Abstract
The progesterone receptor (PGR) is a nuclear receptor transcription factor that is essential for female fertility, in part due to its control of oocyte release from the ovary, or ovulation. In all mammals studied to date, ovarian expression of PGR is restricted primarily to granulosa cells of follicles destined to ovulate. Granulosa cell expression of PGR is induced by the pituitary Luteinizing Hormone (LH) surge via mechanisms that are not entirely understood, but which involve activation of Protein Kinase A and modification of Sp1/Sp3 transcription factors on the PGR promoter. Null mutations for PGR or treatment with PGR antagonists block ovulation in all species analyzed, including humans. The cellular mechanisms by which PGR regulates ovulation are currently under investigation, with several downstream pathways having been identified as PGR-regulated and potentially involved in follicular rupture. Interestingly, none of these PGR-regulated genes has been demonstrated to be a direct transcriptional target of PGR. Rather, in ovarian granulosa cells, PGR may act as an inducible coregulator for constitutively bound Sp1/Sp3 transcription factors, which are key regulators for a discrete cohort of ovulatory genes.
Introduction
The two main functions of the ovary are production of reproductive hormones that synchronize the reproductive cycle and to nurture and release oocytes capable of fertilization and development into live offspring. Both processes occur within ovarian follicles, and progress through highly orchestrated developmental stages to culminate in the simultaneous release of a developmentally-competent oocyte and elevated production of progesterone that primes uterine receptivity.
Progesterone is traditionally known to be essential for pregnancy maintenance (hence ‘pro-gestation’) but it also plays a critical role in the ovary. Its effects are mediated by progesterone receptors (PGRs), which are members of the nuclear receptor superfamily of transcription factors (NR3C3) [Evans, 1988; Schrader and O'Malley, 1972; Tsai and O'Malley, 1994]. PGR has been identified as an important regulator of gene transcription during the peri-ovulatory period, specifically of genes found to be necessary for successful oocyte release from the preovulatory follicle.
Hormonally-regulated expression of progesterone receptor in the ovary
Physiological events in the ovulating follicle
Ovarian follicle development is controlled by pituitary FSH and LH (or treatment with PMSG), which maintain follicle growth up to the preovulatory stage. In these follicles, the somatic cells which surround the fluid-filled antral cavity have differentiated into cumulus cells that encase the oocyte, mural granulosa cells which line the follicle wall and theca cells which lie outside the follicular basement membrane on which the mural granulosa cells sit. FSH stimulates the production of high levels of estrogens and the appearance of LH receptors, predominantly on mural granulosa cells [Eppig et al., 1997; Peng et al., 1991]. The mural cells then respond to the ovulatory LH surge (or treatment with hCG) by ceasing proliferation and undergoing luteinization, a terminal differentiation whereby they become highly steroidogenic, synthesizing elevated progesterone that acts on the uterus to prepare it for implantation and subsequent pregnancy maintenance. The cumulus cells undergo cumulus expansion as a secondary response to EGF-like ligands released by granulosa cells responding to LH [Park et al., 2004]. The unique extracellular matrix that forms envelops the cumulus oocyte complex and is important for successful oocyte maturation and ovulation (reviewed by [Russell and Robker, 2007]). At the same time, the oocyte resumes meiosis and undergoes maturation to become competent for ovulation and fertilization.
LH-induction of progesterone receptor (PGR) expression
Activation of the G-protein coupled LH-receptor (LH-R) on mural granulosa cells in response to the LH surge stimulates adenylate cyclase (AC), increases intracellular cAMP, thereby activating protein kinase A (PKA) and inducing expression of PGR (Figure 1). This induction can be prevented by blockade of the LH surge with pentobarbital [Park and Mayo, 1991] or mimicked by using cell permeable cAMP or by activating adenylate cyclase with high doses of FSH or forskolin [Park-Sarge and Mayo, 1994]. The LH surge via PKA also activates MAPK [Russell et al., 2003; Salvador et al., 2002], which is essential for the induction of PGR, since mice lacking ERK1/2 in granulosa cells fail to express PGR mRNA in response to ovulatory hCG [Fan et al., 2009]. Surge levels of LH also activate protein kinase C (PKC) and inclusion of the PKC activator PMA with forskolin in cultured granulosa cells causes a synergistic induction of PGR [Sriraman et al., 2003].
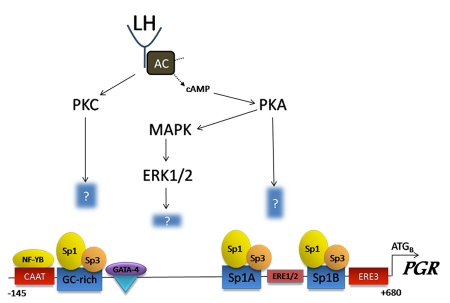
Figure 1. The LH surge transcriptionally regulates PGR expression in rodent granulosa cells.
LH binding to its receptor activates adenylate cyclise (AC), stimulating the production of cAMP and activation of protein kinase A (PKA). The LH surge also activates protein kinase C (PKC), which acts synergistically with PKA to induce PGR mRNA expression. PKA activates MAP kinase (MAPK) and its downstream kinases (ERK1/2) in granulosa cells. The downstream targets of these kinases required for PGR induction are currently unknown. The distal region of the PGR promoter contains a CAAT box which binds NF-YB transcription factor, a GC-rich region which binds Sp1/3, and a GATA site which binds GATA4. The proximal promoter consists of an ERE1/2 site, two Sp1/Sp3binding sites and a series of ERE sites. Interestingly, the only essential regulatory sites are the Sp1/Sp3 sites, and even though estrogen receptors are abundant in granulosa cells, they do not bind the EREs. How the constitutively bound Sp1/Sp3 controls transcription at the PGR promoter is not known, but is thought to involve the recruitment of additional, yet to be identified, coregulatory factors. (Sequence numbering based on the murine PGR promoter and adapted from Sriraman et al., 2003).
PGR expression in response to LH or forskolin only occurs in granulosa cells of preovulatory follicles [Park-Sarge and Mayo, 1994] or those that have been differentiated in FSH plus testosterone or estradiol [Clemens et al., 1998]. The presence of estrogen receptor antagonist ICI182780 during granulosa cell differentiation blunts the induction of PGR by cAMP [Clemens et al., 1998] and ERβ-/- mice exhibit reduced PGR expression due to lower LH-R expression and impaired cAMP production [Deroo et al., 2009].
Within the PGR gene, mRNA expression is regulated by alternate promoters [Kastner et al., 1990; Kraus et al., 1993] and a series of studies on the rodent PGR promoter have identified the key regulatory regions in granulosa cells (Figure 1) [Clemens et al., 1998; Park-Sarge and Mayo, 1994; Park-Sarge et al., 1995; Sriraman et al., 2003]. In contrast to other cell types in which PGR induction has been well characterized, the ERE-like elements do not bind ER in granulosa cells, but interact synergistically with the GC-rich distal promoter [Clemens et al., 1998], with both regions retaining constitutively bound Sp1/Sp3 proteins, which are required for maximal induction of PGR mRNA expression in response to the LH surge [Clemens et al., 1998; Sriraman et al., 2003]. The molecular mechanism by which Sp1/Sp3 controls transactivation of the PGR gene is not known, but is thought to involve recruitment of additional, as yet unidentified, transcriptional regulators to the PGR promoter. Further, how the kinases which are essential for PGR induction modify the transcriptional complex is not known.
PGR mRNA give rise to two protein isoforms, PGR-A (72-94 kDa) and PGR-B (108-120 kDa) [Saqui-Salces et al., 2008; Schrader and O'Malley, 1972] controlled by two distinct translation initiation sites [Conneely et al., 1989; Conneely et al., 1987; Graham and Clarke, 2002; Kastner et al., 1990]. PGR-A and PGR-B are identical except for PGR-A, the shorter form, missing the first 165 amino acids at the amino-terminus that contains one of the three activation function domains possessed by PGR-B [Giangrande and McDonnell, 1999; Graham and Clarke, 2002].
PGR expression in the rodent ovary
PGR mRNA and protein isoforms are expressed in a temporally and cell-type restricted manner in the ovary. PGR mRNA is rapidly yet transiently induced in the rat ovary in vivo [Park and Mayo, 1991] and in primary cultures of rat granulosa cells, peaking after 4-8h in response to LH [Clemens et al., 1998; Natraj and Richards, 1993]. PGR mRNA expression has not been directly localized in the mouse, but the PRlacZ mouse model, whereby the lacZ reporter was placed within exon 1 of the PGR gene, demonstrates that PGR is expressed within 4h of the LH surge, specifically in mural granulosa cells (Figure 2) [Ismail et al., 2002]. PGR protein has also been localized to granulosa cells of mouse preovulatory follicles, transiently up-regulated at 4h and 8h post-hCG [Robker et al., 2000] and undetectable by 12h. Consistent with differences in LH-R expression, PGR is expressed at dramatically higher levels in mural granulosa cells than in cumulus cells (Figure 2) [Ismail et al., 2002; Robker et al., 2000; Teilmann et al., 2006].
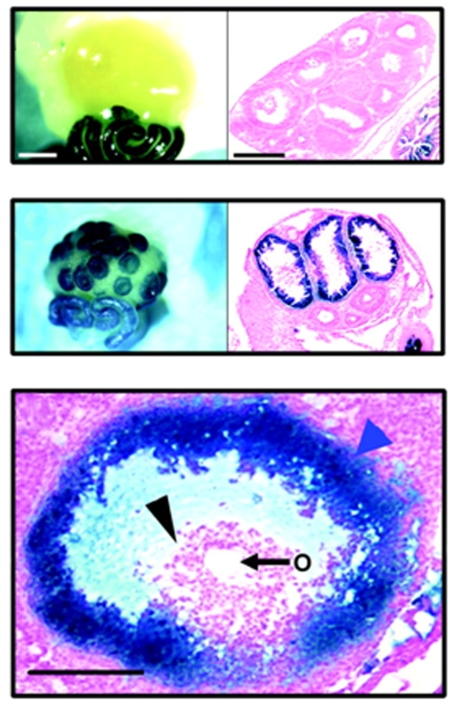
Figure 2. Progesterone receptor (PGR) is expressed in the ovary in a temporal and cell-type specific manner, as demonstrated by PRlacZ mice, which express the lacZ reporter (blue) under the control of the endogenous PGR promoter.
Top panels: Ovaries from mice treated with PMSG do not express PGR, while the oviduct expresses high levels. Middle panels: In response to hCG to mimic the LH surge, lacZ staining is detected within 8h, specifically in the granulosa cells of preovulatory follicles. Lower panel: In preovulatory follicles, lacZ is detected in mural granulosa cells (blue arrow), but not cumulus cells (black arrow) or the oocyte (O). (Reprinted with permission from Ismail et al., 2002). Copyright 2002, The Endocrine Society.
PGR-A and PGR-B specific antibodies were used to show distinct expression of the two protein isoforms in the mouse ovary [Gava et al., 2004]. In contrast to other reports of rodent PGR mRNA and protein, PGR-B was detected throughout the ovary. PGR-A was most highly expressed in granulosa cells of preovulatory follicles and the corpus luteum on the morning of estrus, as well as in the theca of preantral and antral follicles consistent with other observations, including our own of PGR protein in isolated cells within the ovarian theca and stroma in response to the LH surge (Robker, unpublished data). In granulosa cells, however, PGR-A is clearly the more prevalent translated product in the rat [Natraj and Richards, 1993] and mouse [Shao et al., 2003; Teilmann et al., 2006] following the LH surge.
PGR expression in primate and human ovary
Unlike the very transient pattern of PGR expression observed in rodents, primates and humans display more prolonged expression of PGR in the ovary, which is thought to function in the extended luteal phase in these species. Immunohistochemistry of ovaries from rhesus or cynomologus monkeys throughout the menstrual cycle showed PGR protein in granulosa of some primordial and primary follicles, but not granulosa of follicles beyond the primary stage [Hild-Petito et al., 1988]. Highest levels of expression were observed in luteinizing granulosa in follicles that had responded to the LH surge and in theca of follicles at all stages. CL from the early and mid-luteal phases expressed PGR protein, but late-luteal and regressing CL did not [Hild-Petito et al., 1988].
There are very few reports of PGR protein localization in the human ovary. Consistent with all other species examined, PGR is expressed in the dominant follicle at the time of the LH surge [Iwai et al., 1990] and similar to other primates, PGR expression is maintained in the active CL, but not in the late CL [Iwai et al., 1990; Revelli et al., 1996; Suzuki et al., 1994]. PGR is also consistently observed in the theca of some follicles and the stroma [Iwai et al., 1990; Revelli et al., 1996; Suzuki et al., 1994] and interestingly, was detected in a small proportion of primordial follicles and preantral follicles [Revelli et al., 1996]. More work is clearly needed, however, to clarify the expression patterns of PGR mRNA and protein isoforms in the human ovary.
Functional roles of progesterone receptor in the ovary
Receptor antagonists and genetically modified mouse models have been used to delineate the role of PGR in ovarian function. PGR is likely to have multiple roles in the ovary, including modulation of granulosa-lutein cell survival and steroidogenesis, cumulus expansion and oocyte quality, depending on the species. However, studies in many species, including humans, consistently demonstrate that PGR signaling is essential for release of the oocyte at ovulation.
Ovulation
RU486, or mifepristone, is a mixed PGR agonist/antagonist [Chabbert-Buffet et al., 2005], which has been used extensively to experimentally demonstrate the function of PGR both in vitro and in vivo. Administration of RU486 to cycling female mice [Loutradis et al., 1991] or rats [Gaytan et al., 2003; Sanchez-Criado et al., 1990; van der Schoot et al., 1987] prevented or reduced follicle rupture. Similar results occur with more specific unambiguous anti-progestagens: ZK28299 (Onapristone) inhibited ovulation in rats [Uilenbroek, 1991] and Org31710 inhibited ovulation in an in vitro-perfused rat ovary model [Pall et al., 2000]. CDB-2914, which exhibits improved specificity and efficacy as an anti-progestin compared to RU486, due to reduced anti-glucocorticoid activity [Attardi et al., 2004; Attardi et al., 2002; Hild et al., 2000], blocked ovulation in rats [Reel et al., 1998] and severely impaired ovulation in mice [Palanisamy et al., 2006]. These PGR antagonist treatments were effective when delivered 5h [Gaytan et al., 2003] or 1h [Palanisamy et al., 2006] prior to LH administration, concurrent with the LH surge [Loutradis et al., 1991; Pall et al., 2000] or 3.5h post-LH administration [Pall et al., 2000], but not at 7 or 9h post-LH [Pall et al., 2000]. This indicates that PGR is important early in the ovulatory process and becomes superfluous once its cascade of activity has been set in motion.
PGR antagonists also exhibit anti-ovulatory activity in women. Follicular phase administration of RU486 [Liu et al., 1987; Shoupe et al., 1987; Spitz et al., 1993] or CDB-2914 [Stratton et al., 2000] blocks or delays ovulation in a dose-dependent manner. Moreover, women who received CDB-2914 in the mid-follicular phase exhibited a significant incidence of luteinized unruptured follicles [Stratton et al., 2000].
Cumulatively, these studies using PGR antagonists support a large body of work which had previously demonstrated that the progesterone ligand itself is essential for ovulation [Espey et al., 1989; Hibbert et al., 1996; Kohda et al., 1980; Lipner and Greep, 1971; Mori et al., 1977; Snyder et al., 1984].
Targeted deletion of the PGR gene in mice, the PRKO (progesterone receptor knockout) model, has allowed definition of the specific and direct role of PGR in female fertility [Lydon et al., 1995]. PRKO homozygous females develop normally to adulthood, but the ovaries, uterus, mammary gland and brain exhibit altered phenotypes, demonstrating that PGR exerts pleiotropic control over reproductive tissues [Lydon et al., 1996; Lydon et al., 1995]. Within the ovary of PRKO mice there is normal growth and development of ovarian follicles until the preovulatory stage. However, the mice exhibit profound and complete anovulation, even in response to hyperstimulation with gonadotropins, resulting in oocytes retained in unruptured follicles (Figure 3) [Lydon et al., 1995; Robker and Richards, 2000]. Despite impaired follicular rupture, granulosa cells from preovulatory follicles of PRKO mice respond to the LH surge, as demonstrated by the presence of cumulus expansion [Lydon et al., 1995]; normal LH-induced upregulation of COX-2 (cyclooxygenase-2), an enzyme that catalyzes prostaglandin production and is essential for ovulation [Robker et al., 2000]; and differentiation into a luteal phenotype assessed by normal expression of the luteal marker P450 side-chain cleavage enzyme [Robker et al., 2000]. Therefore, PGR is not required for follicle growth and development or granulosa cell differentiation or luteinization, but is specifically and absolutely required for rupture of the preovulatory follicle and oocyte release.
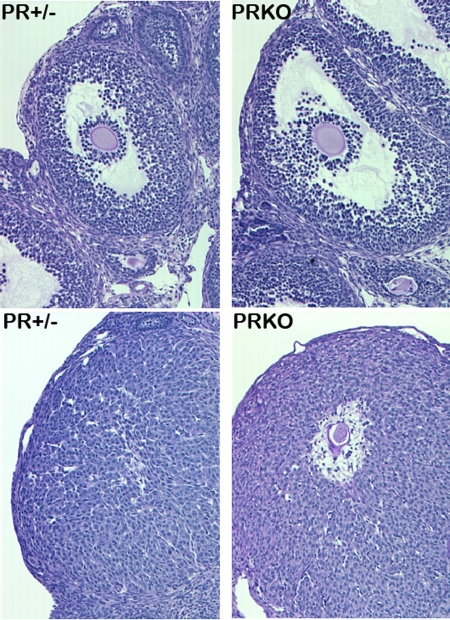
Figure 3. Mice null for PGR (PRKO) demonstrate that PGR is essential for ovulation, particularly follicular rupture, but not follicle growth or luteinization.
Upper panels show ovary sections from mice heterozygous or null for PGR that were treated with PMSG to stimulate preovulatory follicle growth. Lower panels show ovary sections from mice treated with PMSG followed by hCG for 48h to stimulate ovulation and luteinization. Lower right panel shows that in PRKO mice, oocytes remain trapped within luteinized follicles. (Reprinted with permission from Robker et al., 2000).
More recently, mouse models were generated which lack either the PGR-A or PGR-B isoform in order to determine the relative contributions of each to the myriad reproductive phenotypes observed in the PRKO mice. The isoform-specific knockouts were produced using CRE-loxP gene targeting to introduce mutations at either the ATG codon encoding Methionine 1 (M1A) to ablate expression of the PGR-B protein (PRBKO) or Methionine 166 (M166A) to ablate expression of the PGR-A protein (PRAKO) [Mulac-Jericevic et al., 2003; Mulac-Jericevic et al., 2000]. Histology of PRAKO mouse ovaries is similar to PRKO mice, with numerous mature anovulatory follicles containing an intact oocyte [Mulac-Jericevic et al., 2000]. Stimulation of immature PRAKO mice with exogenous gonadotropins further demonstrated a severe impairment in ovulation compared to WT mice, but not as complete as in PRKO mice, where both isoforms were ablated [Conneely et al., 2002; Mulac-Jericevic et al., 2000]. Superovulation was unaffected in PRBKO mice which only express the PGR-A protein [Mulac-Jericevic et al., 2003]. Thus, PRKO mouse models clearly demonstrate the critical importance of the PGR-A isoform in ovulation. Furthermore, the fact that PGR-A alone is capable of supporting ovulation indicates that synergy between PGR-A and PGR-B proteins are not required for regulation of essential P-responsive genes associated with ovulation. An area ripe for investigation is the utilization of microarray techniques to examine differentially expressed genes in PRAKO and PRBKO mice, which will allow PGR-A–selective target genes essential for ovulation to be identified. A recent report has verified that, indeed, when PGR-A or PGR-B is delivered to mouse granulosa cells in vitro, unique genes are induced by each [Sriraman et al., 2009].
Oocyte developmental competence
Even though ovulation is completely blocked in PRKO mice, oocyte developmental competence does not seem to be significantly affected. Oocytes isolated from preovulatory follicles of PMSG-primed PRKO mice can be successfully matured and fertilized in vitro and exhibit normal blastocyst development rates and produce pups following uterine transfer [Robker and Richards, 2000]. However, another study showed that treatment of mice with the PGR antagonist RU486 24h after PMSG resulted in a 50% decrease in the number of two-cell embryos that progressed to the blastocyst stage [Loutradis et al., 1991].
In porcine COCs treated with FSH and LH to induce expression of PGR, RU486 blocked oocyte progression to MII and development to blastocyst [Shimada et al., 2004b]. In this model, PGR is abundantly expressed by cumulus cells and treatment with RU486 also significantly impairs cumulus expansion [Shimada et al., 2004a; Shimada et al., 2004b]. PGR is not normally expressed in cumulus cells of the mouse and in PRKO ovaries cumulus expansion is not overtly affected [Lydon et al., 1995; Robker et al., 2000].
The role of progesterone and specifically PGR in the regulation of cumulus expansion in humans has not been adequately examined. In a single report, analysis of 135 cumulus cell samples collected from 44 IVF patients at the time of oocyte aspiration found that there was no relationship between PGR expression and oocyte fertilization or cleavage rate [Hasegawa et al., 2005]. However, low expression levels of PGR mRNA, regardless of follicular fluid P levels, was correlated with subsequent good embryo quality (defined as embryos with ≥7 blastomeres and <5% fragmentation at d3 post-fertilization) [Hasegawa et al., 2005]. Cumulatively, these studies indicate that a better understanding of the subtle effects of PGR in the peri-conception COC is needed.
Granulosa cell survival and steroidogenesis
PGR has a role in preventing granulosa cell apoptosis during luteinization. Mice treated with RU486 prior to ovulatory hCG have increased caspase-3 activity, a measure of increased apoptosis, in granulosa cells of preovulatory follicles [Shao et al., 2003]. Further, culture of periovulatory mouse [Shao et al., 2003], rat or human [Rung et al., 2005] granulosa cells with RU486 or Org31710 increases DNA laddering, indicative of cellular apoptosis. Caspase-3 activity [Svensson et al., 2001] and TUNEL positivity [Makrigiannakis et al., 2000] have also been reported in human luteinizing granulosa cells in response to either RU486 or Org31710.
Cholesterol synthesis by rat and human periovulatory granulosa cells in vitro is inhibited by RU486 or Org31710 [Rung et al., 2005; Shao et al., 2003; Svensson et al., 2000], suggesting a role for PGR in steroid synthesis during luteinization. In PRKO mice, basal progesterone levels are not different to normal littermates [Chappell et al., 1997], however steroidogenesis and luteal function has not been closely examined in any of the PGR null mouse models. In non-human primates and humans, species with long luteal cycles, it is clear that PGR regulates steroidogenesis in the early corpus luteum (reviewed in [Park and Mayo, 1991; Stouffer, 2003; Stouffer et al., 2007]). Furthermore, based on expression patterns, it is likely that PGR-B in particular regulates luteinization, while PGR-A controls ovulation [Stouffer et al., 2007]. Although PGR has a role in periovulatory granulosa cell survival and steroidogenesis, in primates at least, there is no evidence at present to suggest that these functions contribute to its critical role in follicular rupture.
Peri-ovulatory genes regulated by PGR
The profound anovulation of female PRKO mice has led to intense interest in the identification of PGR-regulated genes. In the ovary this has been pursued primarily via cDNA array hybridization analysis of transcripts from ovarian cells and cultured follicles of PRKO mice compared to heterozygotes, or isolated rodent granulosa cells cultured in the presence or absence of anti-progestins such as CDB-2914 or RU486 (Table 1). The currently identified genes that appear to be regulated by PGR are proteases, growth factors, signal transduction components and transcription factors. For the majority of these genes, their specific function in ovulation remains to be elucidated, and roles in luteinization and/or luteal cell survival cannot be discounted.
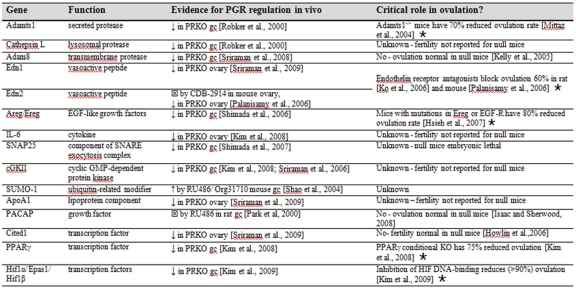
Table 1. PGR regulated genes in the ovary.
A review of the literature has revealed several genes regulated by PGR in the ovary, listed in the order that they appear in the text. The evidence for PGR regulation includes reduced (↓) or increased (↑) expression in PRKO mouse models or inhibition (square encasing x) in response to PGR antagonists. Gene products that have been demonstrated to be critical for ovulation are indicated (star). gc = granulosa cells.
Proteases regulated by PGR
Ovulation requires the action of proteases to facilitate release of the oocyte from within the follicular structure [Butler and Woessner, 1994; Butler et al., 1991] and lack of proteolytic tissue remodeling has been hypothesized to contribute to the anovulatory phenotype of the PRKO mouse [Robker et al., 2000]. At least three proteases (ADAMTS1, cathepsin L and ADAM8) appear to be directly or indirectly regulated by PGR (see Table 1), as they are selectively induced in large ovulatory follicles and absent in follicles from PRKO mice [Robker et al., 2000; Sriraman et al., 2008]. However, to date, ADAMTS1 is the sole protease with a clearly identified role in ovarian function.
The extracellular matrix protease Adamts1 (a disintegrin and metalloproteinase with thrombospondin motif 1) is induced in mural granulosa cells of the mouse ovary in response to ovulatory hCG, an induction that fails to occur in PRKO mice [Robker et al., 2000]. ADAMTS1 protein, the pro-form and the proteolytically-active form, is also deficient in PRKO mice, resulting in reduced cleavage of the matrix protein versican within the COC [Russell et al., 2003]. These results suggest that PGR-mediated induction of Adamts1 regulates ovulatory events, a finding substantiated by the subfertile phenotype of Adamts1 null mice, which release, on average, only 12 oocytes compared to 41 from heterozygote littermates after controlled gonadotropin stimulation [Mittaz et al., 2004]. In addition, Adamts1 null mice exhibit altered cumulus matrix structure, which impacts fertilization in vivo (D. Russell, unpublished data). Ovarian histology of Adamts1 null mice is also reminiscent of that of PRKOS, including luteinized follicles which express P450scc normally, but contain entrapped oocytes [Mittaz et al., 2004].
Growth factors and signal transduction components
A large number of cellular signaling genes have been identified as being PGR-regulated (see Table 1). For the majority, their roles in ovulation are unclear, although the potent vasoactive endothelins (Edn) appear to play an important role. Endothelin 2 (Edn2) is expressed specifically in mural granulosa cells, peaking at 12h post-hCG and then rapidly (within 1h) down-regulated [Palanisamy et al., 2006]. Edn2 is undetectable in ovaries from gonadotropin-stimulated PRKO mice and expression cannot be induced in PRKO primary granulosa cell cultures by treatment with an ovulatory stimulus such as forskolin (Fo) or phorbol myristate acetate (PMA) [Palanisamy et al., 2006]. Endothelin-1 (Edn1) was more recently identified as a PGR-A induced gene in mouse granulosa cells that is not expressed in ovaries from PRKO mice [Sriraman et al., 2009] . Endothelins are likely to be important players in the ovulatory cascade, as treatment with endothelin receptor antagonists severely decrease ovulation rate and result in ovaries with unruptured preovulatory follicles [Ko et al., 2006; Palanisamy et al., 2006].
Other PGR-regulated genes have known roles in the ovary. The EGF-like ligands amphiregulin (Areg) and epiregulin (Ereg) play key roles in triggering cumulus expansion and meiotic resumption in response to ovulatory LH [Ashkenazi et al., 2005; Park et al., 2004]. While Areg null mice have a normal ovulation rate, that of Ereg null mice is greatly reduced [Hsieh et al., 2007]. Inhibition of EGF receptor activation by injecting AG1478 into the ovarian bursa of the rat can modestly, but significantly, reduce ovulation rate in a dose dependent manner [Ashkenazi et al., 2005]. Similarly, a hypomorphic mutation in the EGF-R in mice results in dramatically reduced ovulation [Hsieh et al., 2007].
The cytokine IL-6 is reduced in PRKO mice [Kim et al., 2008], as is synaptosomal-associated protein-25 (SNAP 25), a component of the SNARE exocytosis complex that promotes cytokine secretion by both the COC and granulosa cells [Shimada et al., 2007]. Thus, via regulation of both cytokine production and secretion, PGR may play a role in the inflammatory reaction that is associated with the process of ovulation.
In the case of other putative PGR-regulated signaling molecules, such as cGKII [Kim et al., 2008; Sriraman et al., 2006], SUMO-1 [Shao et al., 2004] and ApoA1 [Sriraman et al., 2009], their roles in ovulation are not clear, or they may be involved in the terminal differentiation of granulosa cells rather than follicular rupture. For instance, pituitary adenylate cyclase activating polypeptide (PACAP) is induced in granulosa cells of preovulatory follicles via LH- and PGR-mediated mechanisms [Ko et al., 1999; Ko and Park-Sarge, 2000; Park et al., 2000]. PACAP null female mice have normal rates of ovulation and fertilization, yet only a small percentage of females have successful implantation, a defect thought to be due to their reduced prolactin and progesterone levels [Isaac and Sherwood, 2008]. Thus, PGR-mediated induction of the PACAP pathway appears to be more important for the establishment of luteinization rather than ovulation.
Transcription factors
Cited1 is a transcription factor which is induced by PGR-A in granulosa cells in vitro and exhibits reduced expression in vivo in PRKO ovaries [Sriraman et al., 2009]. Cited1 expression and function have yet to be characterized in the ovary, but fertility was reported to be normal in one Cited1 null mouse model [Howlin et al., 2006].
Following identification of PPARγ (peroxisome proliferator-activated receptor γ) as a molecular target of PGR [Kim et al., 2008], its role in ovulation was tested using transgenic PR-Cre knock-in mice crossed with PPARγ-floxed mice to delete PPARγ specifically in cells which express PGR. Thus, PPARγ deletion occurred specifically in mural granulosa cells, but not cumulus cells of preovulatory follicles. Follicular development was unaffected. However, the conditional PPARγ-null females (PPARγflox/flox PRcre/+) had severely reduced ovulation rates, with histological analysis of the ovaries showing an absence of CL and abundant unruptured follicles [Kim et al., 2008]. Importantly, PPARγ appears to be responsible for the induction of other genes previously acknowledged as PGR-regulated genes, since Edn2, cGKII and IL-6 mRNA, each of which are disrupted in PRKO ovaries, were also decreased in PPARγflox/flox PRcre/+ ovaries [Kim et al., 2008]. However, expression of the PGR-regulated gene Adamts1 was not altered in PPARγflox/flox PRcre/+ ovaries [Kim et al., 2008], indicating PPARγ only regulates a subset of PGR-regulated genes.
Activating ligands of PPARγ include fatty acid metabolites, such as prostaglandin J2 produced by COX-2 enzyme. Indomethacin and the more specific COX-2 inhibitor NS-398 blocked the induction of the PPARγ-regulated genes (EDN2, cGKII and IL-6) in cultured mouse granulosa cells stimulated with Fo/PMA [Kim et al., 2008]. Thus, it appears that through the induction of PPARγ by LH-PGR regulation and its ligands by COX-2, endocrine and oocyte-controlled signaling pathways converge to promote ovulatory induction of EDN2, cGKII and IL-6 (Figure 4).
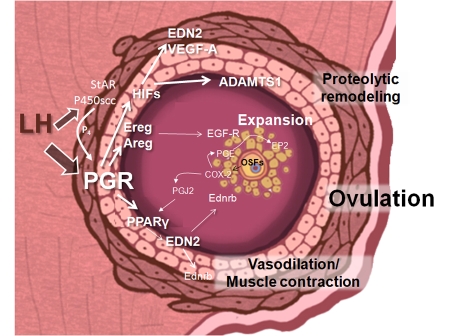
Figure 4. A working model of PGR-dependent pathways that are essential for ovulatory events.
The LH surge induces the expression of PGR specifically in granulosa cells, as well as luteinization-specific genes including steroidogenic enzymes that synthesize progesterone (StAR, P450scc), as well as PGR. Locally secreted progesterone (P4) activates PGR, which then transactivates intermediary genes including Hif1α (which is presumably also dependent on hypoxic conditions), which acts in concert with PGR to induce Adamts1 and VegfA. PGR regulation of EGF-like ligands Areg and Ereg would stimulate EGF-R, including that present on cumulus cells. PGR-mediated PPARγ induction acts upon ligand activation, perhaps supplied by COX-2 generated products such as PGJ2, to transactivate genes including Edn2. Edn2 receptors Ednrb are present on cumulus cells, as well as on ovarian smooth muscle cells. Oocyte-secreted factors (OSFs) regulate the induction of COX-2 and prostaglandin PGE, which acts on EP2 receptors, specifically in cumulus cells. Through this sequential cascade of PGR-initiated gene induction, integrated functional events including protease activation, cumulus expansion, smooth muscle contraction, vascular permeability and angiogenesis all contribute to follicle rupture and oocyte release.
More recently, Hypoxia-Inducible Factor (HIF) transcription factors Hif1α, Epas1 ( formerly known as Hif2α) and Hif1β have been identified as transcriptionally regulated PGR-dependent genes [Kim et al., 2009]. Hif activity is likely to be important for follicular rupture, since treatment of mice with echinomycin, which blocks Hif DNA binding, blocked follicle rupture [Kim et al., 2009]. Echinomycin treatment in vivo also blocked the hCG-stimulated induction of PGR-target genes Adamts1 and endothelin-2, but not Hif1α or Adam8 [Kim et al., 2009], suggesting that HIF transcription factors are also essential mediators of expression for a subset of PGR-regulated genes (Figure 4).
Thus, genes that are PGR-regulated in vivo have been identified primarily via their altered expression in PRKO mice or in response to treatment with PGR antagonists, with the majority yet to be confirmed in replicated studies. Further, whether any of these genes are in fact direct targets of PGR in ovarian cells has not been determined. Studies to date attempting to answer this question have found that in ovarian cells, PGR-regulation of gene expression is complex and currently not well-understood.
PGR regulation of transcription in granulosa cells
Agonistic progestin ligand binding to PGR causes a conformational change and receptor dimerization to facilitate binding to PGR response elements (PRE) in gene promoters [O'Malley, 2005; Spitz, 2006]. The two PGR isoforms, PGR-A and PGR-B, have different transcriptional activities [Giangrande et al., 2000], due to the presence of an additional activation function domain, AF3, on the longer PGR-B isoform [Saqui-Salces et al., 2008; Schrader and O'Malley, 1972]. Aside from this, both isoforms comprise identical ligand binding and DNA-binding domains [Gellersen et al., 2009; Hager et al., 2000; Mulac-Jericevic and Conneely, 2004]. The PGR-A isoform, which has the predominant role in ovulation, can act as both a repressor and activator of transcription, depending on the cell type [Graham and Clarke, 2002; Vegeto et al., 1993]. Interestingly, a mutation in the hinge region of human PGR, which is essential for its regulatory functions, is associated with unexplained infertility in women [Pisarska et al., 2003].
Ligand-activated PGR interacts with and recruits steroid receptor coactivators that modify chromatin structure to facilitate assembly of a macromolecular transcription complex. Further, PGR can be activated in the absence of steroidal ligand by phosphorylation pathways that modulate interactions with coregulator proteins [Rowan et al., 2000; Rowan and O'Malley, 2000]. Interestingly, PGR-A appears to exhibit greater ligand-independent transcriptional activity than PGR-B in mouse granulosa cells in vitro [Sriraman et al., 2009]. As important as the PGR isoform expression, is the suite of coactivators and repressors present in any cellular context. While these have not been exhaustively studied in granulosa cells, the coactivator p120 is induced by gonadotropins in rat granulosa cells and potentiates responsiveness to steroids including progesterone [Yoshino et al., 2006], indicating that this classical nuclear hormone signaling is a key response to the LH surge.
Intriguingly, even though PGR can transduce reporter constructs bearing canonical PRE-dependent promoters in granulosa cells [Doyle et al., 2004], genes identified as PGR-regulated in granulosa cells lack PGR binding response elements in their upstream promoters. Thus, in granulosa cells, unique transcriptional mechanisms are mediated by PGR to regulate a discrete set of peri-ovulatory genes. Of the PGR-regulated genes that have been analyzed to date, their expression in granulosa cells is dependent on GC-box promoter elements that bind specificity protein (Sp1 or Sp3) and early growth response protein-1 (Egr1) transcription factors. Interestingly, these are similar to elements that regulate the induction of PGR itself, but are presumably PGR-independent. Close analysis of the gene promoter of Adamts1, the best characterized granulosa cell PGR-regulated gene (Figure 5), revealed that PGR-mediated induction in mouse granulosa cells requires proximal GC-box elements that bind Sp1/3 [Doyle et al., 2004]. Transactivation of the Adam8 and cGKII genes by LH or its analogues was also found to be disrupted in PRKO mice and could be augmented in vitro by exogenous PGR-A overexpression [Doyle et al., 2004; Sriraman et al., 2008; Sriraman et al., 2006]. As with Adamts1, this activity was dependent on GC-box elements in the proximal promoter and also appears to involve a nuclear factor-1-like element [Sriraman et al., 2008]. Analysis of the rat cathepsin L promoter demonstrated Sp1/3 dependent induction after the LH surge, but the lack of any in vitro experimental progesterone responsiveness in this system prevented determination of a role for PGR [Sriraman and Richards, 2004]. For the cellular signaling factor SNAP25, in vitro induction mediated directly by PGR-A overexpression was also dependent on the GC-box Sp1/3 binding region of the promoter [Shimada et al., 2007]. However, for cGKII, Areg and Ereg the mechanism of PGR-dependent regulation has not been studied. Further, there is evidence to suggest that PGR may only control maintenance of expression or message stability in these genes, rather than the initial induction of expression [Shimada et al., 2006; Sriraman et al., 2006].
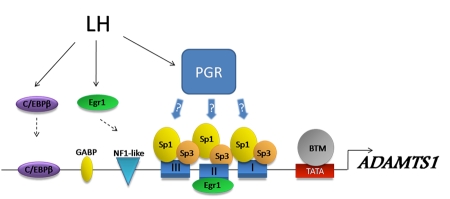
Figure 5. Regulatory elements in the ADAMTS1 promoter, a well-characterized PGR-responsive gene in granulosa cells.
The LH surge induces a suite of transcription factors including C/EBPβ, Egr-1 and PGR. C/EBPβ and Egr-1 have been demonstrated to directly bind to the Adamts1 promoter at sites that are essential for induction in response to ovulatory cAMP (Doyle et al., 2004). In contrast, there are no PREs in the promoter and PGR-dependent transcription is proposed to occur via PGR acting as an inducible coregulator with Sp1/Sp3 transcription factors, which are constitutively bound to a series of GC-rich regions (I, II, and III). BTM = basal transcriptional machinery.
Transcription factors induced downstream of PGR, such as Cited1, PPARγ and HIFs, may also influence expression of what are considered PGR-regulated genes, yet whether these act via direct DNA binding or indirect mechanisms to regulate ovulatory events is not yet known. PPARγ induced by PGR was shown to regulate endothelin-2, cGKII and IL-6 [Kim et al., 2008], while HIF activity was shown to regulate Adamts1 and endothelin-2 [Kim et al., 2009]. It is important to note that blockade of HIF activity with echinomycin also disrupts Sp1/Sp3 transcriptional activity [Vlaminck et al., 2007], so it is unclear whether genes identified by this method are truly HIF-regulated or Sp1-regulated.
Thus, in contrast to all that is known about PGR-regulation of transcription in other reproductive tissues, it is not known how PGR regulates the transcription of its peri-ovulatory gene cohort in granulosa cells. Current evidence based on bioinformatic analysis of PGR-dependent genes and an in depth study of the Adamts1 gene promoter suggest that PGR transcriptional regulation in granulosa cells does not depend on direct DNA binding to proximal promoter elements. Indirect interaction of PGR with gene promoters through binding to Sp1 proteins is a common mechanism of PGR-dependent gene induction, which has mainly been investigated in breast cancer cell lines where direct interaction of Sp1 and PGR has been confirmed [Faivre et al., 2008; Gizard et al., 2006]. Thus, while experiments definitively confirming that PGR interacts with Sp1/3 in granulosa cells are lacking, the evidence for this in other cell types and the consistent dependence on Sp1/3 binding promoter elements for peri-ovulatory PGR-mediated gene induction in granulosa cells suggests that this is the main mechanism for transcription.
Conclusions
PGR is a key specific regulator of ovulation across species. It is induced by the LH surge specifically in granulosa cells of mature preovulatory follicles. A number of granulosa cell PGR-regulated genes have been identified via their mis-expression in PRKO mice or in response to anti-progestins, and a subset appear to be involved in follicular rupture. However, whether these are direct transcriptional targets of PGR or indirectly downstream has yet to be clarified. Interestingly, the genes that have been analyzed lack PREs in the promoter regions, but rather require constitutively bound Sp1/Sp3 transcription factors. Thus, in granulosa cells, PGR may be acting as a “coregulator” of Sp1/Sp3 activity to coordinately induce a discrete cohort of essential ovulatory genes.
Abbreviations
- AC
adenylate cyclase
- ADAMTS1
a disintegrin and metalloproteinase with thrombospondin motif 1
- ApoA1
apolipoprotein A-1
- Areg
amphiregulin
- cAMP
3'-5'-cyclic adenosine monophosphate
- cGKII
cyclic GMP-dependent protein kinase II
- CL
corpora lutea
- COC
cumulus oocyte complex
- COX-2
cyclooxygenase-2
- Edn1
endothelin 1
- Edn2
endothelin 2
- EGF
epidermal growth factor
- Egr1
early growth response protein-1
- Epas1
endothelial PAS domain protein 1
- Ereg
epiregulin
- Fo
forskolin
- FSH
follicle stimulating hormone
- GC
granulosa cell
- hCG
human chorionic gonadotropin
- HIF
hypoxia inducible factor
- IL-6
interleukin 6
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
- P4
progesterone
- PACAP
pituitary adenylate cyclase activating polypeptide
- PGR
progesterone receptor
- PMA
phorbol myristate acetate
- PMSG
pregnant mare serum gonadotropin
- PPARγ
peroxisome proliferator-activated receptor γ
- PRAKO
progesterone receptor A-isoform knockout
- PRBKO
progesterone receptor B-isoform knockout
- PRE
progesterone response element
- PRKO
progesterone receptor knockout
- RGC32
response gene to complement 32
- RUNX1
runt-related transcription factor 1
- SNAP25
synaptosomal-associated protein-25
- SP1/3
specificity protein 1/3
References
- Ashkenazi H., Cao X., Motola S., Popliker M., Conti M., Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Attardi B. J., Burgenson J., Hild S. A., Reel J. R., Blye R. P. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Mol Cell Endocrinol. 2002;188:111–23. doi: 10.1016/s0303-7207(01)00743-2. [DOI] [PubMed] [Google Scholar]
- Attardi B. J., Burgenson J., Hild S. A., Reel J. R. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol. 2004;88:277–88. doi: 10.1016/j.jsbmb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Butler T. A., Woessner J. F., Jr. Gelatinases and endogenous inhibitors in the preovulatory rat ovary. Ann N Y Acad Sci. 1994;732:444–6. doi: 10.1111/j.1749-6632.1994.tb24780.x. [DOI] [PubMed] [Google Scholar]
- Butler T. A., Zhu C., Mueller R. A., Fuller G. C., Lemaire W. J., Woessner J. F., Jr. Inhibition of ovulation in the perfused rat ovary by the synthetic collagenase inhibitor SC 44463. Biol Reprod. 1991;44:1183–8. doi: 10.1095/biolreprod44.6.1183. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N., Meduri G., Bouchard P., Spitz I. M. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005;11:293–307. doi: 10.1093/humupd/dmi002. [DOI] [PubMed] [Google Scholar]
- Chappell P. E., Lydon J. P., Conneely O. M., O'Malley B. W., Levine J. E. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–52. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- Clemens J. W., Robker R. L., Kraus W. L., Katzenellenbogen B. S., Richards J. S. Hormone induction of progesterone receptor (PR) messenger ribonucleic acid and activation of PR promoter regions in ovarian granulosa cells: evidence for a role of cyclic adenosine 3',5'-monophosphate but not estradiol. Mol Endocrinol. 1998;12:1201–14. doi: 10.1210/mend.12.8.0157. [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Mulac-Jericevic B., DeMayo F., Lydon J. P., O'Malley B. W. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–55. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Maxwell B. L., Toft D. O., Schrader W. T., O'Malley B. W. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987;149:493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Kettelberger D. M., Tsai M. J., Schrader W. T., O'Malley B. W. The chicken progesterone receptor A and B isoforms are products of an alternate translation initiation event. J Biol Chem. 1989;264:14062–4. [PubMed] [Google Scholar]
- Deroo B. J., Rodriguez K. F., Couse J. F., Hamilton K. J., Collins J. B., Grissom S. F., Korach K. S. Estrogen receptor β is required for optimal cAMP production in mouse granulosa cells. Mol Endocrinol. 2009;23:955–65. doi: 10.1210/me.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle K. M., Russell D. L., Sriraman V., Richards J. S. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol Endocrinol. 2004;18:2463–78. doi: 10.1210/me.2003-0380. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Wigglesworth K., Pendola F., Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56:976–84. doi: 10.1095/biolreprod56.4.976. [DOI] [PubMed] [Google Scholar]
- Espey L. L., Tanaka N., Woodard D. S., Harper M. J., Okamura H. Decrease in ovarian platelet-activating factor during ovulation in the gonadotropin-primed immature rat. Biol Reprod. 1989;41:104–10. doi: 10.1095/biolreprod41.1.104. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre E. J., Daniel A. R., Hillard C. J., Lange C. A. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22:823–37. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H. Y., Liu Z., Shimada M., Sterneck E., Johnson P. F., Hedrick S. M., Richards J. S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–41. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gava N., Clarke C. L., Byth K., Arnett-Mansfield R. L., deFazio A. Expression of progesterone receptors A and B in the mouse ovary during the estrous cycle. Endocrinology. 2004;145:3487–94. doi: 10.1210/en.2004-0212. [DOI] [PubMed] [Google Scholar]
- Gaytan F., Bellido C., Gaytan M., Morales C., Sanchez-Criado J. E. Differential effects of RU486 and indomethacin on follicle rupture during the ovulatory process in the rat. Biol Reprod. 2003;69:99–105. doi: 10.1095/biolreprod.102.013755. [DOI] [PubMed] [Google Scholar]
- Gellersen B., Fernandes M. S., Brosens J. J. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15:119–38. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- Giangrande P. H., McDonnell D. P. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313; discussion 313-4. [PubMed] [Google Scholar]
- Giangrande P. H., Kimbrel E. A., Edwards D. P., McDonnell D. P. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–15. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizard F., Robillard R., Gross B., Barbier O., Revillion F., Peyrat J. P., Torpier G., Hum D. W., Staels B. TReP-132 is a novel progesterone receptor coactivator required for the inhibition of breast cancer cell growth and enhancement of differentiation by progesterone. Mol Cell Biol. 2006;26:7632–44. doi: 10.1128/MCB.00326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. D., Clarke C. L. Expression and transcriptional activity of progesterone receptor A and progesterone receptor B in mammalian cells. Breast Cancer Res. 2002;4:187–90. doi: 10.1186/bcr450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G. L., Lim C. S., Elbi C., Baumann C. T. Trafficking of nuclear receptors in living cells. J Steroid Biochem Mol Biol. 2000;74:249–54. doi: 10.1016/s0960-0760(00)00100-x. [DOI] [PubMed] [Google Scholar]
- Hasegawa J., Yanaihara A., Iwasaki S., Otsuka Y., Negishi M., Akahane T., Okai T. Reduction of progesterone receptor expression in human cumulus cells at the time of oocyte collection during IVF is associated with good embryo quality. Hum Reprod. 2005;20:2194–200. doi: 10.1093/humrep/dei005. [DOI] [PubMed] [Google Scholar]
- Hibbert M. L., Stouffer R. L., Wolf D. P., Zelinski-Wooten M. B. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci U S A. 1996;93:1897–901. doi: 10.1073/pnas.93.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild S. A., Reel J. R., Hoffman L. H., Blye R. P. CDB-2914: anti-progestational/anti-glucocorticoid profile and post-coital anti-fertility activity in rats and rabbits. Hum Reprod. 2000;15:822–9. doi: 10.1093/humrep/15.4.822. [DOI] [PubMed] [Google Scholar]
- Hild-Petito S., Stouffer R. L., Brenner R. M. Immunocytochemical localization of estradiol and progesterone receptors in the monkey ovary throughout the menstrual cycle. Endocrinology. 1988;123:2896–905. doi: 10.1210/endo-123-6-2896. [DOI] [PubMed] [Google Scholar]
- Howlin J., McBryan J., Napoletano S., Lambe T., McArdle E., Shioda T., Martin F. CITED1 homozygous null mice display aberrant pubertal mammary ductal morphogenesis. Oncogene. 2006;25:1532–42. doi: 10.1038/sj.onc.1209183. [DOI] [PubMed] [Google Scholar]
- Hsieh M., Lee D., Panigone S., Horner K., Chen R., Theologis A., Lee D. C., Threadgill D. W., Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–24. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac E. R., Sherwood N. M. Pituitary adenylate cyclase-activating polypeptide (PACAP) is important for embryo implantation in mice. Mol Cell Endocrinol. 2008;280:13–9. doi: 10.1016/j.mce.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Ismail P. M., Li J., DeMayo F. J., O'Malley B. W., Lydon J. P. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol. 2002;16:2475–89. doi: 10.1210/me.2002-0169. [DOI] [PubMed] [Google Scholar]
- Iwai T., Nanbu Y., Iwai M., Taii S., Fujii S., Mori T. Immunohistochemical localization of oestrogen receptors and progesterone receptors in the human ovary throughout the menstrual cycle. Virchows Arch A Pathol Anat Histopathol. 1990;417:369–75. doi: 10.1007/BF01606025. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 1990;9:1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Sato M., Li Q., Lydon J. P., Demayo F. J., Bagchi I. C., Bagchi M. K. Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28:1770–82. doi: 10.1128/MCB.01556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Bagchi I. C., Bagchi M. K. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150:3392–400. doi: 10.1210/en.2008-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C., Gieske M. C., Al-Alem L., Hahn Y., Su W., Gong M. C., Iglarz M., Koo Y. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–9. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- Kohda H., Mori T., Ezaki Y., Nishimura T., Kambegawa A. A progesterone-dependent step in ovulation induced by human chorionic gonadotrophin in immature rats primed with pregnant mare serum gonadotrophin. J Endocrinol. 1980;87:105–7. doi: 10.1677/joe.0.0870105. [DOI] [PubMed] [Google Scholar]
- Ko C., Park-Sarge O. K. Progesterone receptor activation mediates LH-induced type-I pituitary adenylate cyclase activating polypeptide receptor (PAC(1)) gene expression in rat granulosa cells. Biochem Biophys Res Commun. 2000;277:270–9. doi: 10.1006/bbrc.2000.3667. [DOI] [PubMed] [Google Scholar]
- Ko C., In Y. H., Park-Sarge O. K. Role of progesterone receptor activation in pituitary adenylate cyclase activating polypeptide gene expression in rat ovary. Endocrinology. 1999;140:5185–94. doi: 10.1210/endo.140.11.7149. [DOI] [PubMed] [Google Scholar]
- Kraus W. L., Montano M. M., Katzenellenbogen B. S. Cloning of the rat progesterone receptor gene 5'-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993;7:1603–16. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- Lipner H., Greep R. O. Inhibition of steroidogenesis at various sites in the biosynthetic pathway in relation to induced ovulation. Endocrinology. 1971;88:602–7. doi: 10.1210/endo-88-3-602. [DOI] [PubMed] [Google Scholar]
- Liu J. H., Garzo G., Morris S., Stuenkel C., Ulmann A., Yen S. S. Disruption of follicular maturation and delay of ovulation after administration of the antiprogesterone RU486. J Clin Endocrinol Metab. 1987;65:1135–40. doi: 10.1210/jcem-65-6-1135. [DOI] [PubMed] [Google Scholar]
- Loutradis D., Bletsa R., Aravantinos L., Kallianidis K., Michalas S., Psychoyos A. Preovulatory effects of the progesterone antagonist mifepristone (RU486) in mice. Hum Reprod. 1991;6:1238–40. doi: 10.1093/oxfordjournals.humrep.a137519. [DOI] [PubMed] [Google Scholar]
- Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A., Jr., Shyamala G., Conneely O. M., O'Malley B. W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Lydon J. P., DeMayo F. J., Conneely O. M., O'Malley B. W. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A., Coukos G., Christofidou-Solomidou M., Montas S., Coutifaris C. Progesterone is an autocrine/paracrine regulator of human granulosa cell survival in vitro. Ann N Y Acad Sci. 2000;900:16–25. doi: 10.1111/j.1749-6632.2000.tb06212.x. [DOI] [PubMed] [Google Scholar]
- Mittaz L., Russell D. L., Wilson T., Brasted M., Tkalcevic J., Salamonsen L. A., Hertzog P. J., Pritchard M. A. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004;70:1096–105. doi: 10.1095/biolreprod.103.023911. [DOI] [PubMed] [Google Scholar]
- Mori T., Suzuki A., Nishimura T., Kambegawa A. Inhibition of ovulation in immature rats by anti-progesterone antiserum. J Endocrinol. 1977;73:185–6. doi: 10.1677/joe.0.0730185. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Lydon J. P., DeMayo F. J., Conneely O. M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Conneely O. M. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–46. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B., Mullinax R. A., DeMayo F. J., Lydon J. P., Conneely O. M. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–4. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Natraj U., Richards J. S. Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology. 1993;133:761–9. doi: 10.1210/endo.133.2.8344215. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W. A life-long search for the molecular pathways of steroid hormone action. Mol Endocrinol. 2005;19:1402–11. doi: 10.1210/me.2004-0480. [DOI] [PubMed] [Google Scholar]
- Palanisamy G. S., Cheon Y. P., Kim J., Kannan A., Li Q., Sato M., Mantena S. R., Sitruk-Ware R. L., Bagchi M. K., Bagchi I. C. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20:2784–95. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- Pall M., Mikuni M., Mitsube K., Brannstrom M. Time-dependent ovulation inhibition of a selective progesterone-receptor antagonist (Org 31710) and effects on ovulatory mediators in the in vitro perfused rat ovary. Biol Reprod. 2000;63:1642–7. doi: 10.1095/biolreprod63.6.1642. [DOI] [PubMed] [Google Scholar]
- Park J. Y., Su Y. Q., Ariga M., Law E., Jin S. L., Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–4. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Park J. I., Kim W. J., Wang L., Park H. J., Lee J., Park J. H., Kwon H. B., Tsafriri A., Chun S. Y. Involvement of progesterone in gonadotrophin-induced pituitary adenylate cyclase-activating polypeptide gene expression in pre-ovulatory follicles of rat ovary. Mol Hum Reprod. 2000;6:238–45. doi: 10.1093/molehr/6.3.238. [DOI] [PubMed] [Google Scholar]
- Park-Sarge O. K., Parmer T. G., Gu Y., Gibori G. Does the rat corpus luteum express the progesterone receptor gene? Endocrinology. 1995;136:1537–43. doi: 10.1210/endo.136.4.7534703. [DOI] [PubMed] [Google Scholar]
- Park-Sarge O. K., Mayo K. E. Regulation of the progesterone receptor gene by gonadotropins and cyclic adenosine 3',5'-monophosphate in rat granulosa cells. Endocrinology. 1994;134:709–18. doi: 10.1210/endo.134.2.8299566. [DOI] [PubMed] [Google Scholar]
- Park O. K., Mayo K. E. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol. 1991;5:967–78. doi: 10.1210/mend-5-7-967. [DOI] [PubMed] [Google Scholar]
- Peng X. R., Hsueh A. J., LaPolt P. S., Bjersing L., Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–7. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- Pisarska M. D., Carson S. A., Casson P. R., Tong X., Buster J. E., Kieback D. G. A mutated progesterone receptor allele is more prevalent in unexplained infertility. Fertil Steril. 2003;80:651–3. doi: 10.1016/s0015-0282(03)00755-6. [DOI] [PubMed] [Google Scholar]
- Reel J. R., Hild-Petito S., Blye R. P. Antiovulatory and postcoital antifertility activity of the antiprogestin CDB-2914 when administered as single, multiple, or continuous doses to rats. Contraception. 1998;58:129–36. doi: 10.1016/s0010-7824(98)00067-5. [DOI] [PubMed] [Google Scholar]
- Revelli A., Pacchioni D., Cassoni P., Bussolati G., Massobrio M. In situ hybridization study of messenger RNA for estrogen receptor and immunohistochemical detection of estrogen and progesterone receptors in the human ovary. Gynecol Endocrinol. 1996;10:177–86. doi: 10.3109/09513599609027986. [DOI] [PubMed] [Google Scholar]
- Robker R. L., Russell D. L., Espey L. L., Lydon J. P., O'Malley B. W., Richards J. S. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A. 2000a;97:4689–94. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robker R. L., Richards J. S. Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer-Verlag; 2000b. Progesterone: lessons from the progesterone receptor knockout . [Google Scholar]
- Rowan B. G., Garrison N., Weigel N. L., O'Malley B. W. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol Cell Biol. 2000a;20:8720–30. doi: 10.1128/mcb.20.23.8720-8730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan B. G., O'Malley B. W. Progesterone receptor coactivators. Steroids. 2000b;65:545–9. doi: 10.1016/s0039-128x(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Rung E., Friberg P. A., Shao R., Larsson D. G., Nielsen ECh, Svensson P. A., Carlsson B., Carlsson L. M., Billig H. Progesterone-receptor antagonists and statins decrease de novo cholesterol synthesis and increase apoptosis in rat and human periovulatory granulosa cells in vitro. Biol Reprod. 2005;72:538–45. doi: 10.1095/biolreprod.104.033878. [DOI] [PubMed] [Google Scholar]
- Russell D. L., Robker R. L. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- Russell D. L., Doyle K. M., Ochsner S. A., Sandy J. D., Richards J. S. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–9. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- Salvador L. M., Maizels E., Hales D. B., Miyamoto E., Yamamoto H., Hunzicker-Dunn M. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology. 2002;143:2986–94. doi: 10.1210/endo.143.8.8976. [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado J. E., Bellido C., Galiot F., Lopez F. J., Gaytan F. A possible dual mechanism of the anovulatory action of antiprogesterone RU486 in the rat. Biol Reprod. 1990;42:877–86. doi: 10.1095/biolreprod42.6.877. [DOI] [PubMed] [Google Scholar]
- Saqui-Salces M., Neri-Gomez T., Gamboa-Dominguez A., Ruiz-Palacios G., Camacho-Arroyo I. Estrogen and progesterone receptor isoforms expression in the stomach of Mongolian gerbils. World J Gastroenterol. 2008;14:5701–6. doi: 10.3748/wjg.14.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct. IV. Characterization of purified subunits. J Biol Chem. 1972;247:51–9. [PubMed] [Google Scholar]
- Shao R., Markstrom E., Friberg P. A., Johansson M., Billig H. Expression of progesterone receptor (PR) A and B isoforms in mouse granulosa cells: stage-dependent PR-mediated regulation of apoptosis and cell proliferation. Biol Reprod. 2003;68:914–21. doi: 10.1095/biolreprod.102.009035. [DOI] [PubMed] [Google Scholar]
- Shao R., Zhang F. P., Rung E., Palvimo J. J., Huhtaniemi I., Billig H. Inhibition of small ubiquitin-related modifier-1 expression by luteinizing hormone receptor stimulation is linked to induction of progesterone receptor during ovulation in mouse granulosa cells. Endocrinology. 2004;145:384–92. doi: 10.1210/en.2003-0527. [DOI] [PubMed] [Google Scholar]
- Shimada M., Nishibori M., Yamashita Y., Ito J., Mori T., Richards J. S. Down-regulated expression of A disintegrin and metalloproteinase with thrombospondin-like repeats-1 by progesterone receptor antagonist is associated with impaired expansion of porcine cumulus-oocyte complexes. Endocrinology. 2004b;145:4603–14. doi: 10.1210/en.2004-0542. [DOI] [PubMed] [Google Scholar]
- Shimada M., Yamashita Y., Ito J., Okazaki T., Kawahata K., Nishibori M. Expression of two progesterone receptor isoforms in cumulus cells and their roles during meiotic resumption of porcine oocytes. J Mol Endocrinol. 2004a;33:209–25. doi: 10.1677/jme.0.0330209. [DOI] [PubMed] [Google Scholar]
- Shimada M., Hernandez-Gonzalez I., Gonzalez-Robayna I., Richards J. S. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–65. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Shimada M., Yanai Y., Okazaki T., Yamashita Y., Sriraman V., Wilson M. C., Richards J. S. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487–502. doi: 10.1210/me.2007-0042. [DOI] [PubMed] [Google Scholar]
- Shoupe D., Mishell D. R., Jr., Page M. A., Madkour H., Spitz I. M., Lobo R. A. Effects of the antiprogesterone RU 486 in normal women. II. Administration in the late follicular phase. Am J Obstet Gynecol. 1987;157:1421–6. doi: 10.1016/s0002-9378(87)80236-3. [DOI] [PubMed] [Google Scholar]
- Snyder B. W., Beecham G. D., Schane H. P. Inhibition of ovulation in rats with epostane, an inhibitor of 3 β-hydroxysteroid dehydrogenase. Proc Soc Exp Biol Med. 1984;176:238–42. doi: 10.3181/00379727-176-41865. [DOI] [PubMed] [Google Scholar]
- Spitz I. M. Progesterone receptor antagonists. Curr Opin Investig Drugs. 2006;7:882–90. [PubMed] [Google Scholar]
- Spitz I. M., Croxatto H. B., Salvatierra A. M., Heikinheimo O. Response to intermittent RU486 in women. Fertil Steril. 1993;59:971–5. [PubMed] [Google Scholar]
- Sriraman V., Richards J. S. Cathepsin L gene expression and promoter activation in rodent granulosa cells. Endocrinology. 2004;145:582–91. doi: 10.1210/en.2003-0963. [DOI] [PubMed] [Google Scholar]
- Sriraman V., Rudd M. D., Lohmann S. M., Mulders S. M., Richards J. S. Cyclic guanosine 5'-monophosphate-dependent protein kinase II is induced by luteinizing hormone and progesterone receptor-dependent mechanisms in granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol Endocrinol. 2006;20:348–61. doi: 10.1210/me.2005-0317. [DOI] [PubMed] [Google Scholar]
- Sriraman V., Sinha M., Richards J. S. Progesterone Receptor-Induced Gene Expression in Primary Mouse Granulosa Cell Cultures. Biol Reprod. 2009:(in press). doi: 10.1095/biolreprod.109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriraman V., Eichenlaub-Ritter U., Bartsch J. W., Rittger A., Mulders S. M., Richards J. S. Regulated expression of ADAM8 (a disintegrin and metalloprotease domain 8) in the mouse ovary: evidence for a regulatory role of luteinizing hormone, progesterone receptor, and epidermal growth factor-like growth factors. Biol Reprod. 2008;78:1038–48. doi: 10.1095/biolreprod.107.066340. [DOI] [PubMed] [Google Scholar]
- Sriraman V., Sharma S. C., Richards J. S. Transactivation of the progesterone receptor gene in granulosa cells: evidence that Sp1/Sp3 binding sites in the proximal promoter play a key role in luteinizing hormone inducibility. Mol Endocrinol. 2003;17:436–49. doi: 10.1210/me.2002-0252. [DOI] [PubMed] [Google Scholar]
- Stouffer R. L., Xu F., Duffy D. M. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;12:297–307. doi: 10.2741/2065. [DOI] [PubMed] [Google Scholar]
- Stouffer R. L. Progesterone as a mediator of gonadotrophin action in the corpus luteum: beyond steroidogenesis. Hum Reprod Update. 2003;9:99–117. doi: 10.1093/humupd/dmg016. [DOI] [PubMed] [Google Scholar]
- Stratton P., Hartog B., Hajizadeh N., Piquion J., Sutherland D., Merino M., Lee Y. J., Nieman L. K. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;15:1092–9. doi: 10.1093/humrep/15.5.1092. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Sasano H., Kimura N., Tamura M., Fukaya T., Yajima A., Nagura H. Immunohistochemical distribution of progesterone, androgen and oestrogen receptors in the human ovary during the menstrual cycle: relationship to expression of steroidogenic enzymes. Hum Reprod. 1994;9:1589–95. doi: 10.1093/oxfordjournals.humrep.a138757. [DOI] [PubMed] [Google Scholar]
- Svensson E. C., Markstrom E., Shao R., Andersson M., Billig H. Progesterone receptor antagonists Org 31710 and RU 486 increase apoptosis in human periovulatory granulosa cells. Fertil Steril. 2001;76:1225–31. doi: 10.1016/s0015-0282(01)02891-6. [DOI] [PubMed] [Google Scholar]
- Svensson E. C., Markstrom E., Andersson M., Billig H. Progesterone receptor-mediated inhibition of apoptosis in granulosa cells isolated from rats treated with human chorionic gonadotropin. Biol Reprod. 2000;63:1457–64. doi: 10.1095/biolreprod63.5.1457. [DOI] [PubMed] [Google Scholar]
- Teilmann S. C., Clement C. A., Thorup J., Byskov A. G., Christensen S. T. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol. 2006;191:525–35. doi: 10.1677/joe.1.06565. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Uilenbroek J. T. Hormone concentrations and ovulatory response in rats treated with antiprogestagens. J Endocrinol. 1991;129:423–9. doi: 10.1677/joe.0.1290423. [DOI] [PubMed] [Google Scholar]
- van der Schoot P., Bakker G. H., Klijn J. G. Effects of the progesterone antagonist RU486 on ovarian activity in the rat. Endocrinology. 1987;121:1375–82. doi: 10.1210/endo-121-4-1375. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Shahbaz M. M., Wen D. X., Goldman M. E., O'Malley B. W., McDonnell D. P. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–55. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Vlaminck B., Toffoli S., Ghislain B., Demazy C., Raes M., Michiels C. Dual effect of echinomycin on hypoxia-inducible factor-1 activity under normoxic and hypoxic conditions. Febs J. 2007;274:5533–42. doi: 10.1111/j.1742-4658.2007.06072.x. [DOI] [PubMed] [Google Scholar]
- Yoshino M., Mizutani T., Yamada K., Yazawa T., Ogata-Kawata H., Sekiguchi T., Kajitani T., Miyamoto K. Co-activator p120 is increased by gonadotropins in the rat ovary and enhances progesterone receptor activity. Reprod Biol Endocrinol. 2006;4:50. doi: 10.1186/1477-7827-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]