Abstract
Glycerol kinase (GK) is an enzyme that catalyzes the formation of glycerol 3-phosphate from ATP and glycerol, the rate-limiting step in glycerol utilization. We analyzed the genome of the model organism Drosophila melanogaster and identified five GK orthologs, including two loci with sequence homology to the mammalian Xp21 GK protein. Using a combination of sequence analysis and evolutionary comparisons of orthologs between species, we characterized functional domains in the protein required for GK activity. Our findings include additional conserved domains that suggest novel nuclear and mitochondrial functions for glycerol kinase in apoptosis and transcriptional regulation. Investigation of GK function in Drosophila will inform us about the role of this enzyme in development and will provide us with a tool to examine genetic modifiers of human metabolic disorders.
Keywords: Drosophila melanogaster, Glycerol kinase, Mitochondria, Nucleus, Apoptosis
Introduction
Glycerol kinase (GK) is an enzyme that catalyzes the formation of glycerol 3-phosphate from ATP and glycerol, the rate-limiting step in glycerol utilization [1]. Dihydroxy-acetone and L-glyceraldehyde can also act as acceptors [2]. UTP, and, in the case of the yeast enzyme, ITP and GTP, can act as donors [2]. GK provides a way for glycerol derived from fats or glycerides to enter the glycolytic pathway (Fig. 1). The enzyme can undergo a reversible subunit dissociation between tetramer and dimer [3] in bacteria. GK requires magnesium as a cofactor and is regulated by fructose 1,6-bisphosphate [4].
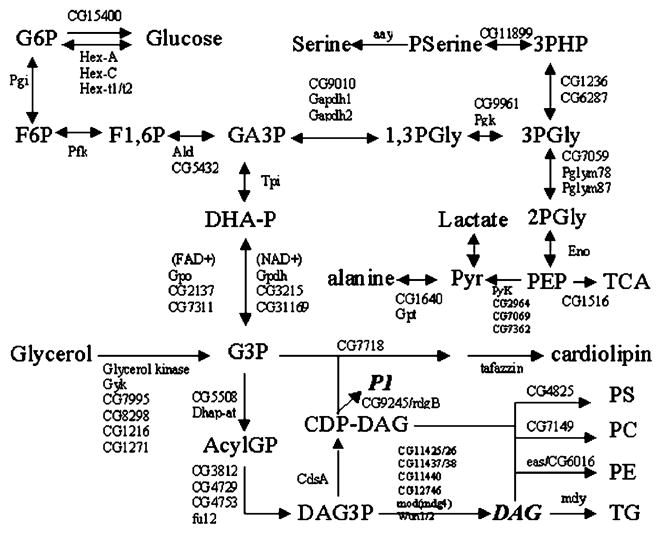
Fig. 1.
Glycerol metabolic pathways in D. melanogaster. Hex, hexokinase; Pgi, phosphoglucose isomerase; Pfk, phosphofructokinase; Ald, aldolase; Tpi, triosephosphate isomerase; Gpdh, NAD+ glycerol 3-phosphate dehydrogenase; Gpo, glycerophosphate oxidase/FAD glycerol 3-phosphate dehydrogenase; aay, astray; Gapdh, glyceraldehydes 3-phosphate dehydrogenase; Pgk, phosphoglycerate kinase; Pglym, phosphoglyceromutase; Eno, enolase; Pyk, pyruvate kinase; Gpt, Glutamate pyruvate transaminase; rdgB, retinal degeneration B; Dhapat, dihydroxyacetone phosphate acyltransferase; CdsA, CDP diglyceride synthetase; eas, easily shocked/ethanolamine kinase; mdy, midway/diacylglycerol O-acyltransferase; wun, wunen/phosphatidate phosphatase. Loci responsible for these activities in Drosophila are shown above and/or below the arrows denoting the reactions.
We have chosen to investigate GK because it is the rate-limiting enzyme and an obligatory step in glycerolipid production. As a consequence, the intermediates do not accumulate under standard conditions. It is known that decreased GK activity directly reduces levels of glycerolipids [5]. In mice, absence of glycerol 3-phosphate dehydrogenase leads to neonatal death [6]. GK knockout mice also die soon after birth [7]. However, the effects of reduction of glycerolipids and thus their role in biological processes are poorly understood.
In mammals, GK activity is most abundant in liver [1], although it is present in adipose tissue under conditions of fasting, obesity, and diabetes [8,9]. Glycerol kinase activity increases 10-fold in adipocytes that lack leptin receptor signaling [10]. Glycerol metabolism has been conserved and localized to the fat body in most insect species. In Drosophila, the fat body is the equivalent of the mammalian liver [11]. This structure expresses all of the enzymes required for carbohydrate and lipid metabolism [12]. Deletion of the fat body leads to death at 5 days of life and female sterility [13]. Its role in glycerol metabolism in the fruitfly is not well known. Incorporation of glycerol 3-phosphate into diacylglycerols and phosphoglycerides is higher in larval stages than adults [14]. GK expression parallels this developmental pattern. In the silkmoth, during the larval–pupal transformation, glycerol accumulation declines in the fat body at both larval and pupal stages in development while total GK activity increases [15]. In Drosophila, disruption of triacylglycerol production through mutations in diacylglycerol acyltransferase leads to death of oocyte nurse cells by apoptosis [16], suggesting that glycerol metabolism is essential for oogenesis. The locust has a complex that acylates phosphoglycerol into phosphatidate [17]. A variety of roles for glycerolipids in development is demonstrated by the differential entry of glycerol into triglycerides versus other glycerolipids between larval and adult stages [18].
Glycerol 3-phosphate is also a major metabolite for mitochondria in insect flight muscle [19]. GK activity has been demonstrated in the muscles of both vertebrates and invertebrates [20]. In the muscle of vertebrates, glycerol is incorporated into glycerolipids and used as energy fuel [21,22]. Flight in Drosophila requires active regulation of glycerolipid biosynthesis during activity [23]. Similarly, glycerol 3-phosphate dehydrogenase is also highly expressed in fat body and flight muscle [24]. All of these enzymes have been shown to colocalize in flight muscle [25]. Disruption of this colocalization leads to flightlessness [26].
Human GK deficiency (GKD, MIM 307030) is an X-linked disorder characterized by increased plasma concentration and urinary excretion of glycerol. It is recognized in two forms: complex GKD, the result of large deletions of multiple contiguous genes, and isolated GKD, resulting from point mutations and small intragenic deletions [1]. Patients who are true GK nulls resulting from gene deletion have facial dysmorphisms and mental retardation [27–32]. They also have seizures and developmental delay as well as hypotonia [30,32–35]. Additional anomalies include abnormal skeleton, spontaneous fractures, and premature loss of abnormal teeth [32,36]. Diabetes has been reported in these pedigrees [1,37,38]. In isolated forms of GKD, the phenotype may vary from a life-threatening childhood metabolic crisis with mental retardation and seizures, to asymptomatic ‘pseudohypertriglyceridemia’ from elevated glycerol levels [1,30]. This phenotypic heterogeneity is clearest within families, where identical mutations may have variable phenotypic manifestations in different family members.
As an initial approach to establishing Drosophila as a model organism for understanding the role of modifier genes on the phenotypic variability observed in individuals with hyperglycerolemia secondary to glycerol kinase deficiency, we have characterized glycerol kinase loci in Drosophila melanogaster.
Experimental procedures
Sequences for each organism were obtained from the following sources: the Unigene database (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=unigene), the Drosophila Genome Project’s release 3.2.0 annotation [111] (http://flybase.org/), the Saccharomyces Genome Database 11/26/2003 annotations (http://www.yeastgenome.org), WormBase gene dump 3/1/2004 (http://www.wormbase.org), and (http://www.genome.org). The ExPASy proteomics server (http://us.expasy.org/) was used for protein sequence analysis. PSORT II (http://www.psort.org/) was used to analyze for subcellular localization sequences. Phylogeny and alignment analyses were performed with the ClustalX [112] and MEGA 3.0 [113]. Pairwise multiple sequence alignment was performed using the Gonnet protein weight matrix. Evolutionary pairwise distances were estimated using a Poisson correction mode and a Grand Payhoff Matrix model with uniform substitution rates. Sequence analysis of the alignments was performed using the Sequencher program (version 4.0.5, Gene-Code, Madison, WI). In silico data mining was performed using the Gene expression omnibus (GEO) database at NCBI (http://www.ncbi.nlm.nih.gov/projects/geo/) as well as supplemental data files from published microarray studies as referenced. For the insect sequence alignments, the numbers after each species name correspond to the scaffold entry number in each organism’s database. For Anopheles gambiae, the annotated protein sequences obtained from http://www.anobase.org/ and ENSEMBL are: ENSANGP00000018137, ENSANGP00000020314, ENSANGP0000 0010758, and ENSANGP00000028995 (also known as ENSANGP00000 013591). For Apis mellifera, a BLAST search was performed using the honeybee proteome database (http://azra.embl-heidel-berg.de/~zdobnov/Bee2/) and the predicted proteins compared by BLAST (http://www.ensembl.org/Multi/blastview) to the annotated sequences at ENSEMBL: Amel 13011 (ENSAPMP00000022551 and ENSAPMP000 00015828), Amel 17342 (ENSAPM P00000004685), Amel 9845 (ENS-APMP0 0000020060, ENSAPMP0000000 3731, and ENS-APMP00000003729), and Amel 3700 (ENSAPMP00000 011946 and ENSAPMP00000011947). For the silkmoth Bombyx mori, predicted protein sequences were obtained by BLAST (http://software.genomics.org.cn/softenv/right/run/index.jsp?class = SilkwormBlast&out = 1): Scaffold 006247, Scaffold001823, Scaffold013907, and Scaffold004254/Scaffold001818. Beetle protein sequences were obtained by BLAST searches of the Tribolium castaneum contig database (http://www.hgsc.bcm.tmc.edu/blast/blast.cgi?organism=Tcastaneum): Contig1229, Contig1078, Contig2615, and Contig1405.
Results
Conserved family of GK genes in Drosophila
We have previously characterized the existence of a well-conserved family of GK genes [39]. Completion of the Drosophila genome sequences [40] has allowed us to identify GK loci in this organism suitable for genetic analysis. Our studies have shown that there are five GK-like genes in D. melanogaster (Fig. 2). One locus, termed Gyk (CG18374, located at 61B2), is the closest homolog to the X-linked GK gene in humans, sharing 53% identity over its entire 510-amino acid length. Another close homolog CG7995 (located at 62B1) shares 48% homology to human GK. In addition, three other loci CG1271 at 63A3, CG 1216 at 61B2, and CG8298 at 48D5 do not share the same degree of homology and contain transmembrane domains according to the Ward laboratory’s Drosophila Membrane Protein Library database (http://www.cbs.umn.edu/fly/). They are closely related to the mammalian glycerol kinase-like pseudogene retroposons, which contain transmembrane domains and lack GK activity [41]. Sequence alignment of all Drosophila GK-like loci with glycerol kinase-like loci from other insect species further confirms the presence of five ancestrally well-conserved loci in this phylum (Fig. 3).
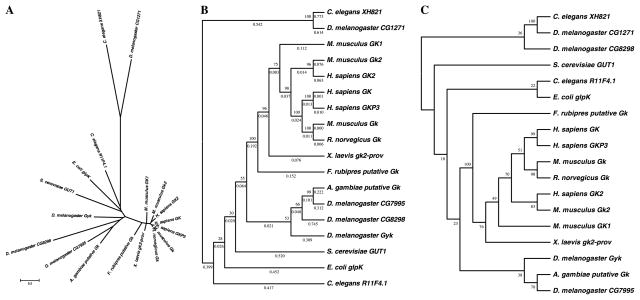
Fig. 2.
Phylogenetic relationship of glycerol kinase (GK) proteins from D. melanogaster and Anopheles to other species. (A) Unrooted protein phylogeny radiation tree for GK proteins. Branch length denotes pairwise estimated evolutionary distances according to scale bar. (B) Unrooted consensus phylogeny of GK proteins showing tree topology not drawn to scale. Data were analyzed using the Neighbor-Joining method. Bootstrap percentage values are located at each node. Numbers below each branch correspond to pairwise estimated evolutionary distance values. (C) Unrooted consensus maximum parsimony tree. Bootstrap percentages are located at each node.
Fig. 3.
Alignment of insect glycerol kinase protein sequences across insect species.
Using the latest version of the Unigene database, we confirmed the presence of five GK-like loci in vertebrates. The gene encoding human GK has been cloned and is comprised of 21 exons that map to chromosome Xp21.3 [42,43]. It was initially suggested that there were six genomic loci in humans with sequence homology to GK [43]. In addition to the X-linked locus at Xp21.3, a single exon non-coding pseudogene was also located at Xq22.1 (GK pseudogene 6, GKP6). Additional chromosome loci at 4q13 (GK2/GKP2) and 4q32.1 (GK pseudogene 3) were identified as intronless genes that encoded two different testis- and brain-specific transcripts that lacked GK activity [41]. The GK2 locus at 4q13 predicted a 553-amino acid protein with a molecular weight of 60.6 kDa.
We have identified an additional locus at 4q26 (LOC201989) that is exclusively expressed in small intestine (Unigene). Initially described as the pseudogene at 1q41 (GK pseudogene 1) [43], it shares 95% homology to the Xp21.3 and the 4q GK loci. A search of the Unigene database did not identify loci for the pseudogenes previously described at chromosome loci 7p (GK pseudogene 4) or 10p (GK pseudogene 5). These have been removed or renamed as “regions” by the HUGO Gene Nomenclature Committee due to lack of sequence data [44]. Another locus at 3q23 (MGC40579) is more widely expressed (Unigene) and only shares 35% homology with GKs. Therefore in humans, based on the sequence data available at this time, there are five different loci identified with homology to GK, similar to the number identified in D. melanogaster.
Glycerol metabolic pathways in Drosophila
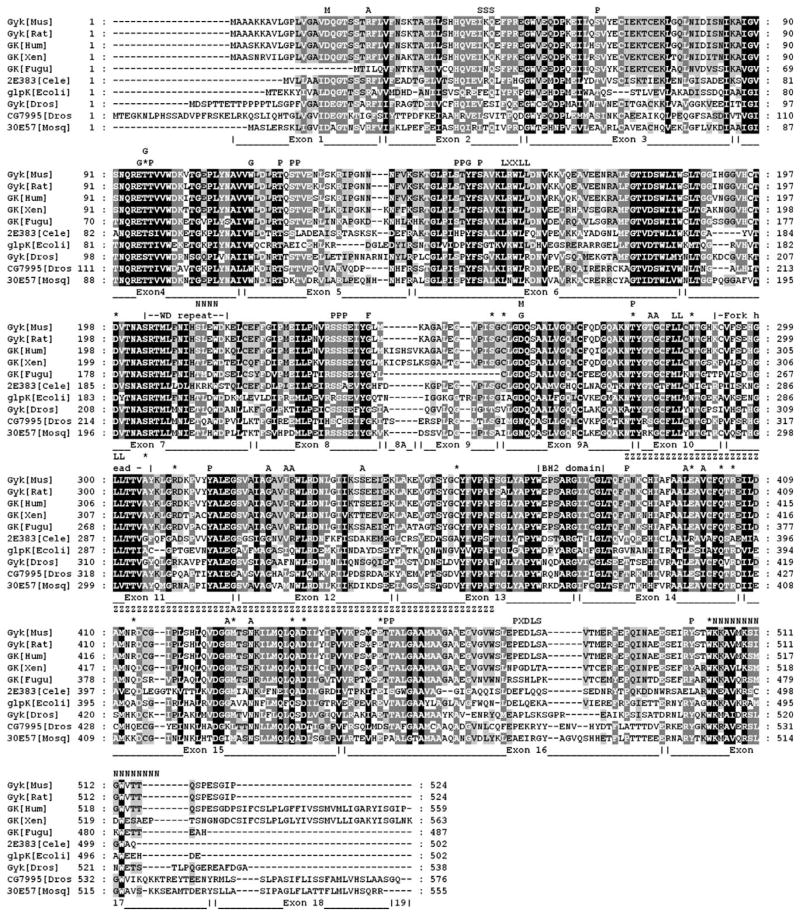
We next determined the suitability of Drosophila as a model for glycerol metabolism studies by identifying the enzymatic pathway components required for glycolysis and glycerolipid biogenesis, and comparing them to their human homologs. A search of the Flybase database identified multiple members of each of the enzymes involved in glycerol metabolism. Fig. 1 shows a schematic representation of the conserved metabolic networks present in Drosophila. Next, we performed a phylogenetic analysis of the Anopheles and Drosophila glycerol kinase-like genes and compared them to those of other species (Fig. 2). Bootstrap analysis identified a clade for the mosquito and Drosophila GK orthologs at a value of 55–99%. Alignment of human, Escherichia coli, Saccharomyces cerevisiae, mouse, Caenorhabditis elegans, and Drosophila glycerol kinase protein sequences confirmed that all key functional residues for ADP and glycerol binding are conserved in CG7995 and Gyk, but not in the remaining three Drosophila GK loci (Fig. 4).
Fig. 4.
Alignment of glycerol kinase protein sequences across species. Limits of each human exon are labeled below the aligned sequences. F, fructose 1,-bisphosphate binding site; P, phosphorylation site; G, glycerol binding residue; *, residue mutated in patients with GKD; M, magnesium binding residue; A, ATP binding residue; SSS, SUMOylation sequence; LXXLL, coactivator interaction domain; LL, dileucine repeats; N, nuclear localization sequence; PXDLS, CtBP binding site. The protein residues encoded by each exon are labeled below the sequences.
Conserved functional domains in Drosophila GK proteins
Protein sequence analysis identified potential protein interaction and phosphorylation modification sites that may regulate GK function. We performed a scan for protein motifs and identified multiple potential phosphorylation sites (Fig. 4). Those marked with a ‘P’ are potential phosphorylation sites recognized by protein kinase C (PKC) and casein kinase II (CKII). Some of these residues fall within regions that carry missense mutations in patients with GKD, identified in Fig. 4 by an asterisk. Two prominent and well-conserved examples are the missense mutations W503K and T278M. The tryptophan at position 503 is adjacent to the potential nuclear localization sequence (NLS) present in GK. This bipartite NLS is located in the C-terminus of CG7995 (Fig. 4). The C. elegans GK homolog R11F4.1 also has the NLS HKRK at residues 198–201. The threonine at position 278 of human GK is part of a well-conserved PKC phosphorylation site at residues 296–298 in CG7995. This residue is conserved across species and mutated in GKD patients with the missense mutation T278M [30].
Many dileucine motifs important for protein–protein interactions and organelle targeting are present (‘LL,’ Fig. 4). Human GK also contains a domain between residues 416 and 490 that has 50% homology to zinc-finger domains. In E. coli, GK forms a stable multi-unit complex, GK exists at physiological concentration in an equilibrium between functional dimers and tetramers [45]. Human GK shares a subunit interface interaction region with E. coli GK. This region, when mutated, increases the thermal stability of the enzyme in Flavobacterium meningosepticum [46]. A PEST sequence in the amino terminus of Gyk shares 88% homology with the same conserved sequence in exon 1 of human GK. Also labeled is a conserved potential site for modification by SUMO-1 (‘SSS,’ Fig. 4). These domains suggest a role for proteolysis in GK activity regulation. Conserved residues required for ATP, fructose 1,6-bisphosphate, and glycerol binding are marked A, F, and G, respectively (Fig. 4). A key glycine residue required for glucose-mediated regulation of GK activity in E. coli located at position 304 (G326 in human GK) [47] is conserved among all species. We identified the presence of an LXXLL motif at residues 154–158 (‘LXXLL,’ Fig. 4), involved in the interaction of many transcriptional coactivators with liganded nuclear hormone receptors, as well as a CtBP binding motif (‘PXDLS,’ Fig. 4) at the protein C-terminus. This latter domain is present and exclusively conserved in mammalian species but absent in other vertebrates and invertebrates (Fig. 4). Both the N- and the C-terminal domains of the FGGY family of carbohydrate kinases adopt a ribonuclease H-like fold that are structurally related to each other [48]. This domain is present in protein subunits of nuclear chromatin remodeling complexes [48]. The presence and evolutionary conservation of these protein domains suggests a potential role for GK in nuclear transcriptional regulation and chromatin remodeling.
Sequence analysis performed using the ExPASy protein sequence analysis website identified several domains associated with a role in mitochondrial apoptosis. A conserved sequence in the C-terminus contains a transmembrane hydrophobic domain homologous to that present in the pro-apoptotic protein Bax (Fig. 5) [49]. This domain mediates the translocation of Bax to the mitochondrial membrane in response to cell death signals and is required for its pro-apoptotic function [50]. Using the PSORTII software, we confirmed the likelihood of human GK localization to mitochondria. This is based on the amino acid composition of an alternatively spliced exon 18 that codes for the Bax-like hydrophobic transmembrane domain [51]. This domain is well conserved across species and is present in CG7995 (Fig. 5). Exon 18 is a tissue-specific, differentially spliced exon present in human, mouse, rat, zebrafish, and Drosophila. Sequences similar to human exon 18 are not present in glycerol kinase loci from C. elegans, E. coli, yeast, or plants. Alternative splicing of exon 18 produces an isoform initially described to be a brain-specific form [52], but which is expressed more widely [53]. This sequence targets GK to mitochondria [51]. CG7995 is the only GK ortholog that contains a sequence with similarity to human GK exon 18, while Gyk lacks this domain. It appears that while in Drosophila independent functions were segregated in these two genes, evolution may have merged them in vertebrates where they are differentially regulated at the splicing level. Other Drosophila proteins that share homology with this domain include the monoamine oxidase CG10561, the LDL receptor LpR1/CG31094, and CG5195. Exon 8A is also differentially spliced, and is present in Xenopus and isoforms expressed in testes and fetal liver [54]. Sequences with similarity to human GK exon 8A are not present in Drosophila GK genes.
Fig. 5.
Alignment of human glycerol kinase exon 18 to homologous sequences in other species. Gk2-prov (Xenopus GK2 homolog), 30E57 (Anopheles GK homolog).
In silico data mining for Drosophila GK-like loci expression
Using an in silico approach, we next pursued data mining analysis of previously unidentified gene expression data available for each of the Drosophila GK-like loci from large-scale microarray studies. These data include the developmental and tissue expression patterns of each of the Drosophila GK loci (Tables 1 and 2). These data have been deposited but not previously identified or analyzed for their relevance to GK function in Drosophila.
Table 1.
Expression patterns of Drosophila glycerol kinase-like genes
| Eye disc | Leg disc | Epidermis | Salivary gland | midgut | Head | Wing disc | Testis | Ovary | Brain 8.5 h embryo | Kc167 | SL2 cells | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CG7995 | ––– | ++ | +++ | + | + | + | + | + | ––– | + | + | + |
| CG18374 (Gyk) | ++ | ++ | ––– | ––– | + (EcR dependent) | + | ––– | + | ––– | ––– | + | + |
| CG1216 | ++ | +++ | ––– | + | ––– | ––– | ––– | + | + | + | + | + |
| CG1271 | ––– | ––– | + | + | +++ (up in metamo) | + | ––– | + | + | ––– | + | + |
| CG8298 | ++ | ++ | ––– | ––– | ––– | + | ––– | + | ––– | ––– | ––– | ––– |
| Ref. | 1 | 1 | 2 | 2, 3 | 2 | 2 | 2 | 4 | 4 | 5 | 4 | 4 |
L. Michaut, S. Flister, M. Neeb, K.P. White, U. Certa, W.J. Gehring, Analysis of the eye developmental pathway in Drosophila using DNA microarrays. Proc. Natl. Acad. Sci. USA 100 (7) (2003) 4024–4029.
T.R. Li, K.P. White, Tissue-specific gene expression and ecdysone-regulated genomic networks in Drosophila. Dev Cell 5 (1) (2003) 59–72.
Unigene database.
GEO, gene expression omnibus.
T. Brody, C. Stivers, J. Nagle, W.F. Odenwald, Identification of novel Drosophila neural precursor genes using a differential embryonic head cDNA screen. Mech Dev. 113 (1) (2002) 41–59.
Table 2.
Developmental pattern of expression
| Embryo | Larval–early pupal | Schneider L2 cells | Adult ovary | Adult testes | Adult head | |
|---|---|---|---|---|---|---|
| CG7995 | + | + | + | – | + | + |
| CG18374 (Gyk) | + | + | + | – | + | + |
| CG1216 | + | + | – | + | + | + |
| CG1271 | – | – | – | – | + | + |
| CG8298 | + | – | – | – | + | + |
| Ref. | 1,2 | 2 | 2 | 1 | 1,2 | 1,2 |
Unigene database.
BDGP database: M. Stapleton, G. Liao, P. Brokstein, L. Hong, P. Carninci, T. Shiraki, Y. Hayashizaki, M. Champe, J. Pacleb, K. Wan, C. Yu, J. Carlson, R. George, S. Celniker, G.M. Rubin, The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes, Genome Res. 12 (8) (2002) 1294–1300.
Gyk is expressed in testis, head, embryo, and larva, as well as the cell line Schneider S2. Based on microarray data across developmental stages, peak expression is in the early larval stage. CG7995 is present in head, embryo, larva, and Schneider S2 cells, but the very early embryo is the stage with peak expression. While CG1271 appears to be widely expressed, it is present in testis, demonstrating a conserved evolutionary function with its mammalian orthologs, which are primarily expressed in testis. CG8298 is expressed in the testis as well as the larval brain and imaginal discs. CG1216 shares little homology at the sequence level with GKs, except in a potassium channel tetramerization domain. However, an overlapping transcription unit exists where the 3′ end of CG1216 shares antisense sequences with the Gyk 3′ end sense transcript. This type of transcript overlap has been previously described for Cs/CG10561 and dopa decarboxylase/ddc loci in Drosophila [55]. CG10561 contains a conserved monoamine oxidase domain associated with dopamine breakdown. As part of the dopa decarboxylase complex, CG10561 is coregulated with other enzymes involved in dopamine synthesis and breakdown. Antisense transcripts have also been described for the human/mouse GK locus in mammals [56,57]. This overlapping transcript relationship potentially links CG1216 with Gyk activity, either as an antisense transcript or as a member of a shared pathway. Most key functional residues are missing in CG1216 suggesting it has no GK activity.
Discussion
Using an in silico approach, our analysis demonstrates the conservation of sequence and presence of glycerol kinase loci in D. melanogaster. The utility of this analysis is best supported by the finding of multiple conserved protein domains that suggest novel functions for this family of proteins, including roles in nuclear and mitochondrial activities. These functions appear to have been preserved across evolution.
GK catalyzes the ATP-dependent reaction from glycerol to glycerol 3-phosphate, a key link between carbohydrate and lipid metabolism [1]. GK is expressed at low levels in every human tissue but expressed at higher levels in liver, kidney, lymphocytes, testis, ovary, and lung [1] (Unigene). GKP2 on 4q13 is testis-specific [58]. GK is primarily expressed in hepatocytes, where it is induced by diabetes and fasting [9], not affected by glucagon, dexamethasone or cAMP [59], but increased by 20% linoleic acid and regulated by glycerol [60]. GK is also present in small intestine [61], and in kidney and pneumocytes its activity is increased by diabetes and normalized by insulin [62]. In adipose tissue there normally are low levels, but these increase in obesity [8] and are regulated by sympathetic innervation [63]. According to the Unigene database, GK transcripts have also been detected in isolated pancreatic beta cells, developing neuroectoderm and brain hippocampus, white blood cells, thymus, fetal heart, uterus, testis, stomach, ovary, skeletal muscle, and breast. In the developing mouse cerebellum, levels increase postnatally and peak at P14 [64]. GK is also selectively expressed in a number of cancers, including colon and squamous cell carcinoma [65].
Products of glycerolipid and sphingolipid metabolism are now known to fulfill second messenger functions in a variety of cellular signaling pathways [66]. The role of diacylglycerol in the regulation of protein kinase C (PKC) activity and its site of interaction with PKC are now well known. Recently, another glycerolipid second messenger, phosphatidic acid, was found to interact with the protooncogenic Raf-1 kinase. In cultured cells, a signal-induced generation of phosphatidic acid was critical for Raf-1 translocation to the cell membrane. Thus, different glycerolipid second messengers appear to regulate distinct targets with exquisite specificity [66]. Although the role of individual glycerolipids has been studied extensively, the relative contributions of their numerous potential downstream effectors remain uncertain.
Lysophosphatidic acid (LPA) and phosphatidic acid (PA) can act to promote cell survival, proliferation, and migration. Phosphatidic acid has been shown to affect migration of neutrophils [67], while lysophosphatidic acid stimulates migration of hematopoetic cells [68], induces neuronal shape changes, and leads to cell proliferation [69]. Mouse lipid phosphate phosphohydrolase (LPP3) affects development [70]. A Drosophila homolog of this gene, wunen (wun), is important for guiding migrating germ cells [71] and is implicated in axon guidance [72]. Other enzymes such as rdgB are expressed in the developing nervous system, suggesting a role in its development [73]. This raises the possibility that phospholipids act as signals to guide cells in Drosophila. In Neurospora crassa glycerol kinase is induced by cold temperature [74]. Glycerol kinase is also upregulated 8-fold during the stress response to dehydration in the nematode Steinerenema feltiae [75].
The role of glycerol in cell survival has recently emerged with convincing evidence of its central role. Mice lacking the mitochondrial G3PDH have decreased survival, secondary to increased levels of cytoplasmic G3PDH compensatory activity [6]. In plants, GK is required for resistance against infection [76]. Flies with prolonged life span also have increased resistance to stress, and in particular, they survive extreme cold environments by increasing their levels of glycerol [77]. The role of GK in survival is further supported by its specific regulation during longevity in dauer larvae from C. elegans [78]. In Drosophila, elevation of diacylglycerol levels leads to increased cell death and shorter lifespan [79]. Mutations in midway, a DGAT involved in diacylglycerol removal into triglycerides, lead to cell death most likely from elevated DAG levels [16]. In photoreceptor cells, deficiency of phosphatidic acid leads to cell death and degeneration [80]. In E. coli mutants, overexpression of GK activity leads to growth arrest and cell death. This is independent of glycerol 3-phosphate levels and depends on metabolites downstream of this metabolite [81].
Drosophila melanogaster is a useful model system for identifying second site modifier genes that genetically interact with the GK locus and their role in biological network regulation. Modifier screens in D. melanogaster have been invaluable for elucidating signal transduction pathways involved in human disease [82]. In Drosophila, 87% of genes known to be involved in human mental retardation are conserved [83]. Drosophila GK loci may be useful to develop model systems for investigations of human GK-related syndromes.
The realization of novel functions for GK from sequence analysis suggests experiments to investigate potential roles for members of this protein family in transcriptional regulation and apoptosis. ASTP or the ATP-stimulated glucocorticoid receptor translocation promoter is a cytosolic protein activity that enhances nuclear uptake of the activated glucocorticoid–glucocorticoid receptor complex (G-GRC) in the presence of ATP and is involved in binding to chromatin [84], where it interacts with the argininerich histones H3 and H4, with preference for H4, via lysine residues [85]. Purification and peptide sequencing identified ASTP as identical to GK [86]. The ability of GK to facilitate glucocorticoid receptor nuclear translocation decreases with aging [87]. While mouse GK knockouts have autonomous glucocorticoid secretion and no resistance to its action [7], the role of GK in glucocorticoid receptor function or the roles of its chromatin interaction are still poorly understood. We have confirmed the nuclear translocation of GK in response to dexamethasone [88]. The glucocorticoid receptor, as a member of the nuclear receptor family of transcription regulators, contains a highly conserved, N-terminal zinc-finger domain that mediates specific binding to target DNA sequences. GK contains a domain that has homology to zinc-finger domains. Many of the missense mutations identified in patients with isolated GKD cluster within this domain. We have also identified an LXXLL domain in GK. This domain is present in coactivators of nuclear receptors. GK also contains a putative bipartite nuclear localization sequence at its C-terminus. The lack of LXXLL motifs in Drosophila and other invertebrates correlates with the absence of most nuclear receptors in these species. However, it is most intriguing that a phosphorylation residue adjacent to this motif (Serine 150) and which mediates receptor subtype specificity for nuclear receptor coactivators [89] has indeed been conserved. In addition, the T278 residue mutated in patients with GKD is part of a consensus site for PKC. PKC potentiates glucocorticoid receptor activity via phosphorylation of GK/ASTP [90]. ASTP is composed of two apparently identical subunits with molecular weights of 48,000 [91]. An additional minor band of about 50 kDa is also observed, consistent with GK being phosphorylated [92]. It remains to be determined if this ASTP function has been conserved in invertebrates as well.
Mitochondrial localization of GK has been previously described in response to apoptotic signals [93,94]. GK activity has been described in brain, particularly associated with mitochondria [1,95,96]. GK is associated with non-synaptic mitochondria [97], and appears to be present in neurons [98], primarily in GABAergic neurons of the cortex and in cerebellar granule cells [99]. In the rat brain, the binding of GK to mitochondria is dependent on pro-apoptotic signals including divalent cations and glucose 6-phosphate, while glycerol 3-phosphate and ATP reduce binding [100]. Blockers of mitochondrial functions, such as oligomycin, dinitrophenol, cyanide, and atractyloside, all inhibit GK activity [100]. Our observations suggest a dynamic subcellular localization for GK. Exon 18 codes for a conserved hydrophobic domain present in many other mitochondrial proteins, including Bax and Bcl-2 [101]. Mitochondrial localization of GK has previously been demonstrated in our laboratory, where it associates in response to calcium-induced cell death [93]. In addition, yeast GK localizes to mitochondria [102]. This evidence, as well as preliminary data in Drosophila showing modifiers of GK phenotypes to be involved in apoptosis [103] suggests GK to be a likely candidate in cell death processes.
The GK-like proteins CG1271, CG8298, and CG1216 do not have any glycerol kinase activity [41]. However, there have been suggestions of function. These proteins interact with the serotonin reuptake transporter, but do not affect its activity [104]. CG1216 contains a K+ channel tetramerization domain/BTB/POZ domain found in GMRP-1, C. elegans R05F9.2, and yeast SRP40, and conserved in Anopheles gambiae protein EAA04715.1. This domain is present in many other classes of proteins, mostly K channels. There are several potential transmembrane domains present (Fig. 6) (http://www.cbs.umn.edu/fly/). The POZ/BTB domain involved in protein interactions is present in zinc-finger proteins [105], and the K+ receptor interaction may be a cell-specific action of apoptosis [106]. Along with a role in neuronal function, a screen for olfactory mutants defective in an avoidance response to benzaldehyde identified a P-element insertion line smi61A in the region of the CG1216/Gyk locus [107]. This mutant interacts with a P-element insertion smi60E in the gene DSC1, a sodium channel [108,109]. In addition, CG1216 is expressed in the fly brain [110].
Fig. 6.
Alignment of divergent glycerol kinase protein sequences. TM, transmembrane domain.
We have shown the presence of previously unidentified protein domains in glycerol kinase and their conservation across species. Their evolutionary preservation is suggestive of functional significance. Our observations open up new avenues for investigation of novel functions and roles for glycerolipid metabolism and GK activity in transcriptional regulation and apoptosis. We have also shown the presence and conservation of GK loci between Drosophila and humans. The presence of conserved protein domains not only provides insights into potential new functions for this family of proteins, but supports the utility of this model organism for in vivo genetic studies of GK function. Ongoing studies are defining the role of these novel potential functions in GK activity and their relationship to phenotypic variability in disease.
Acknowledgments
We thank the McCabe laboratory members past and present, George Jackson, Larry Zipursky, and Utpal Banerjee for all their support and helpful comments. This work was performed as part of the Intercampus Medical Genetics Training Program at UCLA and supported by NIH Training Program Grant T32GM08243 (J.A.M.) and R01HD022563 (E.R.B.M.).
References
- 1.McCabe ERB. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 2001. Disorders of glycerol metabolism. [Google Scholar]
- 2.Hayashi SI, Lin EC. Purification and properties of glycerol kinase from Escherichia coli. J Biol Chem. 1967;242:1030–1035. [PubMed] [Google Scholar]
- 3.de Riel JK, Paulus H. Subunit dissociation in the allosteric regulation of glycerol kinase from Escherichia coli. 1. Kinetic evidence. Biochemistry. 1978;17:5134–5140. doi: 10.1021/bi00617a010. [DOI] [PubMed] [Google Scholar]
- 4.Yu P, Pettigrew DW. Linkage between fructose 1,6-bisphosphate binding and the dimer–tetramer equilibrium of Escherichia coli glycerol kinase: critical behavior arising from change of ligand stoichiometry. Biochemistry. 2003;42:4243–4252. doi: 10.1021/bi027142l. [DOI] [PubMed] [Google Scholar]
- 5.Lamb RG, Bow SJ, Wright TO. Effects of chronic insulin and glucagon exposure on the biosynthesis of glycerolipids by cultured hepatocytes. J Biol Chem. 1982;257:15022–15025. [PubMed] [Google Scholar]
- 6.Brown LJ, Koza RA, Marshall L, Kozac LP, MacDonald MJ. Lethal hypoglycemic ketosis and glyceroluria in mice lacking both the mitochondrial and the cytosolic glycerol phosphate dehydrogenases. J Biol Chem. 2002;36:32899–32904. doi: 10.1074/jbc.M202409200. [DOI] [PubMed] [Google Scholar]
- 7.Huq AH, Lovell RS, Ou CN, Beaudet AL, Craigen WJ. X-linked glycerol kinase deficiency in the mouse leads to growth retardation, altered fat metabolism, autonomous glucocorticoid secretion and neonatal death. Hum Mol Genet. 1997;6:1803–1809. doi: 10.1093/hmg/6.11.1803. [DOI] [PubMed] [Google Scholar]
- 8.Thenen SW, Mayer J. Adipose tissue glycerokinase activity in genetic and acquired obesity in rats and mice. Proc Soc Exp Biol Med. 1975;148:953–957. doi: 10.3181/00379727-148-38667. [DOI] [PubMed] [Google Scholar]
- 9.Kondoh Y, Kawase M, Hirata M, Ohmori S. Carbon sources for D-lactate formation in rat liver. J Biochem. 1994;115:590–595. doi: 10.1093/oxfordjournals.jbchem.a124380. [DOI] [PubMed] [Google Scholar]
- 10.Huan JN, Li J, Han Y, Chen K, Wu N, Zhao AZ. Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J Biol Chem. 2003;278:45638–45650. doi: 10.1074/jbc.M304165200. [DOI] [PubMed] [Google Scholar]
- 11.Sondergaard L. Homology between the mammalian liver and the Drosophila fat body. Trends Genet. 1993;9:193. doi: 10.1016/0168-9525(93)90113-v. [DOI] [PubMed] [Google Scholar]
- 12.Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annu Rev Nutr. 2001;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Municio AM, Odriozola JM, Perez-Albarsanz MA. Biochemistry of development in insects. Incorporation of fatty acids into different lipid classes. Eur J Biochem. 1975;60:123–128. doi: 10.1111/j.1432-1033.1975.tb20983.x. [DOI] [PubMed] [Google Scholar]
- 15.Jungreis AM, Dailey JC, Hereth ML. Alpha-Glycerol phosphatase and glycerol kinase activities in tissues of the silkmoth Hyalophora cecropia during the larval–pupal transformation. Am J Physiol. 1975;229:1448–1454. doi: 10.1152/ajplegacy.1975.229.5.1448. [DOI] [PubMed] [Google Scholar]
- 16.Buszczak M, Lu X, Segraves WA, Chang TY, Cooley L. Mutations in the midway gene disrupt a Drosophila acyl coenzyme A: diacylglycerol acyltransferase. Genetics. 2002;160:1511–1518. doi: 10.1093/genetics/160.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchand C, Lemonde A, Beaudoin AR. Isolation and properties of a glycerophosphate acylating fraction in the fat body of Schistocerca gregaria (Forskal) Can J Biochem. 1977;55:1166–1170. doi: 10.1139/o77-174. [DOI] [PubMed] [Google Scholar]
- 18.Megias A, Municio AM, Perez-Albarsanz MA. Biochemistry of development in insects. Triacyglycerol and phosphoglyceride biosynthesis by subcellular fractions. Eur J Biochem. 1997;72:9–16. doi: 10.1111/j.1432-1033.1977.tb11218.x. [DOI] [PubMed] [Google Scholar]
- 19.Hansford RG, Johnson RN. The nature and control of the tricarboxylate cycle in beetle flight muscle. Biochem J. 1975;148:389–401. doi: 10.1042/bj1480389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newsholme EA, Taylor K. Glycerol kinase activities in muscles from vertebrates and invertebrates. Biochem J. 1969;112:465–474. doi: 10.1042/bj1120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montell E, Lerin C, Newgard CB, Gomez-Foix AM. Effects of modulation of glycerol kinase expression on lipid and carbohydrate metabolism in human muscle cells. J Biol Chem. 2002;277:2682–2686. doi: 10.1074/jbc.M107227200. [DOI] [PubMed] [Google Scholar]
- 22.Guo Z, Jensen MD. Blood glycerol is an important precursor for intramuscular triacylglycerol synthesis. J Biol Chem. 1999;274:23702–23706. doi: 10.1074/jbc.274.34.23702. [DOI] [PubMed] [Google Scholar]
- 23.Strunecka A, Sula J, Myskova H, Kubista V. The influence of flight on the phospholipid metabolism in insect flight muscle. Physiol Bohemoslov. 1981;30:411–416. [PubMed] [Google Scholar]
- 24.Sullivan DT, Donovan FA, Skuse G. Developmental regulation of glycerol-3-phosphate dehydrogenase synthesis in Drosophila. Biochem Genet. 1983;21:49–62. doi: 10.1007/BF02395391. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan DT, MacIntyre R, Fuda N, Fiori J, Barrilla J, Ramizel L. Analysis of glycolytic enzyme co-localization in Drosophila flight muscle. J Exp Biol. 2003;206:2031–2038. doi: 10.1242/jeb.00367. [DOI] [PubMed] [Google Scholar]
- 26.Wojtas K, Slepecky N, von Kalm L, Sullivan D. Flight muscle function in Drosophila requires colocalization of glycolytic enzymes. Mol Biol Cell. 1997;8:1665–1675. doi: 10.1091/mbc.8.9.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheuerle A, Greenberg F, McCabe ER. Dysmorphic features in patients with complex glycerol kinase deficiency. J Pediatr. 1995;126:764–767. doi: 10.1016/s0022-3476(95)70409-4. [DOI] [PubMed] [Google Scholar]
- 28.Hellerud C, et al. Clinical heterogeneity and molecular findings in five Polish patients with glycerol kinase deficiency: investigation of two splice site mutations with computerized splice junction analysis and Xp21 gene-specific mRNA analysis. Mol Genet Metab. 2003;79:149–159. doi: 10.1016/s1096-7192(03)00094-5. [DOI] [PubMed] [Google Scholar]
- 29.Sjarif DR, Revesz T, de Koning TJ, Duran M, Beemer FA, Poll-The BT. Isolated glycerol kinase deficiency and Fanconi anemia. Am J Med Genet. 2001;99:159–160. doi: 10.1002/1096-8628(2000)9999:999<00::aid-ajmg1137>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Dipple KM, Zhang YH, Huang BL, McCabe LL, Dallongeville J, Inokuchi T, Kimura M, Marx JH, Roederer GO, Shih V, Yamaguchi S, Yoshida I, McCabe ERB. Glycerol kinase deficiency: evidence for complexity in a single gene disorder. Hum Genet. 2001;109:55–62. doi: 10.1007/s004390100545. [DOI] [PubMed] [Google Scholar]
- 31.Zhang YH, Huang BL, Niakan KK, McCabe ER, Dipple KM. IL1RAPL1 is associated with mental retardation in patients with complex glycerol kinase deficiency who have deletions extending telomeric of DAX1. Hum Mutat. 2004;24:273. doi: 10.1002/humu.9269. [DOI] [PubMed] [Google Scholar]
- 32.Guggenheim MA, McCabe ERB, Roig M, Goodman SI, Lum GM, Bullen WW, Ringel SP. Glycerol kinase deficiency with neuromuscular, skeletal, and adrenal abnormalities. Ann Neurol. 1980;7:441–449. doi: 10.1002/ana.410070509. [DOI] [PubMed] [Google Scholar]
- 33.Sjarif DR, Dorland L, Sperl W, de Koning TJ, Beemer FA, Poll-The BT, Duran M. Hyperketonaemia in glycerol kinase deficiency. J Inherit Metab Dis. 2000;23:760–764. doi: 10.1023/a:1005680211483. [DOI] [PubMed] [Google Scholar]
- 34.Walker AP, Muscatelli F, Stafford AN, Chelly J, Dahl N, Blomquist HK, Delanghe J, Willems PJ, Stienman B, Monaco AP. Mutations and phenotype in isolated glycerol kinase deficiency. Am J Hum Genet. 1996;58:1205–1211. [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis B, Harbord M, Keenan R, Carey W, Harrison R, Robertson E. Isolated glycerol kinase deficiency in a neonate. J Child Neurol. 1994;9:70–73. doi: 10.1177/088307389400900118. [DOI] [PubMed] [Google Scholar]
- 36.Blomquist HK, Dahl N, Gustafsson L, Hellerud C, Holme E, Homgren G, Matsson L, von Zweigbergk M. Glycerol kinase deficiency in two brothers with and without clinical manifestations. Clin Genet. 1996;50:375–379. doi: 10.1111/j.1399-0004.1996.tb02391.x. [DOI] [PubMed] [Google Scholar]
- 37.Gaudet D, Arsenault S, Perusse L, Vohl MC, St-Pierre J, Bergeron J, Despres JP, Dewar K, Daley MJ, Hudson T, Rioux JD. Glycerol as a correlate of impaired glucose tolerance: dissection of a complex system by use of a simple genetic trait. Am J Hum Genet. 2000;66:1558–1568. doi: 10.1086/302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose CI, Haines DS. Familial hyperglycerolemia. J Clin Invest. 1978;61:163–170. doi: 10.1172/JCI108914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worley KC, King KY, Chua S, McCabe ER, Smith RF. Identification of new members of a carbohydrate kinase-encoding gene family. J Comput Biol. 1995;2:451–458. doi: 10.1089/cmb.1995.2.451. [DOI] [PubMed] [Google Scholar]
- 40.Celniker SE, Wheeler DA, Dronmiller B, Carlson JW, Halpern A, Patel S, Adams M, Champe M, Dugan SP, Frise E, Hodgson A, George RA, Hoskins RA, Laverty T, Muzny DM, Nelson CR, Pacleb JM, Park S, Pfeiffer BD, Richards S, Sodergren EJ, Svirskas R, Tabor PE, Wan K, Stapleton M, Sutton GG, Venter C, Weinstock G, Scherer SE, Myers EW, Gibbs RA, Rubin GM. Finishing a whole-genome shotgun: release 3 of the Drosophila melanogaster euchromatic genome sequence. Genome Biol. 2002;3:RESEARCH0079–RESEARCH0090. doi: 10.1186/gb-2002-3-12-research0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan Y, Decker WK, Huq AHHM, Craigen WJ. Retrotransposition of glycerol kinase-related genes from the X chromosome to autosomes: functional and evolutionary aspects. Genomics. 1999;59:282–290. doi: 10.1006/geno.1999.5874. [DOI] [PubMed] [Google Scholar]
- 42.Guo W, Worley K, Adams V, Mason J, Sylvester-Jackson D, Zhang YH, Towbin JA, Fogt DD, Madu S, Wheeler DA, McCabe ERB. Genomic scanning for expressed sequences in Xp21 identifies the glycerol kinase gene. Nat Genet. 1993;4:367–372. doi: 10.1038/ng0893-367. [DOI] [PubMed] [Google Scholar]
- 43.Sargent CA, Young C, Marsh S, Ferguson-Smith MA, Affara NA. The glycerol kinase gene family: structure of the Xp gene, and related intronless retroposons. Hum Mol Genet. 1994;3:1317–1324. doi: 10.1093/hmg/3.8.1317. [DOI] [PubMed] [Google Scholar]
- 44.Imanishi T, Itoh T, Suzuki Y, O’Donavan C, Fukuchi S, Koyanagi KO, Barrero RA, et al. Integrative annotation of 21,037 human genes validated by full-length cDNA clones. PLoS Biol. 2004;2:e162. doi: 10.1371/journal.pbio.0020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Riel JK, Paulus H. Subunit dissociation in the allosteric regulation of glycerol kinase from Escherichia coli. 2. Physical evidence. Biochemistry. 1978;17:5141–5146. doi: 10.1021/bi00617a011. [DOI] [PubMed] [Google Scholar]
- 46.Sakasegawa S, Takehara H, Yoshioka I, Takahashi M, Kagimoto Y, Misaki H, Sakuraba H, Ohshima T. Increasing the thermostability of Flavobacterium meningosepticum glycerol kinase by changing Ser329 to Asp in the subunit interface region. Protein Eng. 2001;14:663–667. doi: 10.1093/protein/14.9.663. [DOI] [PubMed] [Google Scholar]
- 47.Pettigrew DW, Liu WZ, Holmes C, Meadow ND, Roseman S. A single amino acid change in Escherichia coli glycerol kinase abolishes glucose control of glycerol utilization in vivo. J Bacteriol. 1996;178:2846–2852. doi: 10.1128/jb.178.10.2846-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyer LA, Peterson CL. Actin-related proteins (Arps): conformational switches for chromatin-remodeling machines? Bioessays. 2000;22:666–672. doi: 10.1002/1521-1878(200007)22:7<666::AID-BIES9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 49.Ohira RH. UCLA dissertation. 2004. Expression of ARX and GK, two genes in Xp22-p21. [Google Scholar]
- 50.Wolter KG, Ksu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohira RH, McCabe ERB. Function of two alternatively spliced mRNAs from the Xp21 glycerol kinase (GK) gene. Am J Hum Genet. 2004;S332:#1798. [Google Scholar]
- 52.Huq AHHM, Lovell RS, Sampson MJ, Decker WK, Dinulos MB, Disteche CM, Craigen WJ. Isolation, mapping, and functional expression of the mouse X chromosome. Genomics. 1996;36:530–534. doi: 10.1006/geno.1996.0500. [DOI] [PubMed] [Google Scholar]
- 53.Ohira RH, Dipple KM, McCabe ERB. Expression of alternatively spliced glycerol kinase is altered during development. Am J Hum Genet. 2003;73:S460, #1698. [Google Scholar]
- 54.Zhang YH, Huang BL, McCabe ERB. Tissue-specific expression of alternatively spliced forms of human Xp21 glycerol kinase (GK) gene. Am J Hum Genet. 2004;75:S334, #1810. [Google Scholar]
- 55.Spencer CA, Gietz RD, Hodgetts RB. Overlapping transcription units in the dopa decarboxylase region of Drosophila. Nature. 1986;322:279–281. doi: 10.1038/322279a0. [DOI] [PubMed] [Google Scholar]
- 56.Yelin R, Dahary D, Sorek R, Levanon EY, Goldstein O, Shoshan A, Dibe RA, Biton S, Tamir Y, Khosravi R, Nemzer S, Pinner E, Walach S, Bernstein J, Savitsky K, Rotman G. Widespread occurrence of antisense transcription in the human genome. Nat Biotechnol. 2003;21:379–386. doi: 10.1038/nbt808. [DOI] [PubMed] [Google Scholar]
- 57.Lehner B, Williams G, Campbell RD, Sanderson CM. Antisense transcripts in the human genome. Trends Genet. 2002;18:63–65. doi: 10.1016/s0168-9525(02)02598-2. [DOI] [PubMed] [Google Scholar]
- 58.Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pittner RA, Fears R, Brindley DN. Effects of cyclic AMP, glucocorticoids and insulin on the activities of phosphatidate phosphohydrolase, tyrosine aminotransferase and glycerol kinase in isolated rat hepatocytes in relation to the control of triacylglycerol synthesis and gluconeogenesis. Biochem J. 1985;225:455–462. doi: 10.1042/bj2250455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seitz HJ, Porsche E, Tarnowski W. Glycerolkinase—a regulatory enzyme of gluconeogenesis? Acta Biol Med Germ. 1976;35:141–154. [PubMed] [Google Scholar]
- 61.Croset M, Rajas F, Zitoun C, Hurot JM, Montano S, Mithieux G. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50:740–746. doi: 10.2337/diabetes.50.4.740. [DOI] [PubMed] [Google Scholar]
- 62.Uhal BD, Longmore WJ. Glycerol as a substrate for phospholipid biosynthesis in type II pneumocytes isolated from streptozotocin-diabetic rats. Biochim Biophys Acta. 1988;961:122–128. doi: 10.1016/0005-2760(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 63.Kawashita NH, Festuccia WT, Brito MN, Moura MA, Brito SR, Garofalo MA, Kettelhut IC, Migliorini RH. Glycerokinase activity in brown adipose tissue: a sympathetic regulation? Am J Physiol Regul Integr Comp Physiol. 2002;282:R1185–R1190. doi: 10.1152/ajpregu.00419.2001. [DOI] [PubMed] [Google Scholar]
- 64.Shiraishi Y, Mizutani A, Furuichi T. Postnatal development of the cerebellar circuit and gene expression. Tanpakushitsu Kakusan Koso. 2000;45:262–268. [PubMed] [Google Scholar]
- 65.Wang Z, Liu Y, Mori M, Kulesz-Martin M. Gene expression profiling of initiated epidermal cells with benign or malignant tumor fates. Carcinogenesis. 2002;23:635–643. doi: 10.1093/carcin/23.4.635. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh S, Strum JC, Bell RM. Lipid biochemistry: functions of glycerolipids and sphingolipids in cellular signaling. FASEB J. 1997;11:45–50. doi: 10.1096/fasebj.11.1.9034165. [DOI] [PubMed] [Google Scholar]
- 67.Siddiqui RA, English D. Phosphatidic acid elicits calcium mobilization and actin polymerization through a tyrosine kinase-dependent process in human neutrophils: a mechanism for induction of chemotaxis. Biochim Biophys Acta. 1997;1349:81–95. doi: 10.1016/s0005-2760(97)00085-4. [DOI] [PubMed] [Google Scholar]
- 68.Yanai N, Matsui N, Furusawa T, Okubo T, Obinata M. Sphingosine-1-phosphate and lysophosphatidic acid trigger invasion of primitive hematopoietic cells into stromal cell layers. Blood. 2000;96:139–144. [PubMed] [Google Scholar]
- 69.Moolenaar WH. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 70.Escalante-Alcalde D, Hernandez L, Le Stunff H, Maeda R, Lee HS, Gang-Cheng, Sciorra VA, Daar I, Spiegel S, Morris AJ, Stewart CL. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–4637. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 71.Zhang N, Zhang J, Purcell KJ, Cheng Y, Howard K. The Drosophila protein Wunen repels migrating germ cells. Nature. 1997;385:64–67. doi: 10.1038/385064a0. [DOI] [PubMed] [Google Scholar]
- 72.Kraut R, Menon K, Zinn K. A gain-of-function screen for genes controlling motor axon guidance and synaptogenesis in Drosophila. Curr Biol. 2001;11:417–430. doi: 10.1016/s0960-9822(01)00124-5. [DOI] [PubMed] [Google Scholar]
- 73.Takano N, Owada Y, Suzuki R, Sakagami H, Shimosegawa T, Kondo H. Cloning and characterization of a novel variant (mM-rdgBbeta1) of mouse M-rdgBs, mammalian homologs of Drosophila retinal degeneration B gene proteins, and its mRNA localization in mouse brain in comparison with other M-rdgBs. J Neurochem. 2003;84:829–839. doi: 10.1046/j.1471-4159.2003.01591.x. [DOI] [PubMed] [Google Scholar]
- 74.North MJ. Cold-induced increase of glycerol kinase activity in Neurospora crassa: rapid inactivation of the enzyme in vivo. J Bacteriol. 1974;120:741–747. doi: 10.1128/jb.120.2.741-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gal TZ, Glazer I, Koltai H. Differential gene expression during dessication stress in the insect-killing nematode Steinerenema feltiae. J Parasitol. 2003;89:761–766. doi: 10.1645/GE-3105. [DOI] [PubMed] [Google Scholar]
- 76.Kang L, Li J, Zhao T, Xiao F, Tang X, Thilmony R, He S, Zhou JM. Interplay of the Arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proc Natl Acad Sci USA. 2003;100:3519–3524. doi: 10.1073/pnas.0637377100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luckninbill LS. Selection for longevity confers resistance to low-temperature stress in Drosophila melanogaster. J Gerontol Ser A Biol Sci Med Sci. 1998;53:B147–B153. doi: 10.1093/gerona/53a.2.b147. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Kim SK. Global analysis of dauer gene expression in Caenorhabditis elegans. Development. 2003;130:1621–1634. doi: 10.1242/dev.00363. [DOI] [PubMed] [Google Scholar]
- 79.Xu FY, Taylor WA, Hurd JA, Hatch GM. Etomoxir mediates differential metabolic channeling of fatty acid and glycerol precursors into cardiolipin in H9c2 cells. J Lipid Res. 2003;44:415–423. doi: 10.1194/jlr.M200335-JLR200. [DOI] [PubMed] [Google Scholar]
- 80.Masai I, Suzuki E, Yoon CS, Kohyama A, Hotta Y. Immunolocalization of Drosophila eye-specific diacylgylcerol kinase, rdgA, which is essential for the maintenance of the photoreceptor. J Neurobiol. 1997;32:695–706. doi: 10.1002/(sici)1097-4695(19970620)32:7<695::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 81.Zwaig N, Kistler WS, Lin EC. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970;102:753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.St Johnston D. The art and design of genetic screens: Drosophila melanogaster. Nat Rev Genet. 2002;3:176–188. doi: 10.1038/nrg751. [DOI] [PubMed] [Google Scholar]
- 83.Inlow JK, Restifo LL. Molecular and comparative genetics of mental retardation. Genetics. 2004;166:835–881. doi: 10.1093/genetics/166.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamamoto A, Isohashi F, Okamoto K, Horiuchi M, Mitsui Y, Sakamoto Y. Change in activity of an adenosine triphosphate-stimulated glucocorticoid receptor translocation promoter in the cytosol and nucleus of rat liver under various physiological conditions. Endocrinology. 1986;119:357–361. doi: 10.1210/endo-119-1-357. [DOI] [PubMed] [Google Scholar]
- 85.Okamoto K, Isohashi F, Ueda K, Sakamoto Y. Properties of an adenosine triphosphate-stimulated factor that enhances the nuclear binding of activated glucocorticoid-receptor complex: binding to histone-agarose. Endocrinology. 1989;124:675–680. doi: 10.1210/endo-124-2-675. [DOI] [PubMed] [Google Scholar]
- 86.Okamoto K, Hirano H, Isohashi F. Molecular cloning of rat liver glucocorticoid-receptor translocation promoter. Biochem Biophys Res Commun. 1993;193:848–854. doi: 10.1006/bbrc.1993.1703. [DOI] [PubMed] [Google Scholar]
- 87.Martin R, Borner H, Martin H, Rotzsch W. Effect of cytoplasmic factors on cell nucleus binding of glucocorticoid receptor complexes in the rat liver of two age groups. Z Gerontol. 1993;26:238–242. [PubMed] [Google Scholar]
- 88.Eastman K, Martinez JA, McCabe ERB. Glycerol kinase: role in nuclear translocation of the activated glucocorticoid receptor complex (GRC). American Society Human Genetics Annual Meeting; October 26–30; Toronto, Ontario, Canada. 2004. Abstract 257. [Google Scholar]
- 89.Needham M, Raines S, McPheat J, Stacey C, Ellston J, Hoare S, Parker M. Differential interaction of steroid hormone receptors with LXXLL motifs in SRC-1a depends on residues flanking the motif. J Steroid Biochem Mol Biol. 2000;72:35–46. doi: 10.1016/s0960-0760(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 90.Martin R, al-Scheibani AH, Martin H. Age related changes in cellular signal transduction of glucocorticoid hormones in rat brain. Z Gerontol Geriatr. 1999;32:76–82. doi: 10.1007/s003910050087. [DOI] [PubMed] [Google Scholar]
- 91.Okamoto K, Isohashi F, Ueda K, Kokufu I, Sakamoto Y. Purification and characterization of an adenosine triphosphate-stimulated factor that enhances the nuclear binding of activated glucocorticoid-receptor complex from rat liver. Endocrinology. 1988;123:2752–2761. doi: 10.1210/endo-123-6-2752. [DOI] [PubMed] [Google Scholar]
- 92.Okamoto K, Liu G, Yu WG, Isohashi F. Immunochemical characterization of the ATP-stimulated glucocorticoid-receptor-translocation promoter from various organs of rat. J Biochem (Tokyo) 1994;115:862–867. doi: 10.1093/oxfordjournals.jbchem.a124431. [DOI] [PubMed] [Google Scholar]
- 93.Seltzer WK, McCabe ERB. Subcellular distribution and kinetic properties of soluble and particulate-associated bovine adrenal glycerol kinase. Mol Cell Biochem. 1984;64:51–61. doi: 10.1007/BF00420928. [DOI] [PubMed] [Google Scholar]
- 94.Adams V, Griffin L, Towbin J, Gelb B, Worley K, McCabe ERB. Porin interaction with hexokinase and glycerol kinase: metabolic microcompartmentation at the outer mitochondrial membrane. Biochem Med Metab Biol. 1991;45:271–291. doi: 10.1016/0885-4505(91)90032-g. [DOI] [PubMed] [Google Scholar]
- 95.Tildon JT, Stevenson JH, Jr, Ozand PT. Mitochondrial glycerol kinase activity in rat brain. Biochem J. 1976;157:513–516. doi: 10.1042/bj1570513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jenkins BT, Hajra AK. Glycerol kinase and dihydroxyacetone kinase in rat brain. J Neurochem. 1976;26:377–385. doi: 10.1111/j.1471-4159.1976.tb04491.x. [DOI] [PubMed] [Google Scholar]
- 97.Tildon JT, Roeder LM. Glycerol oxidation in rat brain: subcellular localization and kinetic characteristics. J Neurosci Res. 1980;5:7–17. doi: 10.1002/jnr.490050103. [DOI] [PubMed] [Google Scholar]
- 98.Pascual de Bazan HE, Bazan NG. Phospholipid composition and [14C]glycerol incorporation into glycerolipids of toad retina and brain. J Neurochem. 1976;27:1051–1057. doi: 10.1111/j.1471-4159.1976.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen NH, Brathe A, Hassel B. Neuronal uptake and metabolism of glycerol and the neuronal expression of mitochondrial glycerol-3-phosphate dehydrogenase. J Neurochem. 2003;85:831–842. doi: 10.1046/j.1471-4159.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 100.Kaneko M, Kurokawa M, Ishibashi S. Binding and function of mitochondrial glycerol kinase in comparison with those of mitochondrial hexokinase. Arch Biochem Biophys. 1985;237:135–141. doi: 10.1016/0003-9861(85)90262-0. [DOI] [PubMed] [Google Scholar]
- 101.Isenmann S, Khew-Goodall Y, Gamble J, Vadas M, Wattenberg BW. A splice-isoform of vesicle-associated membrane protein-1 (VAMP-1) contains a mitochondrial targeting signal. Mol Biol Cell. 1998;9:1649–1660. doi: 10.1091/mbc.9.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 103.Martinez JA, McCabe ERB. Apoptosis in glycerol kinase deficiency (GKD): investigations in Drosophila melanogaster; American Society of Human Genetics Annual Meeting; October 26–30; Toronto, Ontario, Canada. 2004. Abstract 76. [Google Scholar]
- 104.Haase J, Killian AM, Magnani F, Williams C. Regulation of the serotonin transporter by interacting proteins. Biochem Soc Trans. 2001;29:722–728. doi: 10.1042/0300-5127:0290722. [DOI] [PubMed] [Google Scholar]
- 105.Collins T, Stone JR, Williams AJ. All in the family: the BTB/POZ, KRAB, and SCAN domains. Mol Cell Biol. 2001;21:3609–3615. doi: 10.1128/MCB.21.11.3609-3615.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuo ML, Huang Y, Liu DP, Liang CC. K(ATP) channel: relation with cell metabolism and role in the cardiovascular system. Int J Biochem Cell Biol. 2005;37:751–764. doi: 10.1016/j.biocel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 107.Anholt RRH, Mackay TFC. The genetic architecture of odor-guided behavior in Drosophila melanogaster. Behav Genet. 2001;31:17–27. doi: 10.1023/a:1010201723966. [DOI] [PubMed] [Google Scholar]
- 108.Fedorowicz GM, Fry JD, Anholt RRH, Mackay TFC. Epistatic interactions between smell-impaired loci in Drosophila melanogaster. Genetics. 1998;148:1885–1891. doi: 10.1093/genetics/148.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kulkarni NH, Yamamoto AH, Robinson KO, Mackay TF, Anholt RR. The DSC1 channel, encoded by the smi60E locus, contributes to odor-guided behavior in Drosophila melanogaster. Genetics. 2002;161:1507–1516. doi: 10.1093/genetics/161.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brody T, Stivers C, Nagle J, Odenwald WF. Identification of novel Drosophila neural precursor genes using a differential expression head cDNA screen. Mech Dev. 2002;113:41–59. doi: 10.1016/s0925-4773(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 111.FlyBase Consortium. The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 2003;31:172–175. doi: 10.1093/nar/gkg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]