Abstract
Progesterone is a critical regulator of normal female reproductive function, with diverse tissue-specific effects in the human. The effects of progesterone are mediated by its nuclear receptor (PR) that is expressed as two isoforms, PRA and PRB, which are virtually identical except that PRA lacks 164 amino acids that are present at the N-terminus of PRB. Considerable in vitro evidence suggests that the two PRs are functionally distinct and in animals, tissue-specific distribution patterns of PRA and PRB may account for some of the diversity of progesterone effects. In the human, PRA and PRB are equivalently expressed in most target cells, suggesting that alternative mechanisms control the diversity of progesterone actions. PR mediates the effects of progesterone by association with a range of coregulatory proteins and binding to specific target sequences in progesterone-regulated gene promoters. Ligand activation of PR results in redistribution into discrete subnuclear foci that are detectable by immunofluorescence, probably representing aggregates of multiple transcriptionally active PR-coregulator complexes. PR foci are aberrant in cancers, suggesting that the coregulator composition and number of complexes is altered. A large family of coregulators is now described and the range of proteins known to bind PR exceeds the complement required for transcriptional activation, suggesting that in the human, tissue-specific coregulator expression may modulate progesterone response. In this review, we examine the role of nuclear localization of PR, coregulator association and tissue-specific expression in modulating progesterone action in the human.
Introduction
Progesterone is an essential regulator of normal human female reproductive function in the uterus, ovary, mammary gland and brain, and also plays an important role in non-reproductive tissues such as the cardiovascular system, bone and the central nervous system, highlighting the widespread role of this hormone in normal physiology [Graham and Clarke, 1997; Graham and Clarke, 2002; Li et al., 2004; Mote et al., 2007]. The effects of progesterone are mediated through the nuclear progesterone receptor (PR), which interacts with transcriptional coregulators [Lonard and O'Malley, 2007], moves into nuclear aggregates [Arnett-Mansfield et al., 2004; Arnett-Mansfield et al., 2007] and regulates gene expression. Although progesterone plays a pivotal role in normal physiology, exposure to its analogues in exogenous hormone formulations is associated with deleterious effects, most notably an increase in breast cancer risk [Beral, 2003; Holtorf, 2009; Horwitz, 2008; Lee et al., 2005; Pike et al., 2007; Rossouw et al., 2002; Santen, 2003]. Given the diverse roles of progesterone in normal tissues and in cancer, developing a detailed understanding of the mechanisms that direct its cell and tissue specificity is a high priority. This review will explore the role of nuclear localization of PR, its association with coregulators, and coregulator complement of target tissues as critical contributors to the selectivity and specificity of progesterone effects in normal and malignant target tissues.
The progesterone receptor
Progesterone effects are mediated by binding to the nuclear progesterone receptor (PR). PR is a member of a large family of ligand-activated nuclear transcription regulators [Escriva et al., 2004; Evans, 1988; McEwan, 2009], which are characterised by organisation into specific functional domains and are conserved, to differing degrees, between species and family members. PR is made up of a central DNA binding domain (DBD) and a carboxyl-terminal ligand-binding domain (LBD). In addition, the receptor contains multiple activation (AF) and inhibitory (IF) function elements, which enhance and repress transcriptional activation of PR by association of these regions with transcriptional coregulators [Edwards, 2000; Huse et al., 1998; McEwan, 2009; McKenna et al., 1999; McKenna and O'Malley, 2002; Sartorius et al., 1994; Vegeto et al., 1993]. Newly-transcribed cytoplasmic PR is assembled in an inactive multi-protein chaperone complex [Smith et al., 1990], which is essential for maintenance of the inactive receptor in a state that is competent to bind ligand [Pratt et al., 2004]. Progestin binding to PR causes a conformational change leading to dissociation of chaperones, dimerization, binding to progestin response elements in the promoters of target genes and recruitment of specific coactivators and general transcription factors, resulting in modulation of transcription of those genes [Beato et al., 1987; Gronemeyer, 1991; Tata, 2002].
In addition to the ligand-activated transcriptional effects discussed above, which reflect the nuclear activity of this receptor, PR also regulates transcription via non-genomic pathways such as activation of second messenger signaling cascades [Lange et al., 1998; Leonhardt et al., 2003; Nilsen and Brinton, 2002; Nilsen and Brinton, 2003]. Ligand-independent activation of PR can occur in a cell-type and promoter-specific manner and provides evidence for regulation of PR via cytoplasmic and membrane generated signals [Daniel et al., 2007; Jacobsen et al., 2005]. The molecular mechanisms of PR action through non-genomic mechanisms, including receptor cross-talk with growth factor signaling pathways, have been reviewed in detail recently [Lange, 2008a; Lange, 2008b], and this review will not explore these issues further.
Progesterone action is mediated by two PR isoforms
In the human, the effects of progesterone are mediated by two distinct forms of the PR transcribed from a single gene by alternate initiation of transcription from two distinct promoters [Gronemeyer et al., 1991; Kastner et al., 1990], giving rise to transcripts encoding two protein isoforms, PRA and PRB. PRA and PRB are identical in sequence, except that PRA lacks 164 amino acids at the N-terminus, making it the shorter of the two proteins [Kastner et al., 1990].
Considerable evidence from transient cotransfection studies into a variety of cell lines suggest that PRA and PRB are functionally unique transcriptional regulators, capable of differentially regulating gene transcription within the same promoter context, and capable of recognising entirely different promoters [Aupperlee and Haslam, 2007; Brayman et al., 2006; Jacobsen et al., 2002; Richer et al., 2002; Tung et al., 1993]. In the mouse, ablation of PRA or PRB has revealed the unique roles for PRA and PRB, demonstrating that PRB is required for normal mammary gland development [Mulac-Jericevic et al., 2003], while PRA is essential for uterine development and reproductive function [Mulac-Jericevic et al., 2000].
Although the precise mechanisms underlying the differential activities of the two human PR isoforms is not fully understood, structure-function studies suggest that the AF3 domain located within the PRB upstream sequence region, which is absent in PRA, [Sartorius et al., 1994] contributes to PRB transcriptional activity by suppressing the activity of an inhibitory domain (ID) contained within the sequences common to PRA and PRB [Abdel-Hafiz et al., 2002; Tung et al., 2006]. Moreover, evidence suggests that the two receptors adopt distinct conformations within the cell [Bain et al., 2000; Bain et al., 2001] allowing PRA to interact with a set of coregulators that are different from those which interact with PRB [Tetel et al., 1999]. This is supported by studies demonstrating that PRA has a higher affinity for the corepressor SMRT than PRB in the presence of PR antagonists, and PRA does not interact directly or with as high an affinity as PRB with the coactivators SRC-1 and SRC-2 upon agonist binding [Giangrande et al., 2000; Heneghan et al., 2007; Molenda-Figueira et al., 2008], potentially contributing to differences in the transcriptional activities of the two human PR isoforms and further enhancing the complexity of this regulatory system.
In addition to the translation start sites driving expression of PRA and PRB, a third start site within the PR gene has been identified at amino acid position 595 that could give rise to a truncated PR isoform, PRC. If expressed, PRC would lack the PR N-terminus and one zinc finger of the DNA binding domain, rendering it transcriptionally inactive but able to bind hormone, dimerize and localize in the nucleus [Wei et al., 1990; Wei et al., 1996]. Although in vitro reports have suggested that PRC may either enhance or antagonize the activities of PRA and PRB [Condon et al., 2006; Wei et al., 1996; Wei et al., 1997], recent evidence suggests that PRC is not expressed in vivo and has no real physiological role in progesterone signaling [Samalecos and Gellersen, 2008].
Relative expression of the PR isoforms contributes to selectivity of PR action. Much of our current understanding of the distinct functions of PRA and PRB derives from models where only a single PR isoform is expressed and in which the PR homodimer is the dominant molecular species. However, in a cell that co-expresses both PR isoforms, there is the potential for 3 molecular species (PRB homodimers, PRA homodimers and PRA-PRB heterodimers) to exist concurrently and to contribute to the complexity of PR action.
In mice, where PRA and PRB are frequently expressed in different cells within the reproductive system [Gava et al., 2004; Mote et al., 2006], the homodimer is clearly a critical contributor to progesterone action, consistent with the divergent and tissue-specific roles identified for these proteins in knockout studies [Mulac-Jericevic et al., 2003; Mulac-Jericevic et al., 2000]. In human physiology, however, the majority of PR positive cells express PRA and PRB at equivalent levels, and cells that express only one PR isoform are uncommon [Mote et al., 1999; Mote et al., 2002; Mote et al., 2007]. This suggests that in the human, progesterone exerts its effects in cells that co-express both PR isoforms, and that the PRA-PRB heterodimer is the predominant molecular species.
It appears that the PR dimer species that predominates within a target cell influences the transcriptional program regulated by the receptor. For example, in vitro studies in cell lines expressing only one PR isoform (PRA or PRB) have demonstrated that the gene sets regulated by PRA and PRB homodimers are largely non-overlapping and that the number of genes uniquely regulated by PRB far exceeds the number regulated by PRA [Richer et al., 2002]. Interestingly, in cell lines co-expressing both PRA and PRB, leading to the prevalence of the heterodimer, a unique and smaller number of genes are progesterone-regulated compared to that seen in cells expressing only one PR isoform [Graham et al., 2005; Richer et al., 2002]. Overall, these findings demonstrate that the PR homodimers and the heterodimer have the capacity to regulate different suites of genes and that the ratio of PR isoform expression plays an important role in influencing the transcriptional program regulated by PR in target tissues.
The PR isoform ratio also influences the capacity of PRA to regulate the activity of PRB. In transfection studies, PRA has been shown to be a dominant inhibitor of the transcriptional activity of PRB [Chalbos and Galtier, 1994; Giangrande and McDonnell, 1999; McDonnell and Goldman, 1994; Meyer et al., 1992; Tung et al., 1993; Vegeto et al., 1993; Wen et al., 1994]. Furthermore, PRA has been shown to similarly regulate the activity of a number of nuclear receptors, including the glucocorticoid, mineralocorticoid, androgen, and estrogen receptors [McDonnell and Goldman, 1994; Tung et al., 1993; Vegeto et al., 1993; Wen et al., 1994]. However, it appears that PRA can only exert this dominant negative effect when it is in very significant excess, as the transient transfection experiments that demonstrated the dominant negative inhibitory activity of PRA were likely to involve higher levels of PRA expression than are ever observed endogenously.
This possibility was explored through the construction of cell lines, normally containing equivalent levels of PRA and PRB in recapitulation of their relative levels observed in human tissues, but in which PRA expression can be induced to be in excess over PRB. In this model system, induction of PRA expression to five-fold excess over PRB had little impact on PR-regulated gene expression [Graham et al., 2005]. In particular, the progestin-regulated gene sets in cells expressing equivalent levels of PRA and PRB, and in cells over-expressing PRA, were largely overlapping [Graham et al., 2005]. This demonstrates that there is no dominant negative effect of PRA, when PRA is in physiological excess of PRB in cells endogenously co-expressing both PR isoforms. Most progesterone target tissues express both PRA and PRB at relative levels that are not vastly different, suggesting that dominant negative inhibition of PRB by PRA is not likely to be a prominent effect in normal human physiology.
PR expression in target tissues
PR proteins are expressed in a variety of human tissues, including the uterus, mammary gland, brain, pancreas, bone, ovary, testes, and tissues of the lower urinary tract [Bland, 2000; Graham and Clarke, 1997; Graham and Clarke, 2002; Han et al., 2009; Ozawa, 2005; Tincello et al., 2009]. The ubiquitous expression of PR highlights the widespread physiological effects that progesterone can exert in a variety of organs throughout the body.
PR expression in non-human species
PRA and PRB proteins were initially demonstrated in the chick oviduct in the early 1970s [Schrader and O'Malley, 1972; Schrader et al., 1972] and many studies have since determined the relative levels of PRA and PRB isoforms in a variety of species including birds, reptiles, rodents and mammals. The majority of these early studies examined PR isoform expression using cell-free methods in organs associated with reproduction, and a dominant expression of one PR isoform was frequently observed. PRB was the dominant isoform in the oviduct of the reproductively active freshwater painted turtle (Chrysemys picta) [Giannoukos et al., 1995; Reese and Callard, 1989] and also the quail [Dufrene et al., 1989], whilst the rabbit uterus expressed only PRB [Lamb et al., 1986]. Conversely, rodent reproductive tissues expressed a predominance of PRA [Schneider et al., 1991; Shyamala et al., 1990]. In the mouse uterus and mammary gland the PRA:PRB ratio was 3:1, whilst in murine vaginal tissue it was 2:1 [Ilenchuk and Walters, 1987; Schneider et al., 1991]. Moreover, the relative expression levels of PRA and PRB varied in different organs within the same species, as the liver of the painted freshwater turtle expressed predominantly PRA, in contrast to the PRB-predominant expression in the oviduct of the same species [Giannoukos et al., 1995], whilst the rat brain, unlike the rodent reproductive organs, expressed predominantly PRB [Kato et al., 1993]. These data suggested the ratio of PRA and PRB to be both species- and tissue-specific. Seasonal changes in the relative expression of PRA and PRB in the turtle oviduct also occur, implying influence by circulating ovarian steroid hormones that fluctuate throughout the reproductive cycle [Giannoukos et al., 1995].
The advent of antibodies with specificity for PRA or PRB demonstrated that the predominance of one PR isoform previously demonstrated in whole tissues by cell-free methods was contributed in part by cell-type specificity of PR isoform expression. For instance, the PRA predominance of mouse uterus identified in cell-free studies is contributed by high expression of PRA in the myometrium and stroma, whereas PRA is absent from luminal epithelial cells, which contain only PRB [Mote et al., 2006]. Both PR isoforms are present in the uterine stroma and myometrial cells, with levels that fluctuate with cyclical systemic hormonal exposure [Mote et al., 2006]. Similarly in the mouse ovary, there are estrous cycle-related changes in PRA:PRB [Gava et al., 2004]. There is also a temporal and/or spatial separation of PRA and PRB expression in rodent mammary epithelial cells during normal mammary gland development [Aupperlee et al., 2005; Kariagina et al., 2007]. In the mouse, PRA was the predominant isoform expressed in the pubertal and mature virgin mammary gland, whilst PRB predominance was observed during pregnancy when PRA and PRB rarely co-localized [Aupperlee et al., 2005]. Distinct PRA and PRB expression has also been reported in the rat mammary gland during development [Kariagina et al., 2007]. PRA was only expressed in luminal epithelial cells, whilst PRB was present in both luminal and myoepithelial cells, indicating a role for PRB in myoepithelial cell regulation [Kariagina et al., 2007]. The cell-specificity of PR isoform expression in animal tissues further supports the demonstration in PR isoform null animals of distinct activities of the isoforms in the mouse, and reflects the likelihood that progesterone action is mediated differentially in PRB-positive cells compared to PRA-positive cells within rodent PR-target tissues in vivo.
PR expression in the human
In contrast to the predominant expression of one PR isoform frequently observed in animal tissues, in normal human tissues in vivo, including the breast and uterus, all PR+ epithelial cells co-expressed PRA and PRB at similar levels [Mote et al., 1999; Mote et al., 2002]. This suggests that co-location and thus cooperative activity of PRA and PRB mediates PR action in the human. This is in contrast to the mouse, as outlined above, where the non-overlapping expression of PRA and PRB and the lack of co-location of the two PR isoforms, strongly suggest the PR homodimer to be the active species [Aupperlee et al., 2005; Mote et al., 2006]. Although PRA and PRB proteins are normally expressed equally in human tissues, there is some evidence for differential hormonal regulation of the two PR isoforms in the glandular epithelial cells of the endometrium. During the secretory phase of the menstrual cycle, when high circulating levels of progesterone are associated with decreased PR expression, PRA was preferentially decreased, resulting in a distinct predominance of PRB in these cells at this time [Mote et al., 1999].
In contrast to the balanced expression of PRA and PRB in normal human tissues, progression of breast and endometrial tissues from normal to malignancy is frequently accompanied by progressive changes in PR isoform expression. In normal breast and in proliferative disease without atypia of the breast, PRA and PRB are co-expressed within the same cells in comparable amounts [Mote et al., 2002], and there is little variation in the cell-to-cell relative expression of PRA and PRB. In atypical breast lesions, however, there is a significant increase in predominant expression of PRA or PRB [Graham et al., 1995; Mote et al., 2002]. Similarly, in hyperplastic areas of endometrial tissue there is increased predominance of one PR isoform, suggesting that lack of coordinated PRA:PRB expression is an early event in endometrial cancer [Arnett-Mansfield et al., 2001]. In breast cancer, PR isoform predominance, especially PRA predominance, is evident in a significant proportion of ductal carcinomas in situ (DCIS) and invasive cancers [Mote et al., 2002], and moreover, there is marked heterogeneity of PRA:PRB expression between neighbouring cells in breast cancers [Mote et al., 2002]. In endometrial cancers one PR isoform is frequently lost, and PR isoform loss is often associated with higher histological grade [Arnett-Mansfield et al., 2001].
In summary, studies of PR expression in both humans and animals indicate that PRA and PRB proteins are expressed in a species-, tissue- and cell type-specific manner, highlighting the need for caution when extrapolating results between species. The data suggest that PRA and PRB are able to work both alone or together to modulate the complex and divergent pathways of progesterone action in normal and malignant physiology, and that the alterations in isoform expression frequently observed in malignancy, will affect PR dimer predominance and thereby modulate transcriptional response.
Nuclear positioning of PR
Both liganded and unliganded PR are located in the cell nucleus, and localize to specific intra-nuclear locations: when liganded, the activated PR moves into nuclear aggregates, or 'foci', whereas unliganded PR is distributed evenly throughout the nucleus. In human tissues, endogenous PR is located in foci only in the secretory phase of the menstrual cycle, when progesterone levels are high [Arnett-Mansfield et al., 2004; Arnett-Mansfield et al., 2007]. Ligand binding is required for PR movement into foci [Arnett-Mansfield et al., 2004], and PR isoforms dimerise when they move into foci [Arnett-Mansfield et al., 2007]. PR foci are tethered to the nuclear matrix, and both DNA binding and nuclear matrix tethering are required for PR to move into foci [Graham et al., 2009]. Within these tethered structures PR is highly mobile, consistent with its rapid occupancy at specific chromatin locations associated with transcription [Graham et al., 2009]. Ligand-bound PR in foci co-locates with nascent RNA, activated RNAPolII, and also with the chromatin remodeling histone acetyl transferase p300 [Arnett-Mansfield et al., 2007] and transcriptional inhibitors inhibit the movement of PR into foci [Arnett-Mansfield et al., 2007], demonstrating the link between PR foci and transcriptional activity. Exposure to Roscovitine, which inhibits recruitment of the p160 coactivator SRC-1, also prevents ligand-dependent foci formation. These data collectively demonstrate that foci contain multiple activated PR-coactivator complexes associated with the basic transcriptional machinery.
Nuclear aggregation of transcription factors, including nuclear receptors, is commonly observed [Zaidi et al., 2004; Zink et al., 2004], due to the structural constraints imposed by nuclear compartmentalization, and to the efficiency gains of co-location of functionally-related molecules. A number of components of nuclear receptor pathways have been shown to aggregate in the nucleus, and transfection of tagged receptors has shown that estrogen receptor α (ERα) [Htun et al., 1999], androgen receptor (AR) [Tyagi et al., 2000], glucocorticoid receptor (GR) [van Steensel et al., 1995] and mineralocorticoid receptor (MR) [Fejes-Toth et al., 1998] form nuclear aggregates when exposed to ligand [Fejes-Toth et al., 1998; Htun et al., 1996; Htun et al., 1999; Tyagi et al., 2000]. Furthermore, various nuclear receptor-associated proteins are located in discrete domains [Baumann et al., 2001; Guo et al., 2000] such as p53, steroid receptor coactivator (SRC), and glucocorticoid receptor interacting protein1 (GRIP1), which localize in the PML nuclear body. The frequent observation of nuclear aggregation of nuclear receptor components supports the view that PR foci identified in human tissues are transcriptional complexes required for progesterone action.
PR moves into different foci in cancer cells and normal cells. While PR foci are detected in both normal human tissues and in cancers, they are different. PR foci in normal endometrium have a median length of 0.65 µm, but are significantly larger in endometrial cancers, with a median length over 1.0 µm [Arnett-Mansfield et al., 2007]. PR isoform composition in foci can be aberrant in cancers, with PRA seldom being found in foci, in contrast to PRB [Arnett-Mansfield et al., 2004], and foci in endometrial cancer are associated with clinical grade [Arnett-Mansfield et al., 2004]. The differences in PR foci in normal and cancer tissues suggest that PR foci in cancers may contain different and/or larger numbers of coregulator proteins, and may be functionally different. The link between PR foci in cancers and clinical features suggest that these observations have physiological and clinical relevance.
The role of PR coregulators in regulating progesterone action in human tissues
It is fascinating to realize that what can be seen at the microscopic level as PR foci, in fact represents active and highly mobile PR molecules engaged in transcriptional regulation of target genes and recruiting key coregulators to achieve this regulation. Binding of ligand to PR leads to receptor dimerization, conformational change, association with specific response element sequences in proximal and distal regions of target genes and regulation of transcription. This transcriptional regulation is a complex multistep process, which involves the sequential recruitment of a number of primary and secondary coactivators possessing a range of enzymatic activities. The complex combinatorial process of chromatin remodeling, coregulator recruitment and initiation of transcription by nuclear receptors is now well characterized and has been described in detail in recent reviews [Lonard et al., 2007; Lonard and O'Malley, 2007; Wolf et al., 2008], so only a brief summary is given here.
The ligand-dependent change in PR conformation promotes recruitment of p160 coactivators (SRC-1/NCoA-1, SRC-2/GRIP1/TIF2, SRC-3/AIB1/ACTR/TRAM1/ p/CIP) [Han et al., 2006; Han et al., 2005; McKenna et al., 1998; Onate et al., 1995], histone acetylases (including p300/CBP) [McKenna et al., 1998], DNA helicases (including components of the SWI/SNF complex) [Vicent et al., 2004], ubiquitin ligases (such as E6-AP) [Nawaz et al., 1999], methylases (CARM1) [Lonard and O'Malley, 2007] and the steroid receptor-specific RNA activator SRA [Lanz et al., 1999]. This brings about histone modification and remodeling of the local chromatin to allow recruitment and activation of the RNA polymerase II holocomplex leading to increased RNA transcription of the target gene [Lonard and O'Malley, 2007].
More than 300 coregulators have now been described and more PR-binding coactivators exist than are required to form a functional PR activation complex. It has been hypothesized that the specific combination of coactivators that associate with PR in a given cell is dependent on their relative abundance, which varies in a tissue-specific manner [Giangrande et al., 2000; Han et al., 2005; Lonard et al., 2007]. Moreover, although no study has yet identified unique coregulators of PRA or PRB, in vitro evidence suggests that they have different affinities for a common set of coregulators, resulting in cell type-specific differences in transcriptional activity [Han et al., 2006; Molenda-Figueira et al., 2008; Tung et al., 2006].
Given that PRA and PRB are equivalently expressed in most human target tissues, yet the effects of progesterone are highly tissue-specific, it is likely that variations in the levels of specific PR coregulators with distinct affinities for the two PR isoforms may provide a mechanism by which a functional predominance of PRA or PRB is achieved. It is clear that the level of coregulator expression is critical in determining the overall transcriptional activity of PR in target tissues, and there is now abundant evidence linking aberrant coregulator expression or activity to diseases including cancer [Lanz et al., 2008]. The identification of aberrant coregulator expression in cancers provides support for the view that recruitment of coregulators to PR is altered in cancers, as evidenced by aberrant PR foci observed in cancers compared to normal tissues. Although the corepressors N-CoR and SMRT play no role in normal PR physiology, they have been found to associate with PR and ER when bound to the mixed antagonists RU38486 and tamoxifen, respectively, to suppress the agonist effects of these compounds [Jackson et al., 1997; Smith et al., 1997]. Decreased expression of these corepressors may contribute to the tamoxifen resistance phenotype [Lavinsky et al., 1998; Smith et al., 1997]. It has also been suggested that differences in PR coregulator expression levels might account for variations in hormone responsiveness seen in the population [Gao et al., 2005]. In summary, variation in the level of both coactivators and corepressors of PR is likely to represent an important mechanism controlling responsiveness to progesterone in normal and malignant tissues in the human. Therefore, determining the tissue distribution and regulation of coregulator expression in the human is likely to be an important key to understanding the diversity of progesterone effects.
The physiological role of PR coregulators in animal tissues
Much of the current understanding about the tissue-specific functions of PR coactivators has been derived from their ablation in animals. These studies have revealed that while some functional redundancy may exist between the p160 coactivator family members, there is a critical requirement for specific isoforms in progesterone target tissues. For example, disruption of the SRC-3 gene in mice causes severe growth and reproductive defects, including attenuation of mammary ductal side-branching [Xu et al., 2000], a developmental process shown to be driven by progesterone [Conneely et al., 2007; Lydon et al., 1995]. SRC-1 null mice also display specific aberrations, some of which are distinct from those observed in the SRC-3 knock-out model, such as attenuated decidual response in the uterus and elevated expression of SRC-2 [Xu et al., 1998]. Mice in which SRC-2 ablation is specifically directed to progesterone target cells reveal an essential role for this coactivator in mediating embryo implantation. These animals are infertile, lack a uterine decidual response and display markedly attenuated ductal side-branching [Mukherjee et al., 2006b]. In a study comparing the tissue-specific requirements for SRC-1 and SRC-3 for progesterone signaling, a PR activity indicator (PRAI) mouse model crossed into either an SRC-1 or SRC-3 null background confirmed that SRC-3 is the primary p160 coactivator for PR in the mammary gland, whereas the role of SRC-1 in modulating progesterone action is most evident in the uterus [Han et al., 2006]. There is clear evidence from combined studies of SRC-1 and SRC-2 null mice, displaying overlapping defects in uterine decidual response [Jeong et al., 2007], that these two coactivators function cooperatively to facilitate progesterone action in this tissue. In summary, these knock-out studies demonstrate that the three SRC isoforms play complementary tissue-specific roles in mediating progesterone action in the rodent, with SRC-1 and SRC-2 both playing essential roles in the uterus and SRC-3 being critical in the mammary gland. Animal model studies analysing the role of numerous other PR coregulators in reproductive function have previously been reviewed [Gao and Nawaz, 2002; Li et al., 2004; Rowan and O'Malley, 2000].
Expression of PR coregulators in normal progesterone target tissues in the human
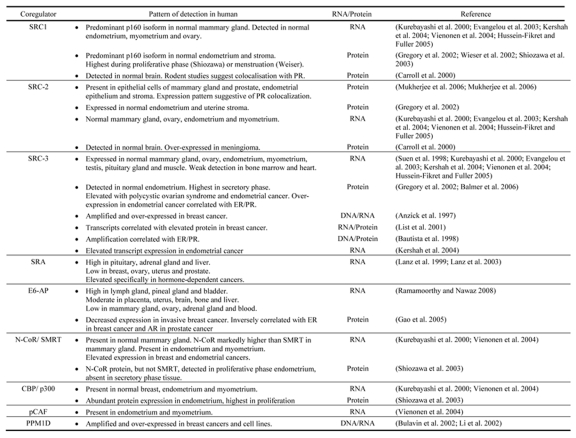
Despite the evidence that physiological response to progesterone in specific target tissues is affected by the combination and expression levels of PR coregulators, a comprehensive profile of coregulator expression is generally unavailable in these tissues in the human. However, some studies of individual coregulators have examined their expression in progesterone-responsive tissues, particularly the mammary gland and reproductive tissues, and in the case of two PR coregulators SRA [Lanz et al., 2003] and E6-AP [Ramamoorthy and Nawaz, 2008], wide-ranging surveys of tissue distribution in the human have been made. The collective findings of these studies are summarized in Table 1. In some cases, functional correlates have been sought, such as fluctuations in coregulator expression during the menstrual cycle or patterns of expression that overlap with PR. These will be discussed. We have attempted to highlight in each case, whether protein or transcript level has been measured, since levels of or fluctuations in transcript expression may not always correlate with what is observed at the protein level.
Table 1. : Reported patterns of PR coregulator expression in human tissues.
The findings of studies reporting expression of known PR coregulators at the transcript or protein level in human tissues are summarised.
The expression of p160 steroid receptor coactivators has been examined in human breast, endometrium, brain and ovary. Kurebayashi et al [Kurebayashi et al., 2000] reported RNA transcript expression for all three SRC isoforms in a small cohort of normal breast tissues. SRC-1 was the most strongly expressed of all SRC coactivators, showing strong expression in all specimens, whereas SRC-2 and SRC-3 showed relatively weak expression. SRC-2 protein is detected in human mammary epithelium and results of PR staining of adjacent sections, coupled with evidence from dual detection in mouse tissues, suggest colocation with PR [Mukherjee et al., 2006a; Mukherjee et al., 2006b]. A number of investigators have also reported on SRC expression in the human endometrium. Transcript expression of all three SRC isoforms is detected in normal human endometrium [Kershah et al., 2004; Vienonen et al., 2004] and expression levels are similar for the three forms [Kershah et al., 2004]. Protein expression of SRC-1 [Gregory et al., 2002; Shiozawa et al., 2003], SRC-2 [Gregory et al., 2002; Mukherjee et al., 2006b] and SRC-3 [Balmer et al., 2006; Gregory et al., 2002] has also been reported in glandular epithelium and stroma in the endometrium, and the study of Gregory et al suggested that although expression levels were similar, SRC-3 staining was slightly lower than SRC-1 or SRC-2. Evidence for fluctuation in SRC expression during the normal menstrual cycle is conflicting. Shiozawa et al reported higher expression of SRC-1 protein in the proliferative phase endometrium [Shiozawa et al., 2003], whereas in another study SRC-1 RNA expression was found to be significantly higher at menstruation, compared to either proliferative or secretory endometrium [Wieser et al., 2002]. The studies of Gregory [Gregory et al., 2002] and Balmer [Balmer et al., 2006] reported no change in SRC-1 protein through the cycle, but observed an increase in SRC-3 in secretory phase endometrium. In summary, while there is suggestive evidence that coactivator expression in the endometrium may fluctuate during the menstrual cycle to modulate hormonal response, more studies are required to clarify the current observations.
Transcripts for SRC1-3 have also been detected in surface epithelium of the normal ovary [Evangelou et al., 2003] and whole normal ovary samples [Hussein-Fikret and Fuller, 2005]. SRC expression is also detectable in the normal brain: immunoblot analysis of a small cohort of normal brain samples revealed consistent expression of SRC-1 and SRC-2, but SRC-3 was absent in all samples [Carroll et al., 2000]. Although limited information is available on the specificity of SRC expression in the normal human brain, studies in rodents have revealed that SRC-1 protein is present in regions of the hypothalamus that are functionally relevant to progesterone action in the brain, and cells that express PR generally also express SRC-1 and CBP, as detected by dual immunofluorescence [Tetel et al., 2007].
Consistent with its role as a fundamental nuclear receptor coactivator, p300/CBP is detectable in all progesterone target tissues examined and there is not strong evidence for fluctuations in expression in normal tissues. CBP transcripts are detected in the normal breast [Kurebayashi et al., 2000] and both CBP and p300 transcripts have been detected in the endometrium and myometrium [Vienonen et al., 2004]. Shiozawa reported abundant expression of p300/CBP protein in normal endometrium, with slightly higher expression observed in proliferative endometrium [Shiozawa et al., 2003].
SRA, a PR coactivator which acts as an RNA, exhibits relatively high expression levels in the human pituitary gland, adrenal gland and liver, and relatively low levels of expression in the breast, ovaries, uterus and prostate [Lanz et al., 2003]. The coregulator E6-AP functions as a coactivator to several nuclear receptors including PR, ER and AR, via its role in the ubiquitin-proteasome pathway, where it is required for the degradation and proper turnover of numerous nuclear hormone receptors [Gao et al., 2005]. E6-AP also directs turnover of other PR-associated coactivators, such as AIB-1/SRC-3 [Mani et al., 2006], suggesting that E6-AP may play an important role in clearing the transcriptional coregulatory complex from a promoter region to allow for the subsequent interaction of activated receptor with a newly-assembled coactivator complex. The finding that the hect domain in E6-AP, which is involved in recruitment of ubiquitin conjugating enzymes, UbcH7 and UbcH8, is not essential for in vitro coactivation of PR [Nawaz et al., 1999], suggests that E6-AP also functions as a coactivator via mechanisms that are independent of its ubiquitin ligase function. Relative expression of E6-AP RNA transcripts in a variety of human tissues was found to be greatest in the lymph node, pineal gland and bladder, moderate in the placenta, uterus, brain, bone and liver, and lowest in the mammary gland, ovary, adrenal gland and blood [Ramamoorthy and Nawaz, 2008].
Transcripts for the PR corepressors N-CoR and SMRT are detectable in mammary gland [Kurebayashi et al., 2000], endometrium and myometrium [Vienonen et al., 2004]. N-CoR was the predominant transcript detected in all three tissue types. As these observations were made in small cohorts of normal tissues collected from clinical specimens, where normal breast samples were obtained from breast cancer specimens by resection of adjacent normal tissue and normal endometrial tissue was derived from hysterectomy samples, it is debatable how normal these tissues may be considered to be, and additional studies in larger cohorts of validated normal tissues would be required to confirm these observations.
Evidence for PR coregulators in disease progression and cancer
There is now strong evidence that altered coregulator expression or activity occurs in a number of human pathologies, including cancers in progesterone target tissues [Lanz et al., 2008]. The general trend for PR coactivators is to be overexpressed and amplified in cancers, suggesting that increased availability of these proteins may enhance PR activity in tissues such as the breast, where it is known to play a role in cancer development. Overexpression in cancers of coregulators that are not normally abundant in a particular normal tissue, for example the breast or uterus, may allow PR to complex with coregulators in cancer tissues that it would not associate with in the corresponding normal tissue, and lead to changed target gene selection.
Among the PR coregulators known to be associated with malignancy, SRC-3 is the best characterized. Initially identified as Amplified In Breast cancer-1 (AIB-1), SRC-3 is often highly expressed and amplified in primary human breast cancers [Anzick et al., 1997] and amplification is correlated with elevated protein level [List et al., 2001]. A screen of 105 unselected specimens of primary breast cancer revealed SRC-3 amplification in approximately 10% of primary tumors and this amplification was correlated with elevated transcript expression [Anzick et al., 1997]. In addition, elevated SRC-3 expression relative to normal mammary epithelium was detected in 58% of tumors in which no amplification was detected [Anzick et al., 1997], indicating that overexpression of SRC-3 by mechanisms other than amplification also occurs frequently in human breast cancers. Bautista et al [Bautista et al., 1998] demonstrated increased SRC-3 copy number in 4.8% of breast tumors and 7.4% of ovarian tumors and amplification in breast cancers was positively correlated with ER and PR. Increased SRC-3 transcript [Kershah et al., 2004] and protein [Balmer et al., 2006] expression was also reported in endometrial cancers and was correlated with ER and PR positivity [Balmer et al., 2006].
Overexpression of SRC-2 and SRC-3 has also been reported in endometrial, prostate and brain cancers [Carroll et al., 2000; Kershah et al., 2004; Lanz et al., 2008], supporting their role in enhanced hormone responsiveness in these malignancies. Gregory et al [Gregory et al., 2002] showed an increased level of expression of SRC-2 and SRC-3 in the endometrium of women with polycystic ovarian syndrome, a condition known to be associated with a higher risk of endometrial cancer. Elevated expression of SRC-2 was reported in meningiomas, compared to normal brain, and expression was correlated with PR expression [Carroll et al., 2000; Lanz et al., 2008].
Altered expression of coregulators that can impact on SRC function has also been reported. The magnesium-dependent protein phosphatase PPM1D has been reported to enhance the intrinsic activity of p160 coactivators and to promote interaction between PR and SRC-1 [Proia et al., 2006]. PPM1D, which enhanced PR activity in vitro [Proia et al., 2006], was amplified and overexpressed in breast cancer cell lines and primary breast cancers [Bulavin et al., 2002; Li et al., 2002]. As described above, the ubiquitin ligase function of E6-AP is required for proper turnover of coactivators, as well as nuclear receptors. E6-AP was lower in invasive breast cancer than in adjacent normal mammary tissue or ductal carcinoma in situ (DCIS) [Gao et al., 2005], suggesting that reduced ubiquitin ligase activity may contribute to the elevation in p160 coactivator levels often observed in breast cancers. The expression of E6-AP in DCIS was not different from normal tissue, suggesting that the downregulation of E6-AP protein is a relatively late event in the development of cancer and is associated with the invasive phenotype [Gao et al., 2005]. Consistent with its role in ER and PR turnover, E6-AP protein level was inversely correlated with expression of ERα, suggesting that loss of this coregulator may augment hormone signaling in breast cancers.
In a survey of several cancer types, SRA was overexpressed in over 90% of ovarian primary tumor samples, 100% of uterine samples, and 90% of breast tumors, compared to increased expression in fewer than 35% of tumors of the pancreas and kidney, suggesting that the upregulation of SRA was specific for hormone-dependent malignancies [Lanz et al., 1999]. This is in contrast to the relatively lower expression of SRA in normal hormone-responsive tissues. Overexpression of SRA in transgenic mice resulted in precocious lobular-alveolar development of the mammary gland during pregnancy, thus supporting the assertion that overexpression of SRA in the breast may enhance progesterone action and in this way could contribute to breast cancer development [Lanz et al., 2003].
Overexpression of transcripts for the corepressors N-CoR and SMRT has been reported in breast [Kurebayashi et al., 2000] and endometrial cancers [Kershah et al., 2004]. Both of these studies are relatively preliminary and the impact of transcript overexpression on protein levels has not been determined. Moreover, since these corepressors only affect PR action in the context of mixed antagonists, their overexpression in progesterone target tissues is unlikely to play a role in progesterone-related breast cancer etiology.
In summary, the studies discussed suggest that amplification and/or altered expression of PR coregulators is a relatively common event in hormone-dependent malignancies, which appears likely to have functional consequences for progesterone signaling in those tissues, particularly if elevated abundance of coregulators enhances steroid responsiveness in cancer tissues.
Summary and conclusion
When bound to progesterone, PR activates target gene transcription in a diverse range of target tissues such as the breast, uterus, brain, central nervous and cardiovascular systems. The effects of progesterone on these target tissues are diverse, and the evidence to date supports the view that coregulators play a critical role in regulating the magnitude and nature of the biological response to progesterone, and contribute to the expression of a diverse subset of progesterone-regulated genes and specificity of progesterone action in target tissues.
The pool of coregulators that are able to associate with PR, and the complex series of steps involved in receptor activation, coregulator association and ultimately transcriptional activation have now been defined by elegant in vitro studies [Lonard et al., 2007; Lonard and O'Malley, 2007]. However, it is clear that there is a surplus of potential PR interactors, and the nature of endogenous PR-coregulator complexes that actually form in true target tissues has not been explored. Delineation of the role of coregulators in the specificity of PR action in target tissues will require demonstrating a functional association between the progesterone receptor and its coregulators in human tissues and identification of the PR coregulators required for progesterone action in human physiology.
Although the expression and role of PR coregulators has been extensively studied in vitro and in animal models, it has become evident that few studies have looked comprehensively at PR coregulator levels and function in human tissues, making it difficult to assess whether the differences in progesterone action can be attributed to specific coregulator interactions with PR. Nevertheless, disparity in PR coregulator expression in various human tissues, such as in the endometrium throughout the different phases of the menstrual cycle, suggest that PR coregulators do play an important role in regulating the tissue-specific response to progesterone.
Demonstration of the co-location of PR and coregulators by in situ methods such as immunofluorescence in target tissues is an essential first step in demonstrating a functional association that potentially contributes to the specificity of PR action. Very few studies in human tissues have explored co-location of PR and coregulators, and of those that have, none have distinguished between expression of the two PR isoforms and the differential coregulator affinities of PRA and PRB. As co-location is a correlative measure of association, direct measurement of PR-coregulator association, ideally performed on primary human cells, may allow for the identification of coregulatory proteins existing in a complex with the PR, providing support for a functional association. Such studies would also contribute to the identification of PR coregulators that are required for progesterone action in the various cell types in which this receptor is active in normal physiology.
It is essential to identify PR coregulators required for PR to move into nuclear foci, as these coregulators are likely to play a direct role in regulating the specific effects of progesterone in normal tissues and in cancers. The aberrant formation of PR foci in cancers suggests that PR forms complexes with different and/or larger numbers of coregulatory proteins in these tissues, potentially leading to functionally different PR foci in normal and malignant cells. An analysis of PR coregulators involved in PR foci formation is required to identify PR coregulators directly involved in modulating progesterone effects in normal tissues and cancers.
A significant challenge in identifying coregulators required for progesterone action arises from the fact that nuclear receptors are highly related, sharing the same structural features and utilizing a common pool of coregulator partners to regulate transcription. All coregulators that have been demonstrated to interact with PR, also interact with numerous other nuclear receptors such as ER and AR, and interact with unrelated transcription factors. Furthermore, evidence suggests that coregulators can control cellular functions outside of the nucleus such as mRNA translation, mitochondrial function, and motility [Dawson et al., 1996].
In summary, it has become clear that studies of coregulators, their interaction with the PR, and the downstream effects of such interactions in progesterone target tissues in the human, are still in their infancy. Further studies are required to uncover the role of PR coregulators in human tissues and the impact of variation in their level of expression on progesterone action in human cells and tissues.
Abbreviations
- ACTR
actin-related protein
- AF
activation function
- AIB-1
amplified in breast cancer-1
- AR
androgen receptor
- CARM1
coactivator-associated arginine methyltransferase 1
- CBP/p300
CREB binding protein
- DBD
DNA-binding domain
- DCIS
ductal carcinoma in situ
- E6-AP
E6-associated protein
- ERα
estrogen receptor α
- GRIP1
glucocorticoid receptor-interacting protein 1
- IF
inhibitory function
- LBD
ligand-binding domain
- NCoA-1
nuclear receptor coactivator 1
- N-CoR
nuclear receptor corepressor
- p/CIP
p300/CBP interacting protein
- PML
promyelocytic leukemia
- PPM1D
protein phosphatase 1D magnesium dependent
- PR
progesterone receptor
- PRA
progesterone receptor A
- PRB
progesterone receptor B
- PRC
progesterone receptor C
- SMRT
silencing mediator for retinoid and thyroid hormone
- SRA
steroid receptor RNA activator
- SRC-1
steroid receptor coactivator-1
- SRC-2
steroid receptor coactivator-2
- SRC-3
steroid receptor coactivator-3
- SWI/SNF
switch/sucrose nonfermentable
- TIF2
transcription intermediary factor 2
- TRAM1
thyroid hormone receptor activator molecule 1
- UbcH7
ubiquitin conjugating enzyme H7
- UbcH8
ubiquitin conjugating enzyme H8
References
- Abdel-Hafiz H., Takimoto G. S., Tung L., Horwitz K. B. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277:33950–6. doi: 10.1074/jbc.M204573200. [DOI] [PubMed] [Google Scholar]
- Anzick S. L., Kononen J., Walker R. L., Azorsa D. O., Tanner M. M., Guan X. Y., Sauter G., Kallioniemi O. P., Trent J. M., Meltzer P. S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–8. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Arnett-Mansfield R. L., Graham J. D., Hanson A. R., Mote P. A., Gompel A., Scurr L. L., Gava N., de Fazio A., Clarke C. L. Focal subnuclear distribution of progesterone receptor is ligand dependent and associated with transcriptional activity. Mol Endocrinol. 2007;21:14–29. doi: 10.1210/me.2006-0041. [DOI] [PubMed] [Google Scholar]
- Arnett-Mansfield R. L., deFazio A., Wain G. V., Jaworski R. C., Byth K., Mote P. A., Clarke C. L. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001;61:4576–82. [PubMed] [Google Scholar]
- Arnett-Mansfield R. L., DeFazio A., Mote P. A., Clarke C. L. Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J Clin Endocrinol Metab. 2004;89:1429–42. doi: 10.1210/jc.2003-031111. [DOI] [PubMed] [Google Scholar]
- Aupperlee M. D., Haslam S. Z. Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology. 2007;148:2290–300. doi: 10.1210/en.2006-1721. [DOI] [PubMed] [Google Scholar]
- Aupperlee M. D., Smith K. T., Kariagina A., Haslam S. Z. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology. 2005;146:3577–88. doi: 10.1210/en.2005-0346. [DOI] [PubMed] [Google Scholar]
- Bain D. L., Franden M. A., McManaman J. L., Takimoto G. S., Horwitz K. B. The N-terminal region of human progesterone B-receptors: biophysical and biochemical comparison to A-receptors. J Biol Chem. 2001;276:23825–31. doi: 10.1074/jbc.M102611200. [DOI] [PubMed] [Google Scholar]
- Bain D. L., Franden M. A., McManaman J. L., Takimoto G. S., Horwitz K. B. The N-terminal region of the human progesterone A-receptor. Structural analysis and the influence of the DNA binding domain. J Biol Chem. 2000;275:7313–20. doi: 10.1074/jbc.275.10.7313. [DOI] [PubMed] [Google Scholar]
- Balmer N. N., Richer J. K., Spoelstra N. S., Torkko K. C., Lyle P. L., Singh M. Steroid receptor coactivator AIB1 in endometrial carcinoma, hyperplasia and normal endometrium: Correlation with clinicopathologic parameters and biomarkers. Mod Pathol. 2006;19:1593–605. doi: 10.1038/modpathol.3800696. [DOI] [PubMed] [Google Scholar]
- Baumann C. T., Ma H., Wolford R., Reyes J. C., Maruvada P., Lim C., Yen P. M., Stallcup M. R., Hager G. L. The glucocorticoid receptor interacting protein 1 (GRIP1) localizes in discrete nuclear foci that associate with ND10 bodies and are enriched in components of the 26S proteasome. Mol Endocrinol. 2001;15:485–500. doi: 10.1210/mend.15.4.0618. [DOI] [PubMed] [Google Scholar]
- Bautista S., Valles H., Walker R. L., Anzick S., Zeillinger R., Meltzer P., Theillet C. In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin Cancer Res. 1998;4:2925–9. [PubMed] [Google Scholar]
- Beato M., Arnemann J., Chalepakis G., Slater E., Willmann T. Gene regulation by steroid hormones. J Steroid Biochem. 1987;27:9–14. doi: 10.1016/0022-4731(87)90288-3. [DOI] [PubMed] [Google Scholar]
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Bland R. Steroid hormone receptor expression and action in bone. Clin Sci (Lond) 2000;98:217–40. [PubMed] [Google Scholar]
- Brayman M. J., Julian J., Mulac-Jericevic B., Conneely O. M., Edwards D. P., Carson D. D. Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol. 2006;20:2278–91. doi: 10.1210/me.2005-0343. [DOI] [PubMed] [Google Scholar]
- Bulavin D. V., Demidov O. N., Saito S., Kauraniemi P., Phillips C., Amundson S. A., Ambrosino C., Sauter G., Nebreda A. R., Anderson C. W., Kallioniemi A., Fornace A. J., Jr., Appella E. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31:210–5. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- Carroll R. S., Brown M., Zhang J., DiRenzo J., Font De Mora J., Black P. M. Expression of a subset of steroid receptor cofactors is associated with progesterone receptor expression in meningiomas. Clin Cancer Res. 2000;6:3570–5. [PubMed] [Google Scholar]
- Chalbos D., Galtier F. Differential effect of forms A and B of human progesterone receptor on estradiol-dependent transcription. J Biol Chem. 1994;269:23007–12. [PubMed] [Google Scholar]
- Condon J. C., Hardy D. B., Kovaric K., Mendelson C. R. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–75. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Mulac-Jericevic B., Arnett-Mansfield R. Progesterone signaling in mammary gland development. Ernst Schering Found Symp Proc. 2007:45–54. [PubMed] [Google Scholar]
- Daniel A. R., Qiu M., Faivre E. J., Ostrander J. H., Skildum A., Lange C. A. Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72:188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. J., Wolman S. R., Tait L., Heppner G. H., Miller F. R. MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol. 1996;148:313–9. [PMC free article] [PubMed] [Google Scholar]
- Dufrene L., Pageaux J. F., Fanidi A., Renoir J. M., Laugier C., Baulieu E. E. Biochemical characterization and subunit structure of quail oviduct progesterone receptor. J Steroid Biochem. 1989;32:703–13. doi: 10.1016/0022-4731(89)90516-5. [DOI] [PubMed] [Google Scholar]
- Edwards D. P. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia. 2000;5:307–24. doi: 10.1023/a:1009503029176. [DOI] [PubMed] [Google Scholar]
- Escriva H., Bertrand S., Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- Evangelou A., Letarte M., Jurisica I., Sultan M., Murphy K. J., Rosen B., Brown T. J. Loss of coordinated androgen regulation in nonmalignant ovarian epithelial cells with BRCA1/2 mutations and ovarian cancer cells. Cancer Res. 2003;63:2416–24. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejes-Toth G., Pearce D., Naray-Fejes-Toth A. Subcellular localization of mineralocorticoid receptors in living cells: effects of receptor agonists and antagonists. Proc Natl Acad Sci U S A. 1998;95:2973–8. doi: 10.1073/pnas.95.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Mohsin S. K., Gatalica Z., Fu G., Sharma P., Nawaz Z. Decreased expression of e6-associated protein in breast and prostate carcinomas. Endocrinology. 2005;146:1707–12. doi: 10.1210/en.2004-1198. [DOI] [PubMed] [Google Scholar]
- Gao X., Nawaz Z. Progesterone receptors - animal models and cell signaling in breast cancer: Role of steroid receptor coactivators and corepressors of progesterone receptors in breast cancer. Breast Cancer Res. 2002;4:182–6. doi: 10.1186/bcr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gava N., Clarke C. L., Byth K., Arnett-Mansfield R. L., deFazio A. Expression of progesterone receptors A and B in the mouse ovary during the estrous cycle. Endocrinology. 2004;145:3487–94. doi: 10.1210/en.2004-0212. [DOI] [PubMed] [Google Scholar]
- Giangrande P. H., McDonnell D. P. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313; discussion 313-4. [PubMed] [Google Scholar]
- Giangrande P. H., Kimbrel E. A., Edwards D. P., McDonnell D. P. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–15. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoukos G., Coho D. W., Callard I. P. Turtle oviduct progesterone receptor: radioligand and immunocytochemical studies of changes during the seasonal cycle. Endocrine. 1995;3:429–437. doi: 10.1007/BF02935649. [DOI] [PubMed] [Google Scholar]
- Graham J. D., Yager M. L., Hill H. D., Byth K., O'Neill G. M., Clarke C. L. Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endocrinol. 2005;19:2713–35. doi: 10.1210/me.2005-0126. [DOI] [PubMed] [Google Scholar]
- Graham J. D., Yeates C., Balleine R. L., Harvey S. S., Milliken J. S., Bilous A. M., Clarke C. L. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995;55:5063–8. [PubMed] [Google Scholar]
- Graham J. D., Clarke C. L. Expression and transcriptional activity of progesterone receptor A and progesterone receptor B in mammalian cells. Breast Cancer Res. 2002;4:187–90. doi: 10.1186/bcr450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. D., Hanson A. R., Croft A. J., Fox A. H., Clarke C. L. Nuclear matrix binding is critical for progesterone receptor movement into nuclear foci. Faseb J. 2009;23:546–56. doi: 10.1096/fj.08-113639. [DOI] [PubMed] [Google Scholar]
- Graham J. D., Clarke C. L. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–19. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- Gregory C. W., Wilson E. M., Apparao K. B., Lininger R. A., Meyer W. R., Kowalik A., Fritz M. A., Lessey B. A. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab. 2002;87:2960–6. doi: 10.1210/jcem.87.6.8572. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Meyer M. E., Bocquel M. T., Kastner P., Turcotte B., Chambon P. Progestin receptors: isoforms and antihormone action. J Steroid Biochem Mol Biol. 1991b;40:271–8. doi: 10.1016/0960-0760(91)90192-8. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991a;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Guo A., Salomoni P., Luo J., Shih A., Zhong S., Gu W., Pandolfi P. P. The function of PML in p53-dependent apoptosis. Nat Cell Biol. 2000;2:730–6. doi: 10.1038/35036365. [DOI] [PubMed] [Google Scholar]
- Han Y., Feng H. L., Sandlow J. I., Haines C. J. Comparing expression of progesterone and estrogen receptors in testicular tissue from men with obstructive and nonobstructive azoospermia. J Androl. 2009;30:127–33. doi: 10.2164/jandrol.108.005157. [DOI] [PubMed] [Google Scholar]
- Han S. J., Jeong J., Demayo F. J., Xu J., Tsai S. Y., Tsai M. J., O'Malley B. W. Dynamic cell type specificity of SRC-1 coactivator in modulating uterine progesterone receptor function in mice. Mol Cell Biol. 2005;25:8150–65. doi: 10.1128/MCB.25.18.8150-8165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. J., DeMayo F. J., Xu J., Tsai S. Y., Tsai M. J., O'Malley B. W. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20:45–55. doi: 10.1210/me.2005-0310. [DOI] [PubMed] [Google Scholar]
- Heneghan A. F., Connaghan-Jones K. D., Miura M. T., Bain D. L. Coactivator assembly at the promoter: efficient recruitment of SRC2 is coupled to cooperative DNA binding by the progesterone receptor. Biochemistry. 2007;46:11023–32. doi: 10.1021/bi700850v. [DOI] [PubMed] [Google Scholar]
- Holtorf K. The bioidentical hormone debate: are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy? Postgrad Med. 2009;121:73–85. doi: 10.3810/pgm.2009.01.1949. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. The Year in Basic Science: update of estrogen plus progestin therapy for menopausal hormone replacement implicating stem cells in the increased breast cancer risk. Mol Endocrinol. 2008;22:2743–50. doi: 10.1210/me.2008-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun H., Holth L. T., Walker D., Davie J. R., Hager G. L. Direct visualization of the human estrogen receptor α reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell. 1999;10:471–86. doi: 10.1091/mbc.10.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun H., Barsony J., Renyi I., Gould D. L., Hager G. L. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc Natl Acad Sci U S A. 1996;93:4845–50. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse B., Verca S. B., Matthey P., Rusconi S. Definition of a negative modulation domain in the human progesterone receptor. Mol Endocrinol. 1998;12:1334–42. doi: 10.1210/mend.12.9.0164. [DOI] [PubMed] [Google Scholar]
- Hussein-Fikret S., Fuller P. J. Expression of nuclear receptor coregulators in ovarian stromal and epithelial tumours. Mol Cell Endocrinol. 2005;229:149–60. doi: 10.1016/j.mce.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Ilenchuk T. T., Walters M. R. Rat uterine progesterone receptor analyzed by [3H]R5020 photoaffinity labeling: evidence that the A and B subunits are not equimolar. Endocrinology. 1987;120:1449–56. doi: 10.1210/endo-120-4-1449. [DOI] [PubMed] [Google Scholar]
- Jackson T. A., Richer J. K., Bain D. L., Takimoto G. S., Tung L., Horwitz K. B. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol Endocrinol. 1997;11:693–705. doi: 10.1210/mend.11.6.0004. [DOI] [PubMed] [Google Scholar]
- Jacobsen B. M., Richer J. K., Schittone S. A., Horwitz K. B. New human breast cancer cells to study progesterone receptor isoform ratio effects and ligand-independent gene regulation. J Biol Chem. 2002;277:27793–800. doi: 10.1074/jbc.M202584200. [DOI] [PubMed] [Google Scholar]
- Jacobsen B. M., Schittone S. A., Richer J. K., Horwitz K. B. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol Endocrinol. 2005;19:574–87. doi: 10.1210/me.2004-0287. [DOI] [PubMed] [Google Scholar]
- Jeong J. W., Lee K. Y., Han S. J., Aronow B. J., Lydon J. P., O'Malley B. W., DeMayo F. J. The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology. 2007;148:4238–50. doi: 10.1210/en.2007-0122. [DOI] [PubMed] [Google Scholar]
- Kariagina A., Aupperlee M. D., Haslam S. Z. Progesterone receptor isoforms and proliferation in the rat mammary gland during development. Endocrinology. 2007;148:2723–36. doi: 10.1210/en.2006-1493. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 1990;9:1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Hirata S., Nozawa A., Mouri N. The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid Biochem Mol Biol. 1993;47:173–82. doi: 10.1016/0960-0760(93)90072-5. [DOI] [PubMed] [Google Scholar]
- Kershah S. M., Desouki M. M., Koterba K. L., Rowan B. G. Expression of estrogen receptor coregulators in normal and malignant human endometrium. Gynecol Oncol. 2004;92:304–13. doi: 10.1016/j.ygyno.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Kurebayashi J., Otsuki T., Kunisue H., Tanaka K., Yamamoto S., Sonoo H. Expression levels of estrogen receptor-α, estrogen receptor-β, coactivators, and corepressors in breast cancer. Clin Cancer Res. 2000;6:512–8. [PubMed] [Google Scholar]
- Lamb D. J., Kima P. E., Bullock D. W. Evidence for a single steroid-binding protein in the rabbit progesterone receptor. Biochemistry. 1986;25:6319–24. doi: 10.1021/bi00368a073. [DOI] [PubMed] [Google Scholar]
- Lange C. A. Challenges to defining a role for progesterone in breast cancer. Steroids. 2008a;73:914–21. doi: 10.1016/j.steroids.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C. A., Richer J. K., Shen T., Horwitz K. B. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:31308–16. doi: 10.1074/jbc.273.47.31308. [DOI] [PubMed] [Google Scholar]
- Lange C. A. Integration of progesterone receptor action with rapid signaling events in breast cancer models. J Steroid Biochem Mol Biol. 2008b;108:203–12. doi: 10.1016/j.jsbmb.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz R. B., McKenna N. J., Onate S. A., Albrecht U., Wong J., Tsai S. Y., Tsai M. J., O'Malley B. W. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27. doi: 10.1016/s0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- Lanz R. B., Chua S. S., Barron N., Soder B. M., DeMayo F., O'Malley B. W. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Mol Cell Biol. 2003;23:7163–76. doi: 10.1128/MCB.23.20.7163-7176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz R. B., Lonard D. M., O'Malley B. W. Nuclear Receptor Coregulators in Human Diseases. New Jersey: World Scientific; 2008. Nuclear receptor coregulators in human diseases. pp. 1–133. [Google Scholar]
- Lavinsky R. M., Jepsen K., Heinzel T., Torchia J., Mullen T. M., Schiff R., Del-Rio A. L., Ricote M., Ngo S., Gemsch J., Hilsenbeck S. G., Osborne C. K., Glass C. K., Rosenfeld M. G., Rose D. W. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci U S A. 1998;95:2920–5. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. A., Ross R. K., Pike M. C. An overview of menopausal oestrogen-progestin hormone therapy and breast cancer risk. Br J Cancer. 2005;92:2049–58. doi: 10.1038/sj.bjc.6602617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt S. A., Boonyaratanakornkit V., Edwards D. P. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68:761–70. doi: 10.1016/s0039-128x(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Li X., Lonard D. M., O'Malley B. W. A contemporary understanding of progesterone receptor function. Mech Ageing Dev. 2004;125:669–78. doi: 10.1016/j.mad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Li J., Yang Y., Peng Y., Austin R. J., van Eyndhoven W. G., Nguyen K. C., Gabriele T., McCurrach M. E., Marks J. R., Hoey T., Lowe S. W., Powers S. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat Genet. 2002;31:133–4. doi: 10.1038/ng888. [DOI] [PubMed] [Google Scholar]
- List H. J., Reiter R., Singh B., Wellstein A., Riegel A. T. Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res Treat. 2001;68:21–8. doi: 10.1023/a:1017910924390. [DOI] [PubMed] [Google Scholar]
- Lonard D. M., Lanz R. B., O'Malley B. W. Nuclear receptor coregulators and human disease. Endocr Rev. 2007b;28:575–87. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- Lonard D. M., O'Malley B. W. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007a;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A., Jr., Shyamala G., Conneely O. M., O'Malley B. W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mani A., Oh A. S., Bowden E. T., Lahusen T., Lorick K. L., Weissman A. M., Schlegel R., Wellstein A., Riegel A. T. E6AP mediates regulated proteasomal degradation of the nuclear receptor coactivator amplified in breast cancer 1 in immortalized cells. Cancer Res. 2006;66:8680–6. doi: 10.1158/0008-5472.CAN-06-0557. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P., Goldman M. E. RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J Biol Chem. 1994;269:11945–9. [PubMed] [Google Scholar]
- McEwan I. J. Nuclear receptors: one big family. Methods Mol Biol. 2009;505:3–18. doi: 10.1007/978-1-60327-575-0_1. [DOI] [PubMed] [Google Scholar]
- McKenna N. J., O'Malley B. W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–74. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- McKenna N. J., Nawaz Z., Tsai S. Y., Tsai M. J., O'Malley B. W. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc Natl Acad Sci U S A. 1998;95:11697–702. doi: 10.1073/pnas.95.20.11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna N. J., Lanz R. B., O'Malley B. W. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–44. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Quirin-Stricker C., Lerouge T., Bocquel M. T., Gronemeyer H. A limiting factor mediates the differential activation of promoters by the human progesterone receptor isoforms. J Biol Chem. 1992;267:10882–7. [PubMed] [Google Scholar]
- Molenda-Figueira H. A., Murphy S. D., Shea K. L., Siegal N. K., Zhao Y., Chadwick J. G., Jr., Denner L. A., Tetel M. J. Steroid receptor coactivator-1 from brain physically interacts differentially with steroid receptor subtypes. Endocrinology. 2008;149:5272–9. doi: 10.1210/en.2008-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote P. A., Balleine R. L., McGowan E. M., Clarke C. L. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84:2963–71. doi: 10.1210/jcem.84.8.5928. [DOI] [PubMed] [Google Scholar]
- Mote P. A., Bartow S., Tran N., Clarke C. L. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72:163–72. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- Mote P. A., Arnett-Mansfield R. L., Gava N., deFazio A., Mulac-Jericevic B., Conneely O. M., Clarke C. L. Overlapping and distinct expression of progesterone receptors A and B in mouse uterus and mammary gland during the estrous cycle. Endocrinology. 2006;147:5503–12. doi: 10.1210/en.2006-0040. [DOI] [PubMed] [Google Scholar]
- Mote P. A., Graham J. D., Clarke C. L. Progesterone receptor isoforms in normal and malignant breast. Ernst Schering Found Symp Proc. 2007:77–107. [PubMed] [Google Scholar]
- Mukherjee A., Soyal S. M., Fernandez-Valdivia R., Gehin M., Chambon P., Demayo F. J., Lydon J. P., O'Malley B. W. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006a;26:6571–83. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Amato P., Allred D. C., Fernandez-Valdivia R., Nguyen J., O'Malley B. W., DeMayo F. J., Lydon J. P. Steroid receptor coactivator 2 is essential for progesterone-dependent uterine function and mammary morphogenesis: insights from the mouse--implications for the human. J Steroid Biochem Mol Biol. 2006b;102:22–31. doi: 10.1016/j.jsbmb.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Lydon J. P., DeMayo F. J., Conneely O. M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Mullinax R. A., DeMayo F. J., Lydon J. P., Conneely O. M. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–4. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Nawaz Z., Lonard D. M., Smith C. L., Lev-Lehman E., Tsai S. Y., Tsai M. J., O'Malley B. W. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:1182–9. doi: 10.1128/mcb.19.2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J., Brinton R. D. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–11. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J., Brinton R. D. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Onate S. A., Tsai S. Y., Tsai M. J., O'Malley B. W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–7. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Ozawa H. Steroid Hormones, their receptors and neuroendocrine system. J Nippon Med Sch. 2005;72:316–25. doi: 10.1272/jnms.72.316. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Wu A. H., Spicer D. V., Lee S., Pearce C. L. Estrogens, progestins, and risk of breast cancer. Ernst Schering Found Symp Proc. 2007:127–50. doi: 10.1007/2789_2007_059. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Galigniana M. D., Morishima Y., Murphy P. J. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004;40:41–58. doi: 10.1042/bse0400041. [DOI] [PubMed] [Google Scholar]
- Proia D. A., Nannenga B. W., Donehower L. A., Weigel N. L. Dual roles for the phosphatase PPM1D in regulating progesterone receptor function. J Biol Chem. 2006;281:7089–101. doi: 10.1074/jbc.M511839200. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S., Nawaz Z. E6-associated protein (E6-AP) is a dual function coactivator of steroid hormone receptors. Nucl Recept Signal. 2008;6:e006. doi: 10.1621/nrs.06006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese J. C., Callard I. P. Two progesterone receptors in the oviduct of the freshwater turtle Chrysemys picta: possible homology to mammalian and avian progesterone receptor systems. J Steroid Biochem. 1989;33:297–310. doi: 10.1016/0022-4731(89)90308-7. [DOI] [PubMed] [Google Scholar]
- Richer J. K., Jacobsen B. M., Manning N. G., Abel M. G., Wolf D. M., Horwitz K. B. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–18. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- Rossouw J. E., Anderson G. L., Prentice R. L., LaCroix A. Z., Kooperberg C., Stefanick M. L., Jackson R. D., Beresford S. A., Howard B. V., Johnson K. C., Kotchen J. M., Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rowan B. G., O'Malley B. W. Progesterone receptor coactivators. Steroids. 2000;65:545–9. doi: 10.1016/s0039-128x(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Samalecos A., Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology. 2008;149:5872–87. doi: 10.1210/en.2008-0602. [DOI] [PubMed] [Google Scholar]
- Santen R. J. Risk of breast cancer with progestins: critical assessment of current data. Steroids. 2003;68:953–64. doi: 10.1016/s0039-128x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- Sartorius C. A., Melville M. Y., Hovland A. R., Tung L., Takimoto G. S., Horwitz K. B. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol. 1994;8:1347–60. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- Schneider W., Ramachandran C., Satyaswaroop P. G., Shyamala G. Murine progesterone receptor exists predominantly as the 83-kilodalton 'A' form. J Steroid Biochem Mol Biol. 1991;38:285–91. doi: 10.1016/0960-0760(91)90099-q. [DOI] [PubMed] [Google Scholar]
- Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct. IV. Characterization of purified subunits. J Biol Chem. 1972a;247:51–9. [PubMed] [Google Scholar]
- Schrader W. T., Toft D. O., O'Malley B. W. Progesterone-binding protein of chick oviduct. VI. Interaction of purified progesterone-receptor components with nuclear constituents. J Biol Chem. 1972b;247:2401–7. [PubMed] [Google Scholar]
- Shiozawa T., Shih H. C., Miyamoto T., Feng Y. Z., Uchikawa J., Itoh K., Konishi I. Cyclic changes in the expression of steroid receptor coactivators and corepressors in the normal human endometrium. J Clin Endocrinol Metab. 2003;88:871–8. doi: 10.1210/jc.2002-020946. [DOI] [PubMed] [Google Scholar]
- Shyamala G., Schneider W., Schott D. Developmental regulation of murine mammary progesterone receptor gene expression. Endocrinology. 1990;126:2882–9. doi: 10.1210/endo-126-6-2882. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Nawaz Z., O'Malley B. W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–66. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Faber L. E., Toft D. O. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990;265:3996–4003. [PubMed] [Google Scholar]
- Tata J. R. Signalling through nuclear receptors. Nat Rev Mol Cell Biol. 2002;3:702–10. doi: 10.1038/nrm914. [DOI] [PubMed] [Google Scholar]
- Tetel M. J., Siegal N. K., Murphy S. D. Cells in behaviourally relevant brain regions coexpress nuclear receptor coactivators and ovarian steroid receptors. J Neuroendocrinol. 2007;19:262–71. doi: 10.1111/j.1365-2826.2007.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel M. J., Giangrande P. H., Leonhardt S. A., McDonnell D. P., Edwards D. P. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol Endocrinol. 1999;13:910–24. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- Tincello D. G., Taylor A. H., Spurling S. M., Bell S. C. Receptor isoforms that mediate estrogen and progestagen action in the female lower urinary tract. J Urol. 2009;181:1474–82. doi: 10.1016/j.juro.2008.10.104. [DOI] [PubMed] [Google Scholar]
- Tung L., Mohamed M. K., Hoeffler J. P., Takimoto G. S., Horwitz K. B. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993;7:1256–65. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- Tung L., Abdel-Hafiz H., Shen T., Harvell D. M., Nitao L. K., Richer J. K., Sartorius C. A., Takimoto G. S., Horwitz K. B. Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol. 2006;20:2656–70. doi: 10.1210/me.2006-0105. [DOI] [PubMed] [Google Scholar]
- Tyagi R. K., Lavrovsky Y., Ahn S. C., Song C. S., Chatterjee B., Roy A. K. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000;14:1162–74. doi: 10.1210/mend.14.8.0497. [DOI] [PubMed] [Google Scholar]
- van Steensel B., Brink M., van der Meulen K., van Binnendijk E. P., Wansink D. G., de Jong L., de Kloet E. R., van Driel R. Localization of the glucocorticoid receptor in discrete clusters in the cell nucleus. J Cell Sci. 1995;108 ( Pt 9):3003–11. doi: 10.1242/jcs.108.9.3003. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Shahbaz M. M., Wen D. X., Goldman M. E., O'Malley B. W., McDonnell D. P. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–55. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Vicent G. P., Nacht A. S., Smith C. L., Peterson C. L., Dimitrov S., Beato M. DNA instructed displacement of histones H2A and H2B at an inducible promoter. Mol Cell. 2004;16:439–52. doi: 10.1016/j.molcel.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Vienonen A., Miettinen S., Blauer M., Martikainen P. M., Tomas E., Heinonen P. K., Ylikomi T. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11:104–12. doi: 10.1016/j.jsgi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Wei L. L., Gonzalez-Aller C., Wood W. M., Miller L. A., Horwitz K. B. 5'-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated "C"-receptor and unique A-receptor messages. Mol Endocrinol. 1990;4:1833–40. doi: 10.1210/mend-4-12-1833. [DOI] [PubMed] [Google Scholar]
- Wei L. L., Hawkins P., Baker C., Norris B., Sheridan P. L., Quinn P. G. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol. 1996;10:1379–87. doi: 10.1210/mend.10.11.8923464. [DOI] [PubMed] [Google Scholar]
- Wei L. L., Norris B. M., Baker C. J. An N-terminally truncated third progesterone receptor protein, PR(C), forms heterodimers with PR(B) but interferes in PR(B)-DNA binding. J Steroid Biochem Mol Biol. 1997;62:287–97. doi: 10.1016/s0960-0760(97)00044-7. [DOI] [PubMed] [Google Scholar]
- Wen D. X., Xu Y. F., Mais D. E., Goldman M. E., McDonnell D. P. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14:8356–64. doi: 10.1128/mcb.14.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser F., Schneeberger C., Hudelist G., Singer C., Kurz C., Nagele F., Gruber C., Huber J. C., Tschugguel W. Endometrial nuclear receptor co-factors SRC-1 and N-CoR are increased in human endometrium during menstruation. Mol Hum Reprod. 2002;8:644–50. doi: 10.1093/molehr/8.7.644. [DOI] [PubMed] [Google Scholar]
- Wolf I. M., Heitzer M. D., Grubisha M., DeFranco D. B. Coactivators and nuclear receptor transactivation. J Cell Biochem. 2008;104:1580–6. doi: 10.1002/jcb.21755. [DOI] [PubMed] [Google Scholar]
- Xu J., Qiu Y., DeMayo F. J., Tsai S. Y., Tsai M. J., O'Malley B. W. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–5. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- Xu J., Liao L., Ning G., Yoshida-Komiya H., Deng C., O'Malley B. W. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A. 2000;97:6379–84. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi S. K., Young D. W., Choi J. Y., Pratap J., Javed A., Montecino M., Stein J. L., Lian J. B., van Wijnen A. J., Stein G. S. Intranuclear trafficking: organization and assembly of regulatory machinery for combinatorial biological control. J Biol Chem. 2004;279:43363–6. doi: 10.1074/jbc.R400020200. [DOI] [PubMed] [Google Scholar]
- Zink D., Fischer A. H., Nickerson J. A. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–87. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]