 10.1038/ncb1740. Demonstrates not only the capacity of UVRAG to recruit a Vps-C complex to mammalian autophagosomes to deliver autophagosomes to lysosomes, but also suggests a role for UVRAG in stimulating endosomal trafficking and membrane fusion through a Vps-C complex interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim BY, Akazawa C. Endosomal trafficking of EGFR regulated by hVps18 via interaction of MVB sorting machinery. Biochem Biophys Res Commun. 2007 doi: 10.1016/j.bbrc.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 69.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raposo G, Marks MS. Melanosomes--dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, Kramer H. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J Cell Sci. 2005;118:3663–3673. doi: 10.1242/jcs.02502. [DOI] [PubMed] [Google Scholar]
- 73.Lindmo K, Simonsen A, Brech A, Finley K, Rusten TE, Stenmark H. A dual function for Deep orange in programmed autophagy in the Drosophila melanogaster fat body. Exp Cell Res. 2006;312:2018–2027. doi: 10.1016/j.yexcr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Kuhlman B, Dantas G, Ireton GC, Varani G, Stoddard BL, Baker D. Design of a novel globular protein fold with atomic-level accuracy. Science. 2003;302:1364–1368. doi: 10.1126/science.1089427. [DOI] [PubMed] [Google Scholar]