Abstract
Background and purpose:
Catechol-O-methyltransferase (COMT) metabolizes compounds containing catechol structures and has two forms: soluble (S-COMT) and membrane-bound (MB-COMT). Here we report the generation of a mouse line that expresses MB-COMT but not S-COMT. We compared the effects of deleting S-COMT only or both COMT forms on the pharmacokinetics of oral L-DOPA.
Experimental approach:
L-DOPA (10 mg·kg−1) and carbidopa (30 mg·kg−1) were given to mice by gastric tube, and samples were taken at various times. HPLC was used to measure L-DOPA in plasma and tissue samples, and dopamine and its metabolites in brain. Immunohistochemistry and Western blotting were used to characterize the distribution of COMT protein isoforms.
Key results:
Lack of S-COMT did not affect the levels of L-DOPA in plasma or peripheral tissues, whereas in the full COMT-knock-out mice, these levels were increased. The levels of 3-O-methyldopa were significantly decreased in the S-COMT-deficient mice. In the brain, L-DOPA levels were not significantly increased, and dopamine was increased only in females. The total COMT activity in the S-COMT-deficient mice was 22–47% of that in the wild-type mice. In peripheral tissues, female mice had lower COMT activity than the males.
Conclusions and implications:
In S-COMT-deficient mice, MB-COMT in the liver and the duodenum is able to O-methylate about one-half of exogenous L-DOPA. Sexual dimorphism and activity of the two COMT isoforms seems to be tissue specific and more prominent in peripheral tissues than in the brain.
Keywords: COMT, S-COMT, MB-COMT, COMT knock-out, pharmacokinetics, L-DOPA, enzyme activity
Introduction
Catechol-O-methyltransferase (COMT) is a widely expressed enzyme that O-methylates catecholamines and other compounds carrying a catechol structure, including catechol-oestrogens (Guldberg and Marsden, 1975; Männistö and Kaakkola, 1999). High COMT activity is found in the liver, kidney and gut wall. A single Comt gene codes for two separate enzymes, the soluble (S-COMT) and membrane-bound (MB-COMT) forms. S-COMT is more abundant in peripheral tissues while MB-COMT prevails in the brain, particularly in humans (Männistö and Kaakkola, 1999).
The two isoforms of COMT have been proposed to have partially distinct roles: MB-COMT is supposed to be primarily involved in the termination of dopaminergic and noradrenergic synaptic neurotransmission at physiologically relevant low concentrations of catecholamines (Roth, 1992). MB-COMT has also been shown to have a higher affinity for catechol substrates and a lower Km value for dopamine than S-COMT. S-COMT, on the other hand, is a high-capacity enzyme isoform indicated by higher Vmax values than those of MB-COMT (Lotta et al., 1995). S-COMT is mainly responsible of the elimination of biologically active or toxic, particularly exogenous, catechols and some hydroxylated metabolites. COMT also acts as an enzymatic detoxifying barrier between the blood and other tissues, shielding them from the detrimental effects of hydroxylated xenobiotics (Männistöet al., 1992; Kaakkola et al., 1994; Männistö and Kaakkola, 1999). COMT modulates some excretory functions in the kidney and intestinal tract by modulating the dopaminergic tone (Kaakkola et al., 1994; Eklöf et al., 1997; Hansell et al., 1998). The same is likely to be true in the brain: COMT activity regulates the levels of dopamine, particularly in frontal cortical areas (Yavich et al., 2007), and may therefore be associated with the modulation of several behavioural and cognitive processes (Tunbridge et al., 2006).
Much of our knowledge of COMT has been generated using selective COMT inhibitors that have been introduced to clinical use as adjuncts of the regular L-DOPA/DOPA decarboxylase (DDC) inhibitor therapy of Parkinson's disease (Männistö and Kaakkola, 1999). COMT inhibitors increase peripheral L-DOPA levels and thus improve its partition to the brain by preventing its conversion to 3-O-methyl DOPA (3-OMD) in peripheral tissues. Accordingly, the dose of L-DOPA can be substantially reduced compared with combination therapy without a DDC inhibitor. The dose interval of L-DOPA can also be prolonged. Further, COMT inhibitors should decrease fluctuations of dopamine formation in the brain (Männistö and Kaakkola, 1999).
To further clarify the importance of COMT, mice with genetic deletion of COMT [COMT-knock-out (COMT-KO) mice] have been developed (Gogos et al., 1998). Under normal conditions, these animals do not show any major disturbances in their behaviour or reproduction. We have recently generated a mouse line that does not produce S-COMT by genetically engineering a mutation in one of the two start codons of the Comt gene. This is the first report where this line will be partially characterized. This S-COMT-deficient mouse line will lead to a greater understanding of the role of the isoforms. The present study aimed at defining the quantitative role of MB-COMT in the pharmacokinetics and metabolism of orally given L-DOPA. To this end, L-DOPA and 3-OMD levels were measured in the plasma, a number of peripheral tissues and brain regions of the wild-type control mice, the S-COMT-deficient and full COMT-KO mice. Also, immunoreactivity to COMT was characterized in peripheral tissues. Both male and female mice were included in the study to explore sex differences in L-DOPA metabolism and the function of COMT. The results of this study suggest that in the mouse, MB-COMT might have a more important role than previously assumed in the peripheral metabolism of L-DOPA.
Methods
Animals
All animal care and experimental procedures were according to the European Community Guidelines for the use of experimental animals (European Communities Council Directive 86/609/EEC), reviewed by the State Provincial Office of Southern Finland and approved by the Animal Experiment Board in conformance with the current legislation.
Both female and male Comt gene-disrupted (COMT-KO mice; age range: 3–6 months), S-COMT-deficient mice (age range: 3–6 months) and the wild-type littermates of both strains were used. Mice were bred in the Laboratory Animal Centre in the University of Helsinki, Finland, breeding heterozygous males and females. To keep the strains viable, they were enriched regularly by mating C57BL/6J females obtained from Harlan, the Netherlands, with heterozygous males. The heterozygous offspring of these couples were used further for breeding. The mice were housed at an ambient temperature of 21–23°C under 12:12 h light cycle with free and continuous access to food pellets and drinking fluid. The phase of the oestrous cycle in female mice was not determined.
Generation of transgenic mouse lines
The Comt gene-disrupted mouse strain was originally generated by Gogos et al. (1998) on mixed 129/Sv × C57BL/6J background and later backcrossed for more than 20 generations on pure C57BL/6J background.
The S-COMT-deficient strain was constructed by a mutation of the methionine coding start codon (ATG) for S-COMT to threonine (ACC) in the Comt gene (Figure 1A). The detailed methodology for producing S-COMT-deficient mice is described in the supplementary material. Briefly, the MET2 sequence (see Figure 1A) was mutated in a plasmid (pLitmus-29, New England Biolabs, MO) that contained a 1.3 kb fragment from the Comt gene including exon2, introducing a new restriction site (BshTI). The fragment was cloned back to engineered restriction sites (Bsp1407/Kpn2I) of pGEM11-based vector containing contiguous flanking Comt gene regions (2.3 kb upstream and 4.2 kb downstream). This vector contained also Loxed Neo and TK cassettes within the 4–5 intron and the diptheria toxin A cassette at the 5′ end of the whole Comt fragment (Figure 1A). The linearized (XhoI/Mph1103I) plasmid was electroporated in ES cells from which neomycin-resistant clones were selected and treated with Cre recombinase. Positive clones were used for blastocyst injection. Chimerical males were mated with C57BL/6J females, and DNA from tail biopsies of F1 pups was typed by PCR (see below). F1 heterozygous mice were mated, and F2 mice of all three genotypes were obtained. Heterozygous and homozygous S-COMT mice are healthy and viable, and they breed normally. This could be expected because the Comt gene-disrupted knock-out mice are also normal (Gogos et al., 1998; Haasio et al., 2003).
Figure 1.
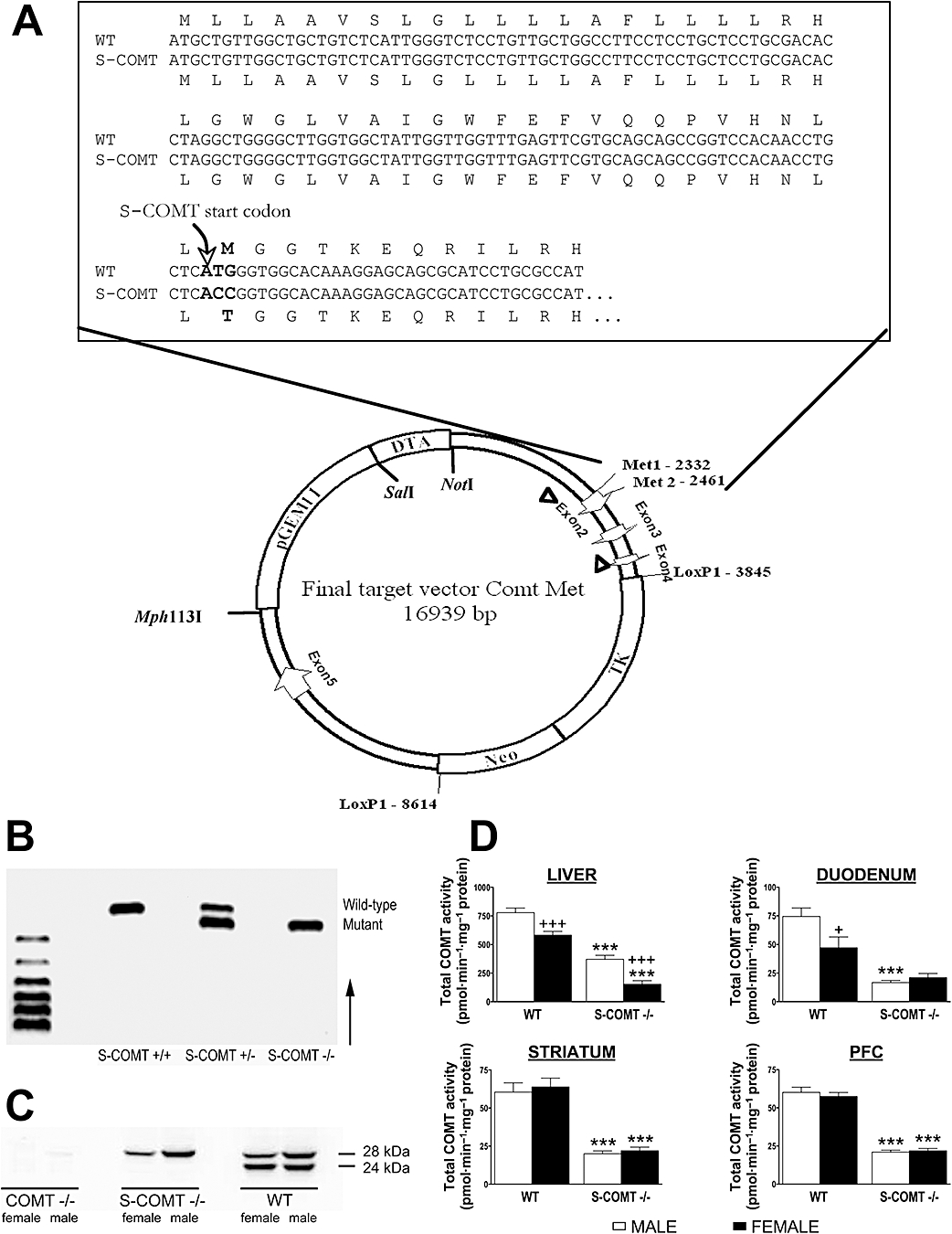
(A) Constructed ∼17 kb targeting vector containing ∼2.5 kb long 5′ homology arm up to Mutated MET2, from Mut-MET2 to floxTK/NEO cassete ∼1.4 kb central arm, ∼4.7 kb floxTKNeo cassette and ∼4 kb short 3′ homology arm. Positions of the Comt exons, vector and selection cassettes are indicated as well as the restriction sites used to linearize and the LoxP1 sites flanking the Neo and TK cassettes removed after recombination in ES cells. Greater detail of the sequence around Met2 in Exon 2 is shown. The DNA sequence of soluble catechol-O-methyltransferase (S-COMT)-deficient mutant is shown, and the sequence of the corresponding protein product is aligned on top (wild type) and bottom (S-COMT). The bases mutated are shown in bold font. A new AgeI restriction site (ACCGGT) for genotyping was introduced along with the Met44Thr mutation. (B) PCR genotyping of S-COMT-deficient animals showing wild-type (S-COMT +/+), heterozygous (S-COMT +/−) and homozygous (S-COMT −/−) genotypes consisting of wild-type allele, mutated allele or both alleles. (C) Western blot analysis visualizing the long (28 kDa) and short (24 kDa) catechol-O-methyltransferase (COMT) transcripts in the liver. (D) Total COMT activities in the liver, duodenum, striatum and prefrontal cortex (PFC) of wild-type (WT) and S-COMT-deficient (S-COMT −/−) mice. +P < 0.05 and +++P < 0.001 differs significantly from corresponding male. ***P < 0.001 differs significantly from corresponding wild-type mice. n (male/female) = 12/12 (WT) and 6/6 (S-COMT −/−).
Genotyping
Genomic DNA was isolated from tail biopsies as described by Laird et al. (1991). The Comt gene-disrupted mice were genotyped as reported in Tammimäki et al. (2008). The details are given in the supplementary material.
The amplified fragments were visualized by ethidium bromide staining under ultraviolet light after electrophoresis in a 2% agarose gel (Figure 1B).
Western immunoblotting
For Western immunoblotting, the liver samples were collected and rinsed in physiological saline solution. Immediately after dissection the tissues were placed in ice-cold centrifuge tubes on dry ice to minimize decomposition. All the tissue samples were frozen and stored in –80°C until analysed. The details are given in the supplementary material.
Assay of COMT activity
Total COMT activity was measured from duodenal and liver samples and from two brain areas [striatum and medial prefrontal cortex (PFC)]. After decapitation of the mice, the brain was rapidly excised, rinsed with ice-cold physiological saline solution and placed on an ice-cooled coronal mouse brain matrix (Stoelting, Wood Dale, IL). A 2-mm coronal slice was cut off with two razor blades, and the dorsal striatum was punched out below the corpus callosum from this slice by a sample corer (i.d. 2 mm) with a plunger (Fine Science Tools GmbH, Heidelberg, Germany). A second 2 mm coronal slice was cut off with two razor blades, and the PFC was cut off with a surgical knife. The brain tissues were immediately placed in frozen centrifuge tubes to be cooled on dry ice. Peripheral tissues were rapidly dissected and placed in pre-cooled plastic centrifuge tubes on dry ice as described earlier in the Western immunoblotting section. Samples were stored in –80°C until assayed. The COMT assay was performed with slight modifications from methods described earlier (Nissinen and Männistö, 1984; Reeniläet al., 1995). Further details of the assay are reported in the supplementary material.
The protein concentration of the samples was determined with Pierce protein assay kit based on bicinchoninic acid method (Pierce, Rockford, IL). Specific activity of COMT is expressed as pmoles vanillic and isovanillic acid formed per minute per milligram of protein in the sample.
COMT immunohistochemistry
Animals and tissue sampling
For tissue sampling of the paraffin-embedded tissues, animals were deeply anaesthetized with chloral hydrate (450 mg·kg−1 i.p.) and then perfused with saline and thereafter with 4% paraformaldehyde solution. After perfusion, the internal organs were removed and placed in 10% paraformaldehyde solution until paraffin embedding and sectioning into 4 mm paraffin-embedded sections using a sliding microtome (Leica SM2000R, Leica Microsystems Inc., Wetzlar, Germany).
Light microscopic immunohistochemistry
For paraffin-embedded mouse tissues, immunohistochemistry was performed using a slightly modified version of the protocol described in Myöhänen et al. (2008). The details are given in the supplementary material.
Pharmacokinetic studies and sample collection
The mice were given L-DOPA (10 mg·kg−1) and the DDC inhibitor carbidopa (30 mg·kg−1) suspended in 0.25% carboxymethylcellulose (CMC) gel with a plastic gastric tube in a volume of 5 mL·kg−1 of body weight. From each mouse, two blood samples were collected in Microvette capillary blood collection tubes (Sarstedt, Nümbrecht, Germany) containing EDTA. The first sample (100 mL) was taken from the saphenous vein and the second, terminal sample (300 mL) from the jugular vein. The two samples from each individual mouse were taken at random time points, and we treated enough mice to get at least six independent samples of each genotype at every time point (0, 30, 60, 90 and 120 min). The blood samples were centrifuged at 4900×g at 4°C for 10 min and the plasma was frozen and stored in –80°C. After the terminal blood sample, the mice were killed by decapitation and the tissues were dissected as described earlier in the Western immunoblotting and COMT activity-assay sections.
Assay of monoamines in plasma and tissue samples
Plasma samples
10 mL of the plasma sample was added to 90 mL of 0.2 M HClO4 to sediment the proteins. The sample was centrifuged for 35 min at 20800×g at 4°C. The supernatant was transferred to 0.5 mL Vivaspin filter concentrators (10,00 MWCO PES; Vivascience AG, Hannover, Germany) and centrifuged additionally for 35 min at 8600×g at 4°C. The filtrate was then analysed using HPLC with electrochemical detection. Details are given in the supplementary material.
Tissue samples
The tissues were prepared as reported previously (Airavaara et al., 2006) and then analysed for monoamines and their metabolites as for the plasma samples.
Vascular space contribution
As the animals were not perfused, the contribution of blood L-DOPA and 3-OMD levels was estimated when judging the tissue concentrations. In case of blood dopamine and its metabolites, blood contribution was negligible. Details of this procedure are given in the supplementary material. We did not recalculate the tissue levels of L-DOPA and 3-OMD. The contribution of the blood to total tissue concentrations was always less than 10% and evidently very similar in all animals. Therefore, comparisons of the three groups were not invalidated.
Statistics
The results are shown as mean ± SEM. Concentration-time curve was calculated from group means as only two blood samples and one tissue sample were collected from each animal. The L-DOPA, dopamine, and metabolite levels and COMT activities were compared with one-way analysis of variance (anova) followed by Scheffe's test. The effect of sex and genotype was calculated using two-way anova followed by Scheffe's test in cases where significant F-values were found. Area under the concentration-time curve (AUC0–120 min) was calculated according to the trapezoidal rule. SAS Institute Statview 5.0 software was used in all calculations.
Materials
L-DOPA, 3-OMD, dopamine, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were from Sigma Chemical Co., (St. Louis, MO), and carbidopa was from Orion Pharma (Espoo, Finland). CMC was from Fluka Chemie (Steinheim, the Netherlands) and chloral hydrate from Merck KgaA (Darmstadt, Germany).
The drug target/receptor nomenclature in this paper follows Alexander et al. (2008).
Results
Generation and characterization of S-COMT-deficient mice
Genetically engineered S-COMT-deficient mice carry a point mutation in Comt gene start codon 2 (corresponding to ‘ATG’ position +30 in exon2 of Comt gene; see Ulmanen and Lundström, 1991), which disables the translation of soluble COMT. The mice were produced and maintained on a C57BL6/J background. Heterozygous and homozygous S-COMT mice were healthy and viable, and they bred normally.
Western immunoblotting of the liver COMT
Western blot analysis of total liver extracts using a polyclonal COMT antibody revealed that the relative intensity of the longer MB-COMT (28 kDa) and the shorter S-COMT (24 kDa) bands was equal in wild-type male mice, while in females, the expression of MB-COMT was slightly weaker than males. As expected, S-COMT-deficient animals expressed only MB-COMT. Similarly to wild-type mice, the relative abundance of MB-COMT was lower in females than in males. No COMT protein was detected in COMT-KO animals (Figure 1C).
COMT activity in peripheral and brain tissues
In both the wild-type and S-COMT-deficient mice, total COMT activity in the liver was lower in female than in male mice (P < 0.001) (Figure 1D). In duodenal samples, sex-related differences were found only in wild-type animals (P < 0.05). Lack of the S-COMT isoform resulted in a clear reduction of total COMT activity in the liver of both sexes [by 74% in females (P < 0.001), by 53% in males (P < 0.001)], whereas in the duodenum, significant reduction was seen only in males (by 78%, P < 0.001). In the striatum and the PFC, a reduction of total COMT activity in S-COMT-deficient animals was also observed (P < 0.001), but no sex-related differences were found. COMT-KO animals had no detectable COMT activity in any tissue or region studied.
COMT protein distribution by immunohistochemistry
In the wild-type mice, COMT was abundantly expressed in the duodenal epithelial cells and microvilli (Figure 2A). Some COMT immunoreactivity was detected in the duodenum of the S-COMT-deficient mice (Figure 2B), but the intensity of COMT immunostaining was noticeably lower than that in the wild-type animals. As expected, no COMT immunoreactivity was detected in the duodenum of the COMT-KO mice (Figure 2C). Similar results were obtained in the liver, where we found high levels of expression of COMT in various types of liver cells in the wild-type mice (Figure 2D), significantly less COMT staining in the S-COMT-deficient mice (Figure 2E) and lack of any immunoreactivity in the COMT-KO mice (Figure 2F). It should be noted that both in the duodenum and the liver of the S-COMT-deficient animals, MB-COMT was seen in intracellular membranes.
Figure 2.
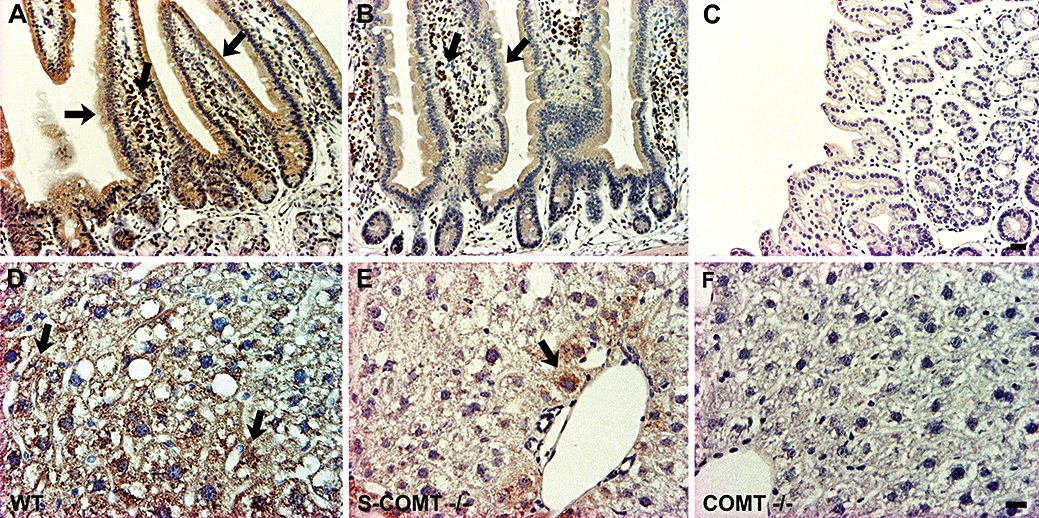
Light-microscopic photomicrographs presenting the distribution of COMT in the duodenal (A–C) and liver (D–F) tissue of wild-type (WT), soluble catechol-O-methyltransferase (S-COMT)-deficient (S-COMT −/−) and catechol-O-methyltransferase (COMT) knock-out (COMT −/−) mice. COMT was visualized with DAB (brown colour) and nuclei were counterstained with Mayer's haematoxylin (blue colour). The lack of S-COMT clearly reduced the intensity of COMT-immunostaining. Moreover, in the S-COMT-deficient liver cells (E), COMT is seen more in the intracellular membranes than the plasma membrane. Scale bars are 20 µm (A–C) and 30 µm (D–F).
Effect of COMT isoforms on absorption and metabolism of L-DOPA after oral administration of L-DOPA and carbidopa
Plasma L-DOPA levels were elevated after a single dose of L-DOPA in both sexes and all genotypes, reaching peak values at 30 min (wild-type and S-COMT-deficient mice) or 60 min (COMT-KO mice) (Figure 3A). Furthermore, the increase in apparent L-DOPA Cmax levels was higher in COMT-KO animals than in S-COMT-deficient mice (∼55% vs. ∼30% compared with their wild-type littermates). In the male mice, the absence of COMT prolonged the elimination of L-DOPA, but the same was not seen in the female mice. Otherwise, there was no sex-related difference in the overall L-DOPA pharmacokinetics. The AUC0–120 min values confirmed that L-DOPA levels were significantly increased only in the COMT-KO mice of both sexes (P < 0.001 in males, P < 0.01 in females).
Figure 3.
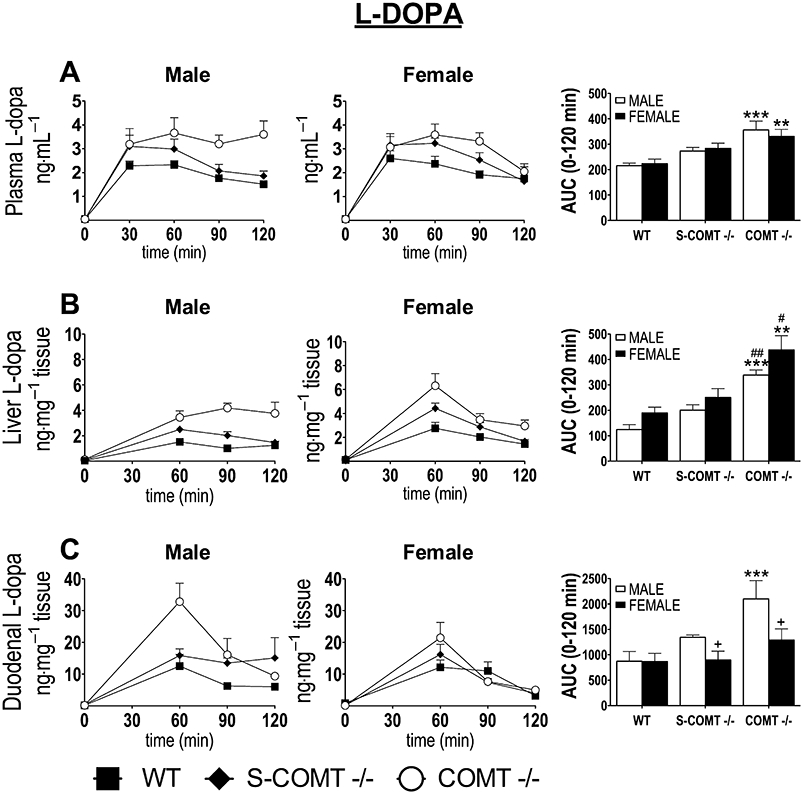
Effect of catechol-O-methyltransferase (COMT) genotype [soluble catechol-O-methyltransferase (S-COMT) deficiency or COMT knock-out] on the time course and AUC0–120 min of the plasma (A), liver (B) and duodenal (C) L-DOPA concentrations after oral administration of L-DOPA and carbidopa (10 mg·kg−1 and 30 mg·kg−1 respectively). The data represent group means ± SEM. +P < 0.05 differs significantly from corresponding male. #P < 0.05 and ##P < 0.01 differs significantly from corresponding S-COMT deficient mice. **P < 0.01 and ***P < 0.001 differs significantly from corresponding wild-type (WT) mice. n (male/female) = 13/13 (WT), 6/7 (S-COMT −/−) and 6/7 (COMT −/−).
Both duodenal and hepatic L-DOPA levels (Figure 3B,C) followed closely the corresponding levels in the plasma, but some striking sex-related differences were observed. In the liver, the absence of both COMT isoforms increased AUC0–120 min values of L-DOPA in both sexes (P < 0.001 in males, P < 0.01 in females) compared with wild-type mice. In the duodenum, significant elevation was observed only in male COMT-KO mice (P < 0.001). L-DOPA levels were very low and no genotype-related differences in peripheral L-DOPA levels were seen under basal conditions (Figure 3).
Effect of COMT isoforms on 3-OMD levels in peripheral tissues after oral administration of L-DOPA and carbidopa
Consistent with COMT activity measurements, plasma 3-OMD levels were significantly reduced in both sexes as a function of decreasing COMT. No 3-OMD was found in the COMT-KO mice. In wild-type mice, the levels of 3-OMD and the respective AUC0–120 min values were lower in females than in males (P < 0.001) (Figure 4A). 3-OMD levels in tissues were similar to those determined in the plasma for both sexes (Figure 4B,C).
Figure 4.
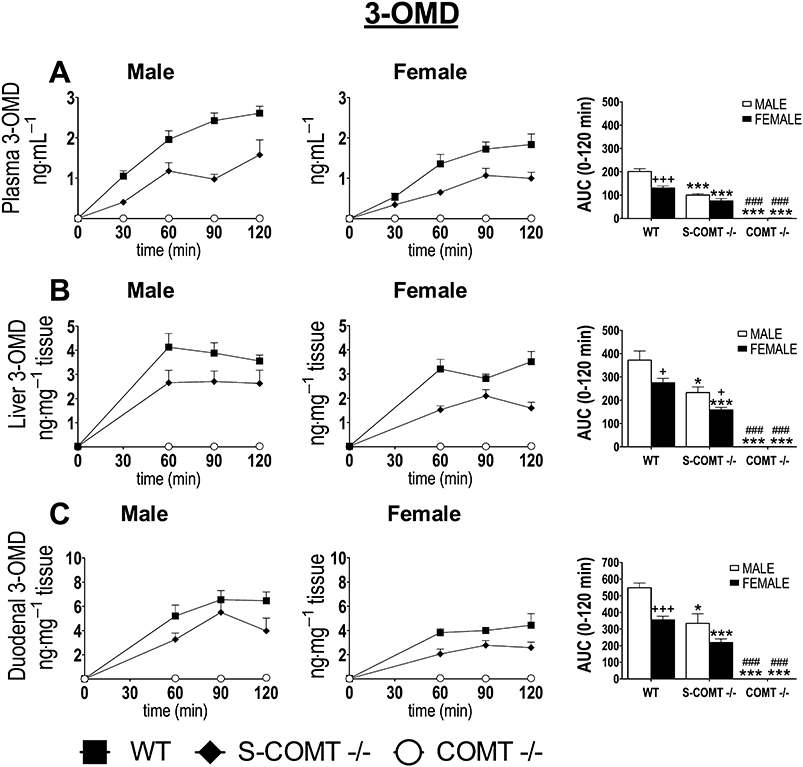
The effect of catechol-O-methyltransferase (COMT) genotype [soluble catechol-O-methyltransferase (S-COMT) deficiency or COMT knock-out] on the time course and AUC0–120 min of the plasma (A), liver (B) and duodenal (C) 3-O-methyl DOPA (3-OMD) levels after oral administration of L-DOPA and carbidopa (10 mg·kg−1 and 30 mg·kg−1 respectively). The data represent group means ± SEM. +P < 0.05 and +++P < 0.001 differs significantly from corresponding male. ###P < 0.001 differs significantly from corresponding S-COMT-deficient mice. *P < 0.05 and ***P < 0.001 differ significantly from corresponding wild-type (WT) mice. n (male/female) = 13/13 (WT), 6/7 (S-COMT −/−) and 6/7 (COMT −/−).
Effect of COMT genotype on L-DOPA, 3-OMD, dopamine, DOPAC and HVA levels in the brain after oral administration of L-DOPA and carbidopa
We did not observe any sex or genotype effects in the AUC0–120 min values of L-DOPA levels in the striatum and the PFC (Figure 5A). The elevation of L-DOPA was not as pronounced as in the peripheral tissues. Also, the apparent Cmax of L-DOPA was generally observed later in the striatum and the PFC than in the plasma (Figure S1, supplementary material). Central 3-OMD levels were strikingly similar to the levels in plasma and peripheral tissues, with the male wild-type mice having the highest 3-OMD concentrations (Figure 5B).
Figure 5.
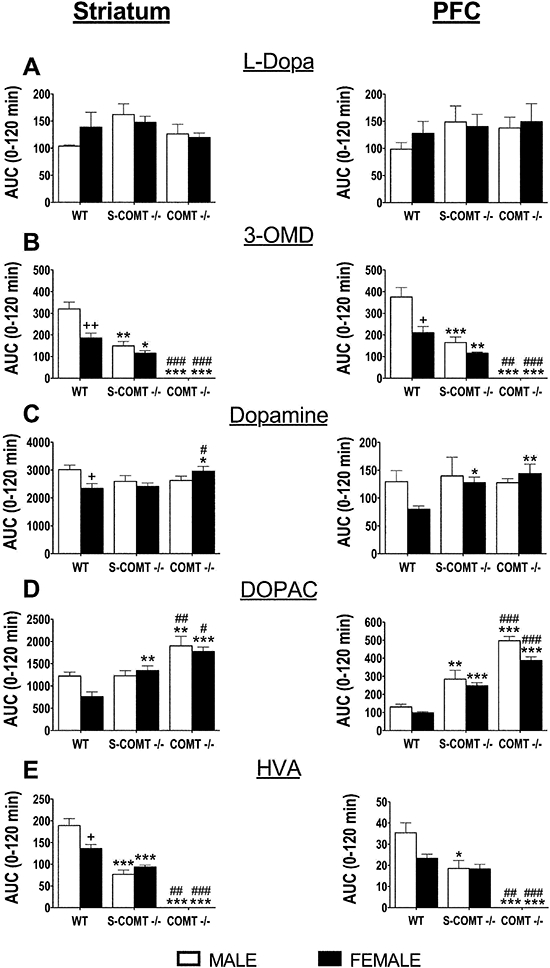
The effect of genotype [soluble catechol-O-methyltransferase (S-COMT) deficiency or catechol-O-methyltransferase (COMT) knock-out] on the AUC0–120 min of striatal and prefrontal cortex (PFC) levels of L-DOPA (A), 3-O-methyl DOPA (3-OMD) (B), dopamine (C), dihydroxyphenylacetic acid (DOPAC) (D) and HVA (E) after oral administration of L-DOPA and carbidopa (10 mg·kg−1 and 30 mg·kg−1 respectively). The data represent group means ± SEM. +P < 0.05 and ++P < 0.01 differ significantly from corresponding male. #P < 0.05, ##P < 0.01 and ###P < 0.001 differ significantly from corresponding S-COMT-deficient mice. *P < 0.05, **P < 0.01 and ***P < 0.001 differ significantly from corresponding wild-type (WT) mice. n (male/female) = 13/13 (WT), 6/7 (S-COMT −/−) and 6/7 (COMT −/−).
In striatal and PFC dopamine levels, no genotype effects were seen under basal conditions (Figure S1C, supplementary material). As for basal dopamine metabolite levels, significant increases of DOPAC (Figure S1D, supplementary material) were seen only in the PFC of the COMT-KO mice in which HVA was absent in both brain areas (Figure S1E, supplementary material). Lack of S-COMT did not significantly modify basal DOPAC or HVA levels in either brain area (Figure S1D,E, supplementary material).
After the administration of L-DOPA, female COMT-KO mice had elevated AUC0–120 min values of dopamine in both the striatum (P < 0.05) and the PFC (P < 0.01) compared with the values in the corresponding wild-type mice, whereas in males the dopamine levels were unaffected by the genotype (Figure 5C). In the striatum, S-COMT deficiency increased the elevation of DOPAC AUC0–120 min values only in the female mice (by 77%, P < 0.01) compared with the wild-type mice (Figure 5D), while the reduction of HVA AUC0–120 min values was seen in both sexes (P < 0.001) of S-COMT-deficient animals (Figure 5E). In the PFC, there was an elevation of DOPAC AUC0–120 min values in both sexes of S-COMT-deficient mice (95% in males, P < 0.01, and 153% in females, P < 0.001) compared with the levels in the corresponding wild-type mice, while the reduction of HVA AUC0–120 min values was seen only in the male mice (P < 0.05). In the COMT-KO mice, the elevation of DOPAC AUC0–120 min values was observed in both sexes, the levels being highest in the PFC of the male mice (281% elevation compared with wild-type mice, P < 0.001). No HVA was detected in the COMT-KO animals.
Discussion
This is the first report of S-COMT-deficient animals and their use to dissect the role of the two COMT forms in L-DOPA pharmacokinetics. Our results suggest a much more important role for MB-COMT in the mouse peripheral tissues than has previously been anticipated (Ellingson et al., 1999; Männistö and Kaakkola, 1999).
In previous Western blot analyses in rodents, S-COMT was at least three times more abundant than MB-COMT in the majority of rat tissues (Tenhunen et al., 1994) and at least twice as abundant than MB-COMT in the hypothalamus and liver of mice (Hill et al., 2007). In our study, both forms of COMT seemed to be equally abundant in the male mouse liver, whereas in the female liver, more S-COMT was present. MB-COMT was seen in the intracellular membranes of the immunostained S-COMT-deficient animals, supporting the earlier cell culture results (Ulmanen et al., 1997).
Our COMT activity assay revealed that the total COMT activity in the liver and the duodenum was lower in the wild-type female than male mice. The apparent gender-related difference in total COMT activity has been seen also in the human liver (De Santi et al., 1998). MB-COMT activity in the S-COMT-deficient mice was sex dependent only in the liver. Our results support only partially the previous findings of Ellingson et al. (1999) in regard to relative activities of S-COMT and MB-COMT. They concluded that the activity of S-COMT was approximately 30-fold to that of MB-COMT in the liver, whereas in our study the relative contribution of S-COMT was much less. Although an increase of MB-COMT in the liver could be compensatory to the absence of S-COMT, our Western blot results provide no evidence of up-regulation. We suggest that S-COMT accounts for ∼50% of the total COMT activity in the male liver and ∼70% in the female liver. Two points may explain these differences. A quantitative biochemical separation of the COMT forms is challenging and prone to contamination, easily leading to overestimation of S-COMT as previously recognized (Ellingson et al., 1999). Also, species- and strain-related variations in COMT activity may occur (Männistö and Kaakkola, 1999; Grice et al., 2007).
In the duodenum, the larger relative reduction in total COMT activity (by 78% in the male vs. by 55% in the female) suggests, in contrast to the liver, an accentuated role for S-COMT in the male mice. In the striatum and the PFC, S-COMT deficiency accounted for 60–70% reduction of total COMT activity. In concurrence with previous studies (Huotari et al., 2002a), no sex-related differences were seen in total COMT activity in the brain tissues studied.
Recent findings by Øverbye and Seglen (2009) may partially explain some of these inconsistencies. Additional variants of COMT, derived by post-translational modifications such as phosphorylation, have been identified. As phosphorylated and non-phosphorylated variants of both S-COMT and MB-COMT were found both in soluble and sedimentable fractions, it was suggested that the COMT variants could be better classified by size than by membrane association. Unfortunately, it is not yet known how phosphorylation affects COMT activity. Also, a new Comt-like gene, COMT2, has recently been identified by Du et al. (2008). This gene is expressed in the outer and inner hair cell of the cochlea as well as in vestibular hair cells and is essential for auditory and vestibular function. The authors suggest that COMT2 may also be expressed elsewhere in the brain and that it would be up-regulated to compensate for an inactive COMT gene. However, several highly sensitive analyses of COMT-KO mice (Huotari et al., 2002a,b; 2004; Tammimäki et al., 2008) have not shown any HVA formation in these mice, ruling out another, generally available COMT-like activity, compensating for the lack of COMT.
In our pharmacokinetic studies, the L-DOPA levels in the plasma and the liver of COMT-KO mice were double of those in the wild-type mice. Visual inspection of the concentration-time curve showed that in male mice, the elimination time of plasma L-DOPA was increased. In females, the levels of the L-DOPA metabolite, 3-OMD, in the plasma were lower than in males, which is in accordance with the lower total COMT activity in the duodenum and the liver. Also in the liver, L-DOPA levels were higher and 3-OMD levels lower in the female than male mice. In the duodenum, a significant genotype-dependent increase in L-DOPA levels was found only in the male COMT-KO mice. Undoubtedly, the importance of COMT in the peripheral metabolism of L-DOPA is greater in male than female mice. The sex-dependent impact of the S-COMT isoform is less clear.
L-DOPA levels in the PFC or the striatum were not genotype- or sex-dependent, although a slight trend towards elevated levels of L-DOPA were seen in the PFC of COMT-KO mice. The 3-OMD levels in both brain areas were much lower in the female than in the male mice. In addition, only the female COMT-KO mice had slightly elevated striatal and PFC dopamine levels compared with the corresponding wild-type animals. 3-OMD is readily transported through the blood–brain barrier and, thus, the brain 3-OMD levels reflect the levels in plasma and peripheral tissues. These results on 3-OMD and L-DOPA levels were consistent with earlier results by Huotari et al. (2002a), who failed to show a significant increase in L-DOPA levels in the brain tissue of COMT-KO animals. Also, in this earlier study, elevated dopamine levels after L-DOPA administration were found only in the PFC of both genders, whereas in the present study, elevation occurred both in the striatum and the PFC, but only in female mice. The gender differences may be due to the various effects of oestrogen on dopamine turnover as explained below. Also, the different dosing regimens may have an effect on the divergent results from these two studies: the bioavailability of oral L-DOPA in our study may have been much lower than in the previous one where L-DOPA was given intraperitoneally (Huotari et al., 2002a).
Sex differences in COMT-KO mice have previously been reported in terms of locomotor effects after administration of amphetamine (Huotari et al., 2004), GBR12909-induced increase in dopamine levels in the striatum (Huotari et al., 2002b), ethanol consumption (Tammimäki et al., 2008) and compensatory expression levels of liver proteins (Tenorio-Laranga et al., 2009). These apparent sex-related differences might be explained by an oestrogen-induced, time- and dose-dependent down-regulation of COMT (Xie et al., 1999). However, Hill et al. (2007) reported unexpectedly that oestrogen up-regulates the expression of COMT in the hypothalamus of male mice but not in the liver and the PFC. It appears that the regulatory action of oestrogen may be tissue specific. These actions of oestrogens and the variability of the expression and distribution of the two different oestrogen receptors (ERα and ERβ) (Albertazzi and Purdie, 2001; Kuhl, 2005) could be one factor contributing to the discrepant responses seen in our study. Sexually dimorphic and tissue-specific actions of oestrogen have also been found to affect dopamine turnover through modulation of the dopamine transporter (McEwen and Alves, 1999). Collectively, these results indicate a more complex, still undefined role for oestrogens in modulating dopamine metabolism through a variety of proteins.
The present results point out some other interesting issues regarding a COMT inhibitor treatment in humans. First, peripheral, particularly hepatic, COMT seems to have a more important role in the metabolism of L-DOPA than the brain COMT. Although L-DOPA levels were clearly elevated in plasma and peripheral tissues of the COMT-KO mice, the striatal and PFC levels were similar in all genotypes. Therefore, COMT inhibitors that can penetrate into the brain may not offer a significant advantage over those confined to the periphery. This has been suggested also earlier based on COMT inhibitor studies (Männistö and Kaakkola, 1999). Second, our study suggests that females might respond differently from males to a COMT inhibitor treatment. However, to the best of our knowledge, the clinical reports have not shown any sex difference in either the therapeutic or the adverse effects of COMT inhibitors. Third, the only minor dopamine elevation in the striatum in the full COMT-KO mice does not invalidate the importance of COMT inhibitors as adjuncts in the L-DOPA treatment of Parkinson's disease. The major effect of peripheral COMT inhibition is to prolong and smooth out the kinetics of L-DOPA entry into the brain. The levels of striatal dopamine are not necessarily elevated, but they are more sustained than without COMT inhibition.
In conclusion, the present results indicate that MB-COMT has a more important role in peripheral L-DOPA metabolism in the mouse than has been previously assumed. Although low oral doses of L-DOPA used in this study may result in an overestimation of the importance of MB-COMT, our results clearly demonstrate that MB-COMT can effectively compensate for the absence of S-COMT. We also found that the relative importance of the two COMT isoforms may be sex and tissue dependent.
Acknowledgments
The authors would like to thank Ms Anna Niemi for excellent technical assistance. Drs Petteri Piepponen and Ilkka Reenilä are gratefully acknowledged for their guidance in the HPLC analyses. This study was supported by the Helsinki University Research Funds, Academy of Finland (No. 210758), Sigrid Juselius Foundation to Pekka T. Männistö and the Finnish Parkinson Foundation to Mikko Käenmäki.
Glossary
Abbreviations:
- 3-OMD
3-O-methyl DOPA
- CMC
carboxymethylcellulose
- COMT
catechol-O-methyltransferase
- DAB
3,3′-diaminobenzidine
- DOPAC
dihydroxyphenylacetic acid
- HVA
homovanillic acid
- MB-COMT
membrane-bound COMT
- PFC
prefrontal cortex
- S-COMT
soluble COMT
- TBS
Tris-buffered saline
Conflict of interest
None.
Supporting information
Text S1 Generation of the mouse line that does not express S-COMT
Text S2 Western immunoblotting
Text S3 Light microscopic immunohistochemistry
Text S4 COMT activity-assay
Text S5 Assay of monoamines in plasma and tissue samples
Text S6 Vascular space contribution
Figure S1 The effect of COMT genotype (S-COMT deficiency or full COMT-ko) on the time course of striatal and PFC levels of L-dopa (A), 3-OMD (B), dopamine (C), DOPAC (D) and HVA (E) after oral administration of L-dopa and carbidopa (10 mg·kg−1 and 30 mg·kg−1, respectively). Data represents group means. n (male/female) = 13/13 (WT), 6/7 (S-COMT −/−), 6/7 (COMT −/−).
Table S1 Primers used for COMT_Met2 Target vector construction and testing.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Airavaara M, Mijatovic J, Vihavainen T, Piepponen TP, Saarma M, Ahtee L. In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse. 2006;59:321–329. doi: 10.1002/syn.20245. [DOI] [PubMed] [Google Scholar]
- Albertazzi P, Purdie PW. The life and times of the estrogen receptors: an interim report. Climacteric. 2001;4:194–202. [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi C, Giulianotti PC, Pietrabissa A, Mosca F, Pasifici GM. Catechol-O-methyltransferase: variation in enzyme activity and inhibition by entacapone and tolcapone. Eur J Clin Pharmacol. 1998;54:215–219. doi: 10.1007/s002280050448. [DOI] [PubMed] [Google Scholar]
- Du X, Schwander M, Moresco EMY, Viviani P, Halle C, Hildebrandt MS, et al. A catechol-O-methyltransferase that is essential for auditory function in mice and humans. Proc Natl Acad Sci USA. 2008;105:14609–14614. doi: 10.1073/pnas.0807219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklöf AC, Holtbäck U, Sundelöf M, Chen S, Aperia A. Inhibition of COMT induces dopamine-dependent natriuresis and inhibition of proximal tubular Na+,K+-ATPase. Kidney Int. 1997;52:742–747. doi: 10.1038/ki.1997.390. [DOI] [PubMed] [Google Scholar]
- Ellingson T, Duddempudi S, Greenberg BD, Hooper D, Eisenhofer G. Determination of differential activities of soluble and membrane-bound catechol-O-methyltransferase in tissues and erythrocytes. J Chromatogr B Biomed Sci Appl. 1999;729:347–353. doi: 10.1016/s0378-4347(99)00125-5. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice DE, Reenilä I, Männistö PT, Brooks AI, Smith GG, Golden GT, et al. Transcriptional profiling of C57 and DBA strains of mice in the absence and presence of morphine. BMC Genomics. 2007;8:76. doi: 10.1186/1471-2164-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 1975;27:135–206. [PubMed] [Google Scholar]
- Haasio K, Huotari M, Nissinen E, Männistö PT. Tissue histopathology, clinical chemistry and behaviour of adult Comt-gene-disrupted mice. J Appl Toxicol. 2003;23:213–219. doi: 10.1002/jat.909. [DOI] [PubMed] [Google Scholar]
- Hansell P, Odlind C, Männistö PT. Different renal effects of two inhibitors of catechol-O-methylation in the rat: entacapone and CGP 28014. Acta Physiol Scand. 1998;162:489–494. doi: 10.1046/j.1365-201X.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- Hill RA, McInnes KJ, Gong ECH, Jones MEE, Simpson ER, Boon WC. Estrogen deficient male mice develop compulsive behavior. Biol Psychiatry. 2007;61:359–366. doi: 10.1016/j.biopsych.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Huotari M, Gogos JA, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, et al. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci. 2002a;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Männistö PT. Effect of dopamine uptake inhibition on brain catecholamine levels and locomotion in catechol-O-methyltransferase-disrupted mice. J Pharmacol Exp Ther. 2002b;303:1309–1316. doi: 10.1124/jpet.102.043042. [DOI] [PubMed] [Google Scholar]
- Huotari M, Garcia-Horsman JA, Karayiorgou M, Gogos JA, Männistö PT. D-amphetamine responses in catechol-O-methyltransferase (COMT) disrupted mice. Psychopharmacology. 2004;172:1–10. doi: 10.1007/s00213-003-1627-3. [DOI] [PubMed] [Google Scholar]
- Kaakkola S, Teräväinen H, Ahtila S, Rita H, Gordin A. Effect of entacapone, a COMT inhibitor, on clinical disability and levodopa metabolism in parkinsonian patients. Neurology. 1994;44:77–80. doi: 10.1212/wnl.44.1.77. [DOI] [PubMed] [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(S1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucl Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Männistö PT, Ulmanen I, Lundström K, Taskinen J, Tenhunen J, Tilgmann C, et al. Characteristics of catechol-O-methyltransferase (COMT) and properties of selective COMT inhibitors. Prog Drug Res. 1992;39:291–350. doi: 10.1007/978-3-0348-7144-0_9. [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, García-Horsman JA, Piltonen M, Männistö PT. Distribution of Prolyl oligopeptidase in the mouse whole-body sections and peripheral tissues. Histochem Cell Biol. 2008;130:993–1003. doi: 10.1007/s00418-008-0468-x. [DOI] [PubMed] [Google Scholar]
- Nissinen E, Männistö P. Determination of catechol-O-methyltransferase activity by high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1984;137:69–73. doi: 10.1016/0003-2697(84)90348-8. [DOI] [PubMed] [Google Scholar]
- Øverbye A, Seglen PO. Phosphorylated and non-phosphorylated forms of catechol O-methyltransferase in rat liver, brain and other tissues. Biochem J. 2009;417:535–545. doi: 10.1042/BJ20081284. [DOI] [PubMed] [Google Scholar]
- Reenilä I, Tuomainen P, Männistö PT. Improved assay of reaction products to quantitate catechol-O-methyltransferase activity by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1995;663:137–142. doi: 10.1016/0378-4347(94)00433-6. [DOI] [PubMed] [Google Scholar]
- Roth J. Membrane-bound catechol-O-methyltransferase: a reevaluation of its role in the O-methylation of the catecholamine neurotransmitters. Rev Physiol Biochem Pharmacol. 1992;120:1–29. doi: 10.1007/BFb0036121. [DOI] [PubMed] [Google Scholar]
- Tammimäki A, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Increase in free choice oral ethanol self-administration in catechol-O-methyltransferase gene-disrupted male mice. Basic Clin Pharmacol Toxicol. 2008;103:297–304. doi: 10.1111/j.1742-7843.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Lundström K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- Tenorio-Laranga J, Männistö PT, Karayiorgou M, Gogos JA, Garcia-Horsman JA. Sex-dependent compensated oxidative stress in the mouse liver upon deletion of catechol O-methyltransferase. Biochem Pharmacol. 2009;77:1541–1552. doi: 10.1016/j.bcp.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Ulmanen I, Lundström K. Cell-free synthesis of rat and human catechol O-methyltransferase. Insertion of the membrane-bound form into microsomal membranes in vitro. Eur J Biochem. 1991;202:1013–1020. doi: 10.1111/j.1432-1033.1991.tb16464.x. [DOI] [PubMed] [Google Scholar]
- Ulmanen I, Peränen J, Tenhunen J, Tilgmann C, Karhunen T, Panula P, et al. Expression and intracellular localization of catechol O-methyltransferase in transfected mammalian cells. Eur J Biochem. 1997;243:452–459. doi: 10.1111/j.1432-1033.1997.0452a.x. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


