Abstract
The Drosophila melanogaster lymph gland is a haematopoietic organ1–3 in which pluripotent blood cell progenitors proliferate and mature into differentiated haemocytes. Previous work4 has defined three domains, the medullary zone, the cortical zone and the posterior signalling centre (PSC), within the developing third-instar lymph gland. The medullary zone is populated by a core of undifferentiated, slowly cycling progenitor cells, whereas mature haemocytes comprising plasmatocytes, crystal cells and lamellocytes are peripherally located in the cortical zone. The PSC comprises a third region that was first defined as a small group of cells expressing the Notch ligand Serrate5. Here we show that the PSC is specified early in the embryo by the homeotic gene Antennapedia (Antp) and expresses the signalling molecule Hedgehog. In the absence of the PSC or the Hedgehog signal, the precursor population of the medullary zone is lost because cells differentiate prematurely. We conclude that the PSC functions as a haematopoietic niche that is essential for the maintenance of blood cell precursors in Drosophila. Identification of this system allows the opportunity for genetic manipulation and direct in vivo imaging of a haematopoietic niche interacting with blood precursors.
The Drosophila lymph gland primordium is formed by the coalescence of three paired clusters of cells that express Odd-skipped (Odd) and arise within segments T1–T3 (Fig. 1a) of the embryonic cardiogenic mesoderm6. At developmental stages 11–12, mesodermal expression of Antp is restricted to the T3 segment (Fig. 1b, c). A fraction of these Antp-expressing cells will contribute to the formation of the dorsal vessel7,8, whereas the remainder, which also express Odd, give rise to the PSC (Fig. 1d, e). By stages 13–16, the clusters coalesce and Antp is observed in 5–6 cells at the posterior boundary of the lymph gland (Fig. 1d, e). The expression of Antp is subsequently maintained in the PSC through the third larval instar (see Fig. 2a). The embryonic stage 16 PSC can also be distinguished by Fasciclin III expression (Fig. 1f, g) and at stage 17 these are the only cells in the lymph gland that incorporate BrdU (Fig. 1h).
Figure 1. Embryonic specification of the PSC by Antp.
a, Schematic representation of the development of the Drosophila lymph gland. T1–T3, three thoracic segments; dv, the dorsal vessel; cb, cardioblast; lgprim, lgsec lgtert, primary, secondary and tertiary lobes, respectively, of the lymph gland; and pc, pericardial cells. b–e, Immunohistochemical analysis showed that the lymph gland is formed by the fusion of the three Odd-positive cell groups (T1, T2 and T3). Antp expression is confined to a group of cells at the posterior boundary of T3 (b, e). The cells that will become larval PSC remain confined to the posterior edge of the embryonic lymph gland (e). f, g, Fas III (Fasciclin III, a homophilic cell adhesion molecule) is upregulated in the PSC. h, In the late embryo, the PSC cells incorporate BrdU. i, Antp expression is maintained in the embryonic col mutant background. j, k, Expression of col, detected by in situ hybridization, in the PSC (WT, arrow in j) is eliminated in an Antp mutant background (arrow in k). l, Antp protein is expressed in the PSC, whereas Hth protein is seen in the rest of the lymph gland. m, n, In the hth mutant background (m), the lymph gland is virtually eliminated (arrow). Overexpression of hth (twist-gal4, UAS-hth; n) causes a reduction in the number of cells in the PSC. All colours correspond to the marker label in each panel. All images were acquired using a ×40 objective with additional × 1.3 (b, c, d) or ×2.5 (e–n) confocal magnification.
Figure 2. The larval PSC functions as a haematopoietic niche to maintain blood precursors in the medullary zone.
a, In wild-type (WT), the PSC consists of a cell cluster located along the posterior edge of the lymph gland. b, Antp expression in the lymph gland is missing in col−(PSC−). The residual expression is in the cardioblasts of the dorsal vessel. c, The PSC is greatly expanded on Antp overexpression (PSCexp). d–k′, In wild type, medullary zone markers (d, g, j) are restricted to the precursor population of cells. These markers are all eliminated in the absence of the PSC (e, h, k). Rarely, a much reduced medullary zone can be seen (example shown in k′). Expansion of the PSC causes expansion of the medullary zone (f, i). l–n, In wild-type (l), BrdU incorporation is limited to the cortical zone. In the absence of PSC, cells in more medial regions incorporate BrdU (m), whereas in an expanded PSC genotype (n), the number of cells in S-phase is greatly reduced. o–q, In wild-type (o), differentiating cells reside in the peripheral cortical zone. In the absence of a PSC (p), differentiated cells are found throughout the lymph gland lobe. On expansion of the PSC (q), these cells are restricted to a thin layer along the distal edge. The colour of molecular markers for each row corresponds to those of the side labels (the label ‘domelessGFP’ corresponds to the expression of GFP under the control of domeless-gal4; ‘SerLacZ’ is expression of β-galactosidase under the control of the Ser9.5 (ref. 4) enhancer). All images were acquired using a 40× objective. The PSC panels (b, e, h, k, k′, m, p) also reflect an additional ×1.5 confocal magnification.
Previous studies have identified the transcription factor Collier (Col) as an essential component regulating PSC function9. The gene for this protein is initially expressed in the entire embryonic lymph gland anlagen and by stage 16 is refined to the PSC. In col mutants, the PSC is initially specified, but is entirely lost by the third larval instar. To address further the role of Antp and Col in embryonic lymph gland development, we investigated the expression of each gene in the loss-of-function mutant background of the other. We found that loss of col does not affect embryonic Antp expression (Fig. 1i). In contrast, col expression is absent in the PSC of Antp mutant embryos (Fig. 1j, k), establishing that Antp functions genetically upstream of Col in the PSC.
In imaginal discs, the expression of Antp is related to that of the homeodomain cofactor Homothorax (Hth)10. In the embryonic lymph gland, Hth is initially expressed ubiquitously but is subsequently downregulated in PSC cells, which become Antp-positive (Fig. 1l). In hth loss-of-function mutants, the lymph gland is largely missing (Fig. 1m), whereas misexpression of hth causes loss of PSC and the size of the embryonic lymph gland remains relatively normal (Fig. 1n). We conclude that a mutually exclusive functional relationship exists between Antp and Hth in the lymph gland such that Antp specifies the PSC, whereas Hth specifies the rest of the lymph gland tissue. Interestingly, knocking out the mouse homologue of Hth, Meis1, eliminates definitive haematopoiesis11,12. Meis1 is also required for the leukaemic transformation of myeloid precursors overexpressing HoxB913.
Although lymph gland development is initiated in the embryo, the establishment of zones and the majority of haemocyte maturation takes place in the third larval instar. At this stage, Antp continues to be expressed in the wild-type PSC (Fig. 2a). To investigate how the loss of PSC cells affects haematopoiesis, we first examined Antp expression in third instar col mutant lymph glands. In this background, all Antp-positive PSC cells are missing (Fig. 2b), consistent with the previously described role for col in PSC maintenance9. Overexpression of Antp within the PSC increases the size of PSC from the usual 30–45 cells to 100–200 cells (Fig. 2c). These PSC cells are scattered over a larger volume, often forming two or three large cell clusters rather than the single, dense population seen in wild type.
To determine the role of PSC in haematopoiesis, we investigated the expression pattern of various markers in lymph glands of larvae of the above genotypes, which either lack a PSC or have an enlarged PSC. The status of blood cell progenitors was directly assessed using the medullary-zone-specific markers4 ZCL2897, DE-cadherin (Shotgun) and domeless-gal4 (Fig. 2d–k′). In col mutant lymph glands, expression of these markers is absent or severely reduced (Fig. 2e, h, k, k′) and when the PSC is expanded, the medullary zone is greatly enlarged (Fig. 2f, i). Our previous work demonstrated that medullary zone precursors are relatively quiescent4, a characteristic similar to the slowly cycling stem cell or progenitor populations in other systems14. BrdU incorporation in the wild-type lymph gland is largely restricted to the cortical zone4 (Fig. 2l), but in third-instar col mutants incorporation of BrdU is increased relative to wild type and becomes distributed throughout the lymph gland (Fig. 2m), suggesting that the quiescence of the medullary zone haematopoietic precursors is no longer maintained in the absence of the PSC. Similarly, when the PSC domain is expanded, BrdU incorporation is significantly suppressed throughout the lymph gland (Fig. 2n).
We next used P1 and ProPO as markers for plasmatocytes and crystal cells, respectively, to assess the extent of haemocyte differentiation within lymph glands of the above genotypes. Loss of the PSC does not compromise haemocyte differentiation; rather, mature plasmatocytes and crystal cells are found abundantly within the lymph gland. Furthermore, the distribution of these differentiating cells is not restricted to the peripheral region that normally constitutes the cortical zone and many cells expressing ProPO and P1 can be observed medially throughout the region normally occupied by the medullary zone (Fig. 2o, p). Increasing the PSC domain causes a concomitant reduction in the differentiation of haemocytes (Fig. 2q).
In summary, loss of the PSC causes a loss of medullary zone markers, a loss of the quiescence normally observed in the wild-type precursor population and an increase in cellular differentiation throughout the lymph gland. Similarly, increased PSC size leads to an increase in the medullary zone, a decrease in BrdU incorporation and a decrease in the expression of maturation markers. We conclude that the PSC functions as a haematopoietic niche that maintains the population of multipotent blood cell progenitors within the lymph gland. The observed abundance of mature cells in the absence of the PSC suggests that the early blood cell precursors generated during the normal course of development will differentiate in the absence of a PSC-dependent mechanism that normally maintains progenitors as a population. This situation is reminiscent of the Drosophila15 and Caenorhabditis elegans16 germ lines in which disruption of the niche does not block differentiation per se, but lesser numbers of differentiated cells are generated as a result of the failure to maintain stem cells. It is also interesting to note that col mutant larvae are unable to mount a lamellocyte response to immune challenge9. We speculate that this could be because of the loss of precursor cells that are necessary as a reserve to differentiate during infestation.
Recent work on several vertebrate and invertebrate developmental systems has highlighted the importance of niches14,17 as unique microenvironments in the maintenance of precursor cell populations. Examples include haematopoietic18,19, germline20 and epidermal21 stem cell niches that provide, through complex signalling interactions, stem cells with the ability to self-renew and persist in a non-differentiated state. The work presented in this report demonstrates that the PSC is required for the maintenance of medullary zone haematopoietic progenitors. The medullary zone represents a group of cells within the lymph gland that are compactly arranged and express the homotypic cell-adhesion molecule, DE-cadherin4. These cells are pluripotent, slowly cycling and undifferentiated and are capable of self-renewal. It is presently uncertain whether Drosophila has blood stem cells capable of long-term repopulation as haematopoietic stem cells are in vertebrates. Nevertheless, it is clear that the maintenance of medullary zone cells as precursors is niche dependent.
In order for the PSC to function as a haematopoietic niche there should exist a means by which the PSC can communicate with precursors. As such, a signal emanating from the PSC and sensed by the medullary zone represents an attractive model of how this might occur. Although we have reported that Ser (ref. 5) and Upd3 (ref. 4) are expressed in the PSC, preliminary analysis suggests that elimination of either of these ligands alone will not cause the phenotype seen for Antp and col mutants. We therefore investigated the haematopoietic role of several signalling pathways and identified the hedgehog (hh) signalling pathway as a putative regulator in the maintenance of blood cell progenitors. The hhts2 lymph gland (Fig. 3d) is remarkably similar in its phenotype to that seen for Antp hypomorphic22 (Fig. 3b) or col loss-of-function (Fig. 3c) mutants (compare to wild type in Fig. 3a). Blocking Hh signalling in the lymph gland through the expression of a dominant-negative form of the downstream activator Cubitus inter-ruptus (Ci, the Drosophila homologue of Gli) also causes a phenotype similar to that observed in Antp and col loss-of-function backgrounds. This is true when expressed either specifically in the medullary zone (Fig. 3e) or throughout the lymph gland (Fig. 3f).
Figure 3. A Hedgehog signal from the PSC is required for the maintenance of the precursor cell population of the medullary zone.
a, Wild-type (WT) expression of the cortical zone markers P1 (green) and ProPO (red). b, Third-instar Antp-mutant22 larvae show a lymph gland phenotype similar to that seen in col mutants (c). hhts2 (d) shows an identical phenotype (compare to c). Misexpression of dominant-negative Ci (e, f) phenocopies Antp, col and hh loss-of-function lymph gland phenotypes (b–d). g–i, Hh expression (green) is restricted to the PSC in the second (g) and third instars (h, i). All cells expressing Antp (red in i) also express Hh (yellow in i). A few dispersed cells (arrowhead) in the cortical zone also initiate Hh expression (i). j–l, The PSC (Antp in green) is present in hhts2 mutants (k, l) as in wild-type (j). m, Hh expression marks the PSC and Ptc marks the medullary zone. n–p, Ptc and Ci (red) expression co-localizes with the medullary zone marker domeless-gal4, UAS-GFP (labelled ‘Dome’). Ci forms a gradient with highest staining intensity near the PSC. The asterisk marks the PSC. Haematopoietic markers for each panel are colour-coded. All images were acquired using a ×40 objective. Panels (c, d, k and l) reflect an additional ×1.5 confocal magnification. The confocal magnification used for the close-up of the PSC in panel (i) was ×3.
Consistent with the above functional results, Hh protein is expressed in the second instar PSC (Fig. 3g) and continues to be expressed in third instar PSC cells (Fig. 3h, i). In the hhts2 mutant background, the PSC cells continue to express Antp at the restrictive temperature (Fig. 3j–l) indicating that, unlike col and Antp, Hh is not essential for the specification of the PSC. Rather, Hh constitutes a component of the signalling network that allows the PSC to maintain the precursor population of the medullary zone. Consistent with this notion, downstream components of the Hh pathway, the receptor Patched (Ptc) (Fig. 3m, n) and activated Ci (Fig. 3o, p), are found in the medullary zone. On the basis of both functional and expression data, we propose that Hh in the PSC signals through activated Ci in medullary zone cells, thereby keeping them in a quiescent precursor state.
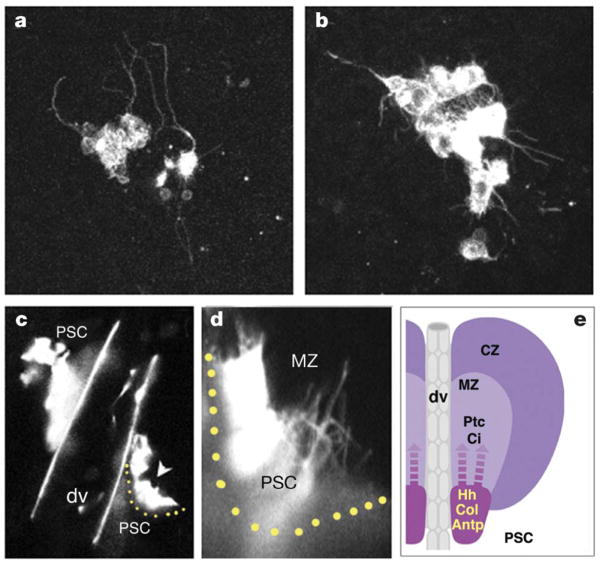
The Hh pathway has been studied extensively in the context of animal development23. Although the Hh signal does not disperse widely on secretion, many studies have shown that this signal can be transmitted over long distances24. The mechanism by which this occurs is not fully clear and this is also true of how the PSC delivers Hh to medullary zone progenitors. However, when labelled with green fluorescent protein (GFP), we find that PSC cells extend numerous thin processes over many cell diameters (Fig. 4). The morphology of the PSC cells, taken together with the long-range function of Hh revealed by the mutant phenotype, indicates that the long cellular extensions may deliver Hh to receiving cells not immediately adjacent to the PSC. In this respect, the Drosophila haematopoietic system shows remarkable similarity to the C. elegans germline25. In both cases, precursors are maintained as a population over some distance from the niche and in both instances, the niche cells extend long processes when interacting with the precursors.
Figure 4. PSC cells exhibit extensive processes that project into the lymph gland.
a, b, GFP expressed exclusively in PSC cells (using Antp-gal4, UAS-mCD8–GFP) reveals the presence of numerous thin processes that extend over several cell diameters into the medullary zone. c, d, Analysis of PSC morphology in live animals. Whole-mount live third instar larva showing GFP expression in the PSC. A close-up view (d) of the region indicated by the arrowhead in (c), showing processes extending into the lymph gland. dv, dorsal vessel. e, A schematic representation of the PSC region of the lymph gland as a niche involved in the maintenance of medullary zone progenitors. All images were acquired using a ×40 objective except panel c, which was taken with a ×10 objective. Panels a,b and c and d, reflect additional ×6, ×2.5, and ×8 confocal magnification, respectively.
Several studies have highlighted the importance of homeodomain proteins in stem cell development and leukaemias26,27. Likewise, the role of Hh in vertebrate and invertebrate stem cell maintenance has recently received much attention28–30. The work presented here describes direct roles for Antp in the specification and Hh in the functioning of a haematopoietic niche. The medullary zone cells are blood progenitors that are maintained in the lymph gland at later larval stages by Hh, a signal that originates in the PSC (Fig. 4e). The maintenance of these progenitors provides the ability to respond to additional developmental or immune-based haematopoietic signals. On the basis of these findings, understanding the specific roles of Hh signalling and Hox genes in the establishment and function of vertebrate haematopoietic niches warrants further investigation. The identification of a haematopoietic niche in Drosophila will allow future investigation of in vivo niche/precursor interactions in a haematopoietic system that allows direct observation, histological studies and extensive genetic analysis.
METHODS
Fly stocks and crosses
The following Drosophila strains (donors in parentheses) were used in the described experiments: Antp-gal4/TM3, Sb (Cohen, S.M.), GFP-trap line ZCL2897 (Cooley, L.), hhts2 (Moses, K.), UAS-Cicell (Basler, K.), col1/CyO, twist-lacZ and col1; P(col5-cDNA)/CyO-TM6B, Tb (Crozatier, M.), dome-less-gal4 (PG14; Noselli, S.) and hth64-1/TM6B and UAS-hth (Salzberg, A.). The following stocks were obtained from the Bloomington Stock Centre: UAS-Antp; Df(2R)knSA3; Antp25 red1 e1/TM3, Sb; Antp17/TM3, Sb; UAS-2xEYFP; hs-gal4/CyO and w; P{tubP-gal80[ts]}10. In collier loss-of-function experiments, dome-less-gal4, UAS-2xEYFP (X chromosome) was placed in the background of heterozygotes carrying the col1 allele over the deficiency Df(2R)knSA3. The ZCL2897 intron trap line (X chromosome) was crossed into the background of col1 allele homozygotes. In overexpression studies, UAS-Antp was driven by Antp-gal4 and ZCL2897 was placed in this background to visualize the status of the medullary zone. Similarly, UAS-hth was driven by twist-GAL4 in hth overexpression studies. For Antp loss-of-function, a heteroallelic combination (Antp25/Antp17)22 that survives to the late third instar was used. For misexpression of UAS-Cicell, these flies were crossed to domeless-gal4; P{tubP-gal80[ts]}10, grown at 18 °C until hatching, and then transferred to 29 °C until dissection. Additionally, UAS-Cicell was crossed to hs-gal4, grown at 18 °C until mid-second instar, heat shocked at 37 °C for 2 h, and then returned to 18 °C until dissection. For hhts2 experiments, the full phenotype is observed when the larvae are shifted to the non-permissive temperature in the mid-second instar.
Immunohistochemistry
Embryos and lymph glands were stained as previously described5,7. The following antibodies were used: rabbit anti-Hth (Salzberg, A.), rabbit anti-Hh (Ingham, P.), mouse anti-Antp, mouse anti-Ptc and mouse anti-FasIII (Developmental Studies Hybridoma Bank), rat anti-ProPO antibody (Müller, H.), mouse anti-P1 (Ando, I.), rat anti-DE-cadherin (Hartenstein, V.) and 2A12 rat anti-Ci (Holmgren, R.). Samples were imaged using a BioRad Radiance 2000 confocal with LaserSharp 2000 acquisition software.
Imaging PSC
For imaging the PSC using membrane GFP (Antp-gal4, UAS-mCD8-GFP), lymph glands were dissected in ice-cold 1× PBS and fixed in 4% formaldehyde/1× PBS on ice for five minutes. Lymph glands were then placed on a slide in 1× PBS, a cover slip was placed and the tissue was immediately imaged using standard confocal microscopy techniques. For live imaging of the PSC, individual whole Antp-gal4, UAS-GFP larvae were washed in water, placed on a slide dorsal-side up in glycerol, and then a cover slip was placed with sufficient pressure to immobilize the larva long enough for the acquisition of confocal sections through the PSC.
Acknowledgments
We thank R. Cripps for drawing our attention to Antennapedia expression in the developing cardiogenic mesoderm. We thank M. Crozatier, M. Meister and A. Vincent for useful discussions. We thank all members of the Banerjee and Hartenstein laboratories for their helpful comments and suggestions. In particular, we thank S. A. Sinenko for assistance with immunohistochemistry and S.-H. Jung for her initial experiments on Hedgehog. We appreciate the help of all those who provided us with reagents: W. Gehring, S. M. Cohen, L. Cooley, K. Moses, M. Crozatier, S. Noselli, A. Salzberg, H. Müller, I. Ando, K. Basler and R. Holmgren. This work was supported by an NIH grant to U.B. and V.H., and by NIH training grant support to J.A.M.-A. and C.J.E.
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Rizki TM. The circulatory system and associated cells and tissues. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. Academic Press; London: 1978. pp. 397–452. [Google Scholar]
- 2.el Shatoury HH. The structure of the lymph gland of Drosophila larvae. Roux Arch EntwMech Organ. 1955;147:489–495. doi: 10.1007/BF00576000. [DOI] [PubMed] [Google Scholar]
- 3.Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 4.Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 5.Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nature Genet. 2004;36:1019–1023. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- 7.Lo PC, Skeath JB, Gajewski K, Schulz RA, Frasch M. Homeotic genes autonomously specify the anteroposterior subdivision of the Drosophila dorsal vessel into aorta and heart. Dev Biol. 2002;251:307–319. doi: 10.1006/dbio.2002.0839. [DOI] [PubMed] [Google Scholar]
- 8.Ryan KM, Hoshizaki DK, Cripps RM. Homeotic selector genes control the patterning of seven-up expressing cells in the Drosophila dorsal vessel. Mech Dev. 2005;122:1023–1033. doi: 10.1016/j.mod.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Crozatier M, Ubeda JM, Vincent A, Meister M. Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2004;8:E196. doi: 10.1371/journal.pbio.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casares F, Mann RS. The ground state of the ventral appendage in Drosophila. Science. 2001;293:1477–1480. doi: 10.1126/science.1062542. [DOI] [PubMed] [Google Scholar]
- 11.Hisa T, et al. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J. 2004;23:450–459. doi: 10.1038/sj.emboj.7600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azcoitia V, Aracil M, Martinez AC, Torres M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol. 2005;280:307–320. doi: 10.1016/j.ydbio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel CA, Jacobs Y, Cleary ML. HoxA9-mediated immortalization of myeloid progenitors requires functional interactions with TALE cofactors Pbx and Meis. Oncogene. 2000;19:608–616. doi: 10.1038/sj.onc.1203371. [DOI] [PubMed] [Google Scholar]
- 14.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 15.Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 16.Kimble J, Crittenden SL The C. elegans Research Community. Germline proliferation and its control. WormBook. 2005 August 15; doi: 10.1895/wormbook.1.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 18.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: lessons from the Drosophila germline. J Cell Sci. 2005;118:665–672. doi: 10.1242/jcs.01680. [DOI] [PubMed] [Google Scholar]
- 21.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott MK, Kauffman TC. The relationship between the functional complexity and the molecular organization of Antennapedia locus of Drosophila melanogaster. Genetics. 1986;114:919–942. doi: 10.1093/genetics/114.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 24.Chuang PT, Kornberg TB. On the range of hh signaling. Curr Opin Genet Dev. 2002;10:515–522. doi: 10.1016/s0959-437x(00)00121-0. [DOI] [PubMed] [Google Scholar]
- 25.Crittenden SL, Leonhard KA, Byrd DT, Kimble J. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol Biol Cell. 2006;17:3051–3061. doi: 10.1091/mbc.E06-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramovich C, Humphries RK. Hox regulation of normal and leukemic hematopoietic stem cells. Curr Opin Hematol. 2005;12:210–216. doi: 10.1097/01.moh.0000160737.52349.aa. [DOI] [PubMed] [Google Scholar]
- 27.Kieusseian A, et al. Expression of Pitx2 in stromal cells is required for normal hematopoiesis. Blood. 2006;107:492–500. doi: 10.1182/blood-2005-02-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Kalderon D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature. 2001;410:599–604. doi: 10.1038/35069099. [DOI] [PubMed] [Google Scholar]
- 29.Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Bhardwaj G, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nature Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]