Abstract
Background and purpose:
Dietary anthocyanins hold great promise in the prevention of chronic disease but factors affecting their bioavailability remain poorly defined. Specifically, the role played by transport mechanisms at the intestinal and blood–brain barriers (BBB) is currently unknown.
Experimental approach:
In the present study, 16 anthocyanins and anthocyanidins were exposed to the human efflux transporters multidrug resistance protein 1 (MDR1) and breast cancer resistance protein (BCRP), using dye efflux, ATPase and, for BCRP, vesicular transport assays.
Key results:
All test compounds interacted with the BCRP transporter in vitro. Of these, seven emerged as potential BCRP substrates (malvidin, petunidin, malvidin-3-galactoside, malvidin-3,5-diglucoside, cyanidin-3-galactoside, peonidin-3-glucoside, cyanidin-3-glucoside) and 12 as potential inhibitors of BCRP (cyanidin, peonidin, cyanidin-3,5-diglucoside, malvidin, pelargonidin, delphinidin, petunidin, delphinidin-3-glucoside, cyanidin-3-rutinoside, malvidin-3-glucoside, pelargonidin-3,5-diglucoside, malvidin-3-galactoside). Malvidin, malvidin-3-galactoside and petunidin exhibited bimodal activities serving as BCRP substrates at low concentrations and, at higher concentrations, as BCRP inhibitors. Effects on MDR1, in contrast, were weak. Only aglycones exerted mild inhibitory activity.
Conclusions and implications:
Although the anthocyanidins under study may alter pharmacokinetics of drugs that are BCRP substrates, they are less likely to interfere with activities of MDR1 substrates. The present data suggest that several anthocyanins and anthocyanidins may be actively transported out of intestinal tissues and endothelia, limiting their bioavailability in plasma and brain.
Keywords: anthocyanin, anthocyanidin, flavonoid, ABC transporter, MDR1, P-glycoprotein, BCRP
Introduction
Anthocyanins are flavonoids widely distributed in fruits and vegetables that exhibit antioxidative (Garcia-Alonso et al., 2005; Tarozzi et al., 2007; Salminen and Heinonen, 2008) and anti-inflammatory (Wang and Mazza, 2002; Zafra-Stone et al., 2007; Kim et al., 2008) bioactivities, among others (Shukitt-Hale et al., 2008). A growing body of evidence from animal, epidemiological and clinical investigations suggests that anthocyanins confer neuroprotection (Joseph et al., 1999; Youdim et al., 2004; Matsunaga et al., 2009), but it is not clear how these compounds distribute to tissues and cross the blood–brain barrier (BBB). Daily dietary intake of anthocyanins has been estimated at ∼100 mg in Western countries (Thomasset et al., 2009) and can rise several fold depending on seasonal and lifestyle factors (Wu et al., 2006; Chun et al., 2007).
Based on the current understanding, anthocyanins are either directly absorbed from the gastrointestinal tract (McGhie and Walton, 2007), or are first metabolized by the gut microflora, leading to anthocyanidins and phenolic acid metabolites (Tsuda et al., 1999; Felgines et al., 2002). Native anthocyanins, plus one methylated form, have been identified in rat brain following dietary supplementation with a blackberry extract. Of these, the brain concentration of cyanidin-3-glucoside even exceeded its plasma concentrations (Talavera et al., 2005), suggesting good central nervous system (CNS) bioavailability of selected compounds. A recent study has confirmed the presence of anthocyanins in brains from pigs fed a regular diet, further strengthening this view (Kalt et al., 2008). Uptake in rat brain occurs only 30 min after oral administration (Talavera et al., 2005) and involves regions important for learning and memory (Andres-Lacueva et al., 2005). In pig cerebellum, anthocyanin concentration does not rise uniformly, which implies that anthocyanin absorption may become saturated at high dosage levels (Kalt et al., 2008).
Reports on neuroprotective effects of berry constituents have renewed interest in the mechanisms of BBB penetration and entry into the CNS, by means of a bilitranslocase-like carrier (Talavera et al., 2005) or by cellular transporters for glycosylated anthocyanins (Passamonti et al., 2005). Specifically, the permeability of the BBB may be affected by efflux transporters expressed at the endothelial surface (Youdim et al., 2004).
Efflux transporters of the ATP-binding cassette (ABC) gene family are major determinants of drug distribution to, and elimination from the CNS, of intestinal absorption, and of hepatobiliary excretion (Kusuhara and Sugiyama, 2002; Sun et al., 2003). Localized in the apical membranes of BBB endothelial cells, enterocytes and hepatocytes (Eisenblatter et al., 2003; Dupuy et al., 2006), they mediate the active extrusion of nutrients, toxins, drugs and many metabolites back into the capillary lumen and intestine (del Moral et al., 1998; Begley, 2004; Xu et al., 2005; Breedveld et al., 2006; Fricker, 2008). Moreover, transporters have been implicated in drug interactions that increase the likelihood of adverse effects (Fromm, 2003; Shitara et al., 2005). Among the ABC efflux transporters are the multidrug resistance protein 1 (MDR1, P-glycoprotein) and the breast cancer resistance protein (BCRP). Both are prominently expressed in organs important for absorption (small intestine), distribution (placenta and BBB) and elimination (liver, kidney, small intestine) of drugs (Gottesman et al., 1996; Mao and Unadkat, 2005), and are also found in several tumours (Schinkel and Jonker, 2003; Robey et al., 2007).
We sought to determine whether anthocyanins are substrates for ABC transporters, and whether anthocyanins may interfere with the transport of other substrates. To this end, 16 anthocyanidins and anthocyanins were tested for in vitro effects on the human efflux transporters BCRP and MDR1. Compounds were selected so as to include structurally diverse and yet common representatives abundant in dietary sources, such as malvidin-glucoside (glc) from red grapes and cyanidin-glc from elderberries and blackberries (Bub et al., 2001; Wu et al., 2004; Felgines et al., 2005). We also chose substances found in grapes, strawberries and bilberries, as these have shown positive effects in rodent studies of neuroprotection (Joseph et al., 1999; Ramirez et al., 2005; Shukitt-Hale et al., 2006; Duffy et al., 2008; Rahman et al., 2008; Williams et al., 2008; Matsunaga et al., 2009). Interactions were studied in dye extrusion and ATPase assays, and in vesicular transport assays for BCRP. In combination, these assays give insight into different types of transporter interaction and specify substances' affinities for the ABC transporters under study. Observed inhibitory and substrate-type effects on the BCRP transporter imply a potential of anthocyanidins to alter pharmacokinetics of drugs, and may explain limitations in their plasma and brain bioavailability.
Methods
Assays
Dye extrusion assay
For assessing effects on BCRP, Hoechst fluorescent dye 33342 was added to BCRP transporter-overexpressing MCF7-MX cells (Solvo Biotechnology, Budaörs, Hungary). Modulators of the BCRP transporter activity reduce the rate of Hoechst 33342 extrusion and cause its accumulation inside the cells. Following DNA intercalation, a fluorescent signal may be detected that is proportional to BCRP transporter inhibition. Briefly, cells (1 × 105 per well of standard 96-well tissue culture plates) were incubated with Hoechst 33342 (50 µM) plus the test compound or, alternatively, with DMSO for non-inhibited controls, or Ko143 (1 µM) as a reference inhibitor, at 37°C for 15 min in Hank's balanced salt solution (HBSS). Fluorescence of accumulated Hoechst 33342 inside the cells was measured in real time at excitation and emission wavelengths of 355 and 460 nm, respectively, using a BMG Labtech FluoStar Optima fluorescence photometer (BMG Labtech, Offenburg, Germany).
For quantification of effects on MDR1, calcein-AM was added to MDR1-overexpressing K562-MDR cells (Solvo Biotechnology, Budaörs, Hungary). Modulators of MDR1 activity cause accumulation of calcein-AM inside the cell, whereupon it is cleaved by non-specific esterases to form the fluorescent low permeability dye, calcein. The increase of fluorescent signal inside the cell is proportional to MDR1 inhibition, Briefly, K562-MDR cells (8 × 104 cells per well) and calcein-AM (0.25 µM) were incubated with the test compound or, alternatively, with DMSO for non-inhibited controls, or verapamil (60 µM) as a reference inhibitor, at 37°C for 8 min in HBSS. Fluorescence of accumulated calcein inside the cells was measured at excitation and emission wavelengths of 485 and 538 nm respectively. After the incubation period, propidium iodide was added to all wells (0.01 mg·mL−1) and fluorescence was measured at 530 and 630 nm excitation and emission wavelengths, respectively, to assess potential cytotoxic effects of the substances tested.
ATPase assay
ATPase activity of wild-type human BCRP and MDR1 was measured according to a protocol modified from Sarkadi et al. (1992), by colorimetric detection of inorganic phosphate, the byproduct of ABC transporter function. Membrane preparations from human BCRP transporter- and human MDR1 transporter-expressing Spodoptera frugiperda (Sf9) ovarian cells were supplied by SOLVO Biotechnology (Budaörs, Hungary).
For assessing BCRP and MDR1 transporter-related ATPase activity, incubations were carried out in the presence and absence of sodium orthovanadate (Na3VO4) (1.2 mM), a known ABC transporter inhibitor, to distinguish between background ATPase activity and transporter-related ATPase activity. The ATPase assay was performed according to the manufacturer's protocol. Briefly, membranes (20 µg per well) were preincubated at 37°C for 10 min with the test substance or the solvent DMSO in the absence and presence of vanadate, and reactions were started by adding MgATP. Sulphasalazine (10 µM) and verapamil (40 µM) served for activation of BCRP and MDR1 membranes respectively. Final concentrations of assay compounds amounted to 10 mM MgCl2, 40 mM 3-(N-morpholino)propanesulphonic acid (MOPS)-Tris (pH 7.0), 50 mM KCl, 5 mM dithiothreitol, 0.1 mM EGTA, 4 mM sodium azide, 1 mM ouabain and 5 mM ATP. Reactions were stopped after 10 min at 37°C by the addition of the colour-developing agent followed by a blocking reagent. Another 30 min later, absorbance at 620 nm was detected using a BMG Labtech FluoStar Optima fluorescence photometer (BMG Labtech, Offenburg, Germany). Phosphate standards (0.4 and 0.8 pmol per well) for OD calibration were included on each 96-well plate. For calculation of relative ATPase activity, baseline ATPase activity (ctrl 1), Na3VO4-insensitive ATPase activity (ctrl 2), ATPase activity of fully activated membranes (ctrl 3) and Na3VO4-insensitive ATPase activity of fully activated membranes (ctrl 4) were also determined. The negative control for MDR1 was provided by membrane preparations expressing β-galactosidase (Beta-gal) and for BCRP we used Sf9 cell membranes from cells infected with a baculovirus containing a defective BCRP gene (defBCRP).
Vesicular transport assay
Vesicular transport assays were performed as previously described (Bodo et al., 2003). Membrane vesicles in the inside-out orientation prepared from baculovirus-infected Sf9 cells overexpressing human BCRP transporters were supplied by SOLVO Biotechnology. 3H-oestrone-3-sulphate was used as a radiolabelled, low permeability, reporter substrate which is transported into the vesicles. Incubations were carried out in the absence and presence of 4 mM ATP to distinguish between transporter-related uptake and passive diffusion into the vesicles. The reference inhibitor Ko143 (300 nM) served as a positive control and defBCRP as negative control. Briefly, membrane vesicle preparations were preincubated with reporter substrate and the test substance or, alternatively, the solvent DMSO at 37°C for 10 min in assay buffer, containing 10 mM MgCl2, 10 mM Tris-Cl (pH 7.0) and 250 mM sucrose. Addition of MgATP, or assay buffer for background controls, started the reaction. After 1 min, reactions were stopped by the addition of ice-cold assay buffer. Vesicles were separated by immediate filtration on a 96-well filter plate (0.65 µM, Millipore, Billerica, MA, USA), filter plates were washed, dried and reporter substrate inside the filtered vesicles was quantified by liquid scintillation, using a Perkin Elmer MicroBeta Trilux liquid scintillation counter (Perkin Elmer, Waltham, MA, USA).
Final assay concentrations were 0.07, 0.21, 0.62, 1.85, 5.55, 16.67, 50 and 150 µM for the dye extrusion assays and 0.41, 1.2, 3.7, 11, 33, 100 and 300 µM for ATPase and vesicular transport assays. For each concentration tested, assays were performed in triplicate.
Data analysis
Dye extrusion assay
The rate of dye accumulation was defined as the slope of the fluorescence versus time plot. For calculation of relative transporter inhibition (%), values from DMSO and control inhibitors (Ko143 and verapamil) were defined as 0% and 100% inhibition, respectively, by the following equation:
IC50 was defined as the test substance concentration required for inhibiting the transport of the reporter substrate by 50%. Efficacy describes the maximal inhibition achieved by a test compound in per cent of the maximal inhibition observed in the presence of the reference inhibitors Ko143 and verapamil.
ATPase assay
For calculation of relative activation and inhibition, the vanadate-sensitive baseline ATPase activity and the maximal vanadate-sensitive ATPase activity after stimulation with 10 µM sulphasalazine, for BCRP, or 40 µM verapamil, for MDR1, were defined as 0% and 100% transporter ATPase activity respectively. Relative activation (%) was calculated as follows:
EC50 was defined as the test substance concentration needed to reach 50% of its own maximal activation and efficacy was defined as the compound's maximal activation, as compared with the activation by the respective reference.
Relative inhibition values (%) were obtained as follows:
 |
IC50 was defined as the test substance concentration at which half-maximal inhibition occurred and efficacy was defined as the maximal inhibitory effect achieved by the test substance relative to the baseline activity.
Vesicular transport assay
Substrate transport relative to the non-inhibited control (%) was calculated according to the following equation:
IC50 was defined as the test substance concentration required for inhibiting transport of the reporter substrate by 50%. Efficacy specifies the maximal inhibition achieved by the test compound in per cent of the maximal activity.
ISIS/Draw V2.1.4 (MDL Information Systems, CA, USA) served to illustrate chemical structures of anthocyanidins (Figure 1).
Figure 1.
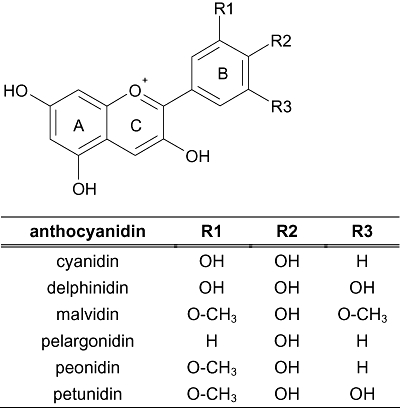
Chemical structures of tested anthocyanidins.
Statistics
GraphPad Prism 4.03 (GraphPad Sofware Inc., San Diego, CA, USA) was used for curve fitting, determination of reaction parameters and statistical analysis.
To address a putative structural effect of tested anthocyanins' sugar component on BCRP ATPase activation and vesicular transport, substances were grouped by the presence or absence of sugar moieties, that is, anthocyanins versus anthocyanidins. EC50 and IC50 values, respectively, were compared with an unpaired t-test following confirmation of Gaussian distributions. To correct for unequal variances (F-test), Welch's correction was performed. In order to minimize the risk of type I error, a Bonferroni correction was applied. Statistical significance was set at P= 0.05.
Materials
Cyanidin, cyanidin-3,5-diglucoside (cyanidin-3,5-diglc), cyanidin-3-galactoside (cyanidin-3-gal), cyanidin-3-glucoside (cyanidin-3-glc), cyanidin-3-rutinoside (cyanidin-3-rut), delphinidin, delphinidin-3-glucoside, malvidin, malvidin-3,5-diglucoside, malvidin-3-galactoside, malvidin-3-glucoside, peonidin, peonidin-3-glucoside, pelargonidin, pelargonidin-3,5-diglucoside and petunidin were purchased from Extrasynthese (Genay, France). Stock solutions of the flavonoids (15 mM) and serial dilutions were prepared in DMSO.
Radiochemicals were purchased from Perkin Elmer (Waltham, MA, USA). Other chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) or were of analytical grade.
Results
None of the test compounds showed any affinity for either the BCRP or the MDR1 transporter in the dye efflux assay. For the remaining BCRP and MDR1 assays, calculated reaction parameters are shown in Table 1.
Table 1.
Reaction parameters derived from MDR1 or BCRP ATPase and BCRP vesicular transport (VT) assays
| Test compound | MDR1 ATPase |
BCRP ATPase |
BCRP VT |
|
|---|---|---|---|---|
| Inhibition [IC50] (eff.) | Inhibition [IC50] (eff.) | Activation [EC50] (eff.) | Inhibition [IC50] (eff.) | |
| Cyanidin | 58 (100) | 7.6 (100) | – (–) | 5 (100) |
| Delphinidin | 104 (100) | 87 (100) | – (–) | 13 (98) |
| Malvidin | 220 (62) | 49 (100) | 1.1 (59) | 3 (98) |
| Pelargonidin | 83 (100) | 76 (79) | – (–) | 17 (97) |
| Peonidin | 100 (100) | 30 (100) | – (–) | 4 (96) |
| Petunidin | 230 (65) | 193 (87) | 3.3 (125) | 5 (100) |
| Cyanidin-3-glc | – (–) | – (–) | 119 (142) | 49 (87) |
| Cyanidin-3-gal | – (–) | – (–) | 15 (237) | 25 (89) |
| Cyanidin-3-rut | – (–) | – (35) | – (–) | 95 (73) |
| Cyanidin-3,5-diglc | – (–) | 43 (100) | – (–) | 83 (76) |
| Delphinidin-3-glc | – (–) | – (44) | – (–) | 47 (93) |
| Malvidin-3-glc | – (42) | – (35) | – (–) | 43 (94) |
| Malvidin-3-gal | – (24) | – (14) | 6.3 (73) | 31 (88) |
| Malvidin-3,5-diglc | – (–) | – (–) | 12 (87) | 23 (81) |
| Pelargonidin-3,5-diglc | – (14) | – (24) | – (–) | 130 (66) |
| Peonidin-3-glc | – (20) | – (–) | 45 (133) | 24 (93) |
Half-maximal inhibition and activation values are displayed in µM. Efficacies are given in brackets at the highest anthocyanidin/anthocyanin concentration tested.
–, no effect observed; BCRP, breast cancer resistance protein; MDR1, multidrug resistance protein 1.
In BCRP ATPase assays, baseline ATPase activity was stimulated by several anthocyanidins and their glycosides (Table 1). The presence or absence of a sugar moiety failed to predict half-maximal ATPase activation values in this assay (P= 0.1504, t= 1.78, df = 4, pcorr= 0.3008). With the exception of cyanidin-3,5-diglc, however, only anthocyanidins elicited BCRP ATPase inhibition at IC50 < 50 µM. Potent inhibitors comprised cyanidin and peonidin, intermediate inhibition was exhibited by pelargonidin and delphinidin, with minimal inhibition observed for cyanidin-3-rut, delphinidin-3-glc, malvidin-3-glc, pelargonidin-3,5-diglc, malvidin-3-gal (eff. <44%). Interestingly, two compounds, malvidin and petunidin, which stimulated the BCRP ATPase at low concentrations (1–3 µM), displayed bimodal activities, inhibiting ATPase activity at higher concentrations (IC50= 49 µM and 193 µM). Of the identified inhibitors, delphinidin, cyanidin, malvidin and peonidin inhibited both the maximal ATPase activity after sulphasalazine stimulation, and the baseline ATPase activity at higher concentrations.
In MDR1 ATPase assays, cyanidin-3-glc, cyanidin-3-gal and delphinidin-3-glc showed weak stimulation of both baseline and verapamil-induced activity (eff. = 35%, 23% and 24% respectively). Only cyanidin, pelargonidin, peonidin and delphinidin fully inhibited verapamil-stimulated ATPase activity within the tested concentration range (<300 µM). Partial and very weak MDR1 inhibition was observed for malvidin, petunidin, malvidin-3-glc, malvidin-3-gal, peonidin-3-glc and pelargonidin-3,5-diglc. Finally, cyanidin-3-rut, cyanidin-3,5-diglc and malvidin-3,5-diglc did not exhibit any measurable inhibitory effects on MDR1 ATPase.
In view of the overall poor affinities of these compounds for MDR1, further characterizations by transport assays to rule out non-specific stimulation of ATPase were conducted only for BCRP. All test compounds inhibited BCRP-mediated transport of 3H-oestrone-3-sulphate into vesicles, dose-dependently (far right hand column, Table 1). Effects were most pronounced for malvidin, peonidin, petunidin and cyanidin with essentially identical IC50 values and full efficacy. The remainder of the compounds tested were less potent with IC50 values ranging from 13 M to 130 M and efficacies from 98% to 66%. With regard to structural features in the vesicular transport assay, anthocyanidins and their glycosides exhibited a significant difference in mean IC50 values (P= 0.0029, t= 4.05, df = 9, pcorr= 0.0058).
Discussion and conclusions
We used a combination of assays to examine the interactions of 16 flavonoids with two key proteins involved in the transport of xenobiotics.
Passive permeability is a prerequisite for eliciting a response in dye extrusion assays as has previously been shown for other compounds (Polli et al., 2001; Mahar Doan et al., 2002). The absence of effects in the calcein-AM and Hoechst 33342 assays, suggests that anthocyanins and their aglycones cannot cross the cell membrane passively and, therefore, will not reach the substrate binding site of the transporter.
We identified seven potent stimulants of the BCRP ATPase activity, with half-maximal activation in the low micromolar range for two anthocyanidins. For four flavonoids, stimulation of baseline ATPase activity exceeded 100% as defined by the reference activator substrate. While this was in part a non-specific effect that was also observed in the defBCRP negative control for cyanidin-3-gal and cyanidin-3-glc (data not shown), actual effects may be lower for these compounds. However, petunidin and peonidin-3-glc emerged as genuine stimulants of BCRP ATPase activity that led to a higher level of activation than is achieved with 10 µM sulphasalazine. Stimulation of ATPase activity by substrates is considered a direct correlate of the actual transport process (Sarkadi et al., 1992; Mao and Unadkat, 2005) and therefore an indicator of substrate functionality. However, stimulation of BCRP- and MDR1-associated ATPase activity may, on occasion, occur independently of substrate transport (Adachi et al., 2001; Ozvegy et al., 2002). In other cases, induction of ABC transporter activity has been observed (Zhou et al., 2004). This appears unlikely for anthocyanins and anthocyanidins, as reporter substrate transport was not increased in the vesicular transport assay. In addition to some stimulators of BCRP ATPase, potent inhibitors were also identified, with IC50 values of cyanidin, peonidin, cyanidin-3,5-diglc and malvidin ranging from 7.6 to 49 µM. Moreover, malvidin and petunidin, both of which stimulated baseline ATPase activity at low concentrations (EC50 of 1.1 and 3.3 µM respectively), inhibited sulphasalazine-stimulated ATPase activity at higher concentrations (IC50 values of 49 and 193 µM respectively). It cannot be entirely ruled out, however, that these two anthocyanidins are not true inhibitors, but rather slowly transported substrates which compete with the assay substrate for transport. With only one exception, cyanidin-3,5-diglc, the absence of sugar moieties was associated with higher levels of BCRP ATPase inhibition, as compared with those achieved by other compounds.
Results of the vesicular transport assays confirmed significantly higher affinities of anthocyanidins for BCRP (mean IC50= 7.8 µM) when compared with affinities of glycosylated test compounds (mean IC50= 55.0 µM, P= 0.0029, t= 4.05, df = 9). Vesicular transport assays also served to verify affinities of all compounds for BCRP. Moreover, none of the tested anthocyanins or aglycones enhanced transport of the reporter substrate. This further supports the hypothesis that the seven compounds activating BCRP ATPase are true BCRP substrates, rather than mere stimulants of BCRP activity.
In contrast to our results for BCRP ATPase, the effects of the test compounds on MDR1 ATPase activity were weak. Three compounds enhanced both baseline and verapamil-stimulated MDR1 ATPase activity, but the latter effect is rarely observed in practice. Our findings are best explained by non-specific interactions with the MDR1 transporter, such as stimulation of an endogenous ATPase. For these flavonoids, a slight stimulation of ATPase activity in the beta-gal control membranes was also observed. All tested aglycones reached IC50 values from 58 to 230 µM for MDR1 ATPase inhibition. Thus, anthocyanidins are either moderate MDR1 inhibitors or slowly transported MDR1 substrates, while even lower levels of inhibition were observed for glycosylated compounds.
A variety of dietary polyphenols are known to interact with BCRP or MDR1 in vitro. Of these, the stilbene resveratrol, the flavanons hesperetin (Cooray et al., 2004) and naringenin (Zhang et al., 2004a), the flavons luteolin and chrysin (Imai et al., 2004), and the flavonols kaempferol and quercetin (Zhang et al., 2004b) have emerged as inhibitors of BCRP, among others. The isoflavone genistein is considered as a natural substrate that competitively inhibits drug efflux by BCRP (Imai et al., 2004). With respect to MDR1, resveratrol (Nabekura et al., 2005), hesperetin, naringenin, chrysin (Mitsunaga et al., 2000), the tea catechins epicatechin gallate (ECG) and epigallocatechin gallate (EGCG), plus the anthocyanidin cyanidin (Kitagawa, 2006) have previously displayed inhibitory activity. Regarding bimodal functions, as observed for malvidin and petunidin with respect to BCRP ATPase activity, similar effects have been noted for quercetin and kaempferol on MDR1 activity (Mitsunaga et al., 2000; Yoo et al., 2007). Polyphenolic MDR1 stimulants include (-)-epicatechin (Wang et al., 2002) and the flavonol galangin (Critchfield et al., 1994).
Owing to the mostly semi-quantitative and heterogenous assays employed in many earlier studies (Conseil et al., 1998), however, a cautious comparison of flavonoid affinities for BCRP and MDR1 is warranted. Among the consistent findings was the modulatory role of sugar moieties on efflux transporter activity. Thus, for most flavonoids that interacted with either BCRP or MDR1, the corresponding glycosides proved either inactive, or less active (Mitsunaga et al., 2000; Imai et al., 2004; Zhang et al., 2004b; Kitagawa, 2006; Yoo et al., 2007). In a further parallel to earlier work on other polyphenols (Imai et al., 2004; Ahmed-Belkacem et al., 2005; Katayama et al., 2007; Brand et al., 2008), we noted prominent effects of anthocyanidins on BCRP (Figure 2). As BCRP ATPase inhibition was up to sevenfold that for MDR1, and substrate-like behaviour was limited to BCRP, we suggest that anthocyanidins and anthocyanins exhibit BCRP-specific functions. This may be relevant to the intestinal barrier and to brain microvessels where BCRP is expressed at higher levels than MDR1 (Albermann et al., 2005; Hilgendorf et al., 2007; Dauchy et al., 2008). Despite the difference in transporter expression, however, the role played by BCRP in substrate efflux at the BBB in vivo is less firmly established (Lee et al., 2005; Zhao et al., 2009). Few investigations have addressed the selectivity of xenobiotics for BCRP, relative to MDR1. Distinct affinities are currently being discussed for the substrates flavopiridol, mitoxantrone and topotecan (Litman et al., 2000; Doyle and Ross, 2003; Nakanishi et al., 2003), plus the inhibitory fumitremorgin C analogue Ko143 and tariquidar analogues (Allen et al., 2002; Kuhnle et al., 2009). For these agents, anthocyanidins may alter the pharmacokinetics, depending on their mode of interaction with BCRP.
Figure 2.
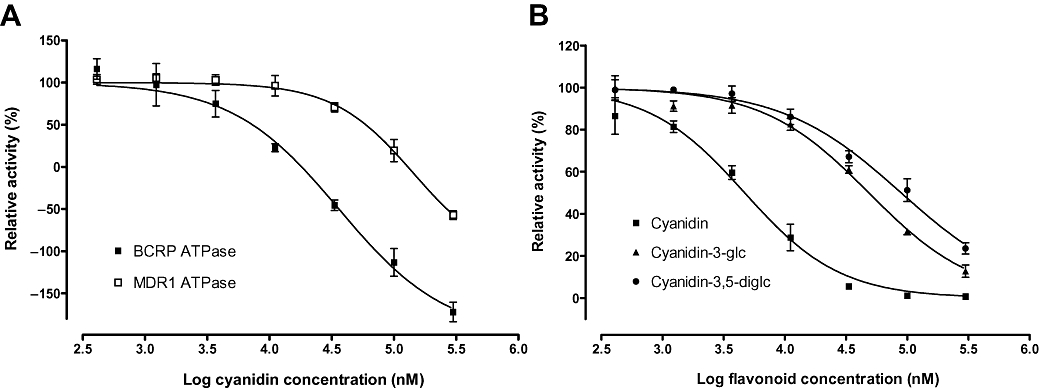
Dose-response curves for cyanidin with respect to BCRP and MDR1 ATPase inhibition (A), and for cyanidin, cyanidin-3-glc and cyanidin-3-diglc with respect to inhibition of BCRP vesicular transport (B). BCRP, breast cancer resistance protein; MDR1, multidrug resistance protein 1.
BCRP has been implicated in multidrug resistance of tumours (Ahmed-Belkacem et al., 2005; Robey et al., 2007), and co-determines responsiveness to the treatment of CNS disease (Loscher and Potschka, 2005; Lazarowski et al., 2007). Targeting of BCRP may affect barriers in the placenta (Gupta et al., 2004), secretory organs (Sarkadi et al., 2004), the digestive tract and the brain (Maliepaard et al., 2001) with regard to permeability for toxins (Jonker et al., 2005), other dietary compounds (Mao and Unadkat, 2005; Sugimoto et al., 2005; Cervenak et al., 2006) and environmental carcinogens (van Herwaarden et al., 2006).
While substrate-type affinity of anthocyanidins and anthocyanins for BCRP is liable to limit their intestinal absorption and, possibly, brain uptake, the moderate levels of interaction with MDR1 would appear not to be able to reduce bioavailability. Moreover, anthocyanidins may compete with and thereby limit transport of drugs with high affinity for BCRP.
Compounds that cause efflux transporter inhibition may on the one hand dismantle protection against toxins (Kusuhara and Sugiyama, 2007), or may pose a risk of food–drug interactions including those mediated by dietary supplements (Zhang et al., 2004b; Alvarez et al., 2009). On the other hand, transporter inhibitors have restored chemosensitivity to tumour cells (Schinkel and Jonker, 2003; Zhang et al., 2004b) and may enhance brain uptake of drugs. As adjuvants to therapy, inhibitors thus hold promise in the pharmacotherapy of epilepsy (Aronica et al., 2005; Loscher and Potschka, 2005; Lazarowski et al., 2007) and primary brain tumours (Aronica et al., 2005; Breedveld et al., 2006; de Vries et al., 2007).
However, for inhibition of BCRP and MDR1 transporters by anthocyanidins to take effect, we would need to assume that anthocyanidins enter intestinal epithelial and brain endothelial cells at micromolar concentrations. Existing data point to relatively high anthocyanin concentrations in the intestine (Kahle et al., 2006), whereas peak plasma concentrations are in the nanomolar range, and less than 1% is usually excreted in urine (Matsumoto et al., 2001; McGhie and Walton, 2007; Mullen et al., 2008). These estimates may be considered conservative in that analytical challenges are posed (i) by anthocyanin accumulation in tissues as shown for rats (El Mohsen et al., 2006); (ii) by metabolic transformation to molecular structures that are not routinely detected; and (iii) by protein binding (Satué-Gracia et al., 1997; Murkovic et al., 2000).
For extrapolations to the in vivo situation, functional genetic variation in transporter proteins must also be taken into account as a likely confounder of the functionalities addressed (Siddiqui et al., 2003; Pauli-Magnus and Kroetz, 2004; Kusuhara and Sugiyama, 2007). More detailed investigations of anthocyanin and anthocyanidin transporter binding sites and interaction mechanisms are desirable, including studies on reversibility of effects. Possible mechanisms include competitive inhibition or steric blockage of substrate binding to the transporter or ATPase, and allosteric effects on substrate recognition, translocation, dissociation or ATP hydrolysis. Other areas of future research would include the effects of natural exposure to combinations of anthocyanins, as in standardized extracts, and to anthocyanin degradation products such as phenolic acids. Finally, direct transport assays and studies on BCRP knockout mice to rationalize our assumptions and comparative studies involving other members of the ABC transporter family are warranted.
In summary, we have demonstrated moderate to high affinities of anthocyanins and anthocyanidins for the human efflux transporter BCRP, and moderate to low affinities for MDR1. These results add to our understanding of anthocyanin bioavailability and put into perspective the potential of those flavonoids to interfere with the transport and the pharmacokinetics of other MDR1 and BCRP substrates.
Acknowledgments
This investigation was funded by the German Federal Ministry of Education, Science, Research and Technology, BMBF – grant no. 0313848C.
Glossary
Abbreviations:
- ABC
ATP-binding cassette
- BBB
blood–brain barrier
- BCRP
breast cancer resistance protein
- calcein-AM
calcein O,O′-diacetate tetrakis (acetoxymethyl) ester
- diglc
diglucosides
- ECG
epicatechin gallate
- ECGC
epigallocatechin gallate
- gal
galactoside
- glc
glucoside
- HBSS
Hank's Balanced Salt Solution
- MDR
multidrug resistance
- MDR1
multidrug resistance protein 1
- rut
rutinoside
- Sf9
Spodoptera frugiperda
Conflicts of interest
None.
References
- Adachi Y, Suzuki H, Sugiyama Y. Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-glycoprotein. Pharm Res. 2001;18:1660–1668. doi: 10.1023/a:1013358126640. [DOI] [PubMed] [Google Scholar]
- Ahmed-Belkacem A, Pozza A, Munoz-Martinez F, Bates SE, Castanys S, Gamarro F, et al. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005;65:4852–4860. doi: 10.1158/0008-5472.CAN-04-1817. [DOI] [PubMed] [Google Scholar]
- Albermann N, Schmitz-Winnenthal FH, Z'Graggen K, Volk C, Hoffmann MM, Haefeli WE, et al. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol. 2005;70:949–958. doi: 10.1016/j.bcp.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- Alvarez AI, Real R, Perez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2009 doi: 10.1002/jps.21851. in press. [DOI] [PubMed] [Google Scholar]
- Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Redeker S, van Vliet EA, Ramkema M, Scheffer GL, et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46:849–857. doi: 10.1111/j.1528-1167.2005.66604.x. [DOI] [PubMed] [Google Scholar]
- Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10:1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B. Differential modulation of the human liver conjugate transporters MRP2 and MRP3 by bile acids and organic anions. J Biol Chem. 2003;278:23529–23537. doi: 10.1074/jbc.M303515200. [DOI] [PubMed] [Google Scholar]
- Brand W, van der Wel PA, Rein MJ, Barron D, Williamson G, van Bladeren PJ, et al. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos. 2008;36:1794–1802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Bub A, Watzl B, Heeb D, Rechkemmer G, Briviba K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur J Nutr. 2001;40:113–120. doi: 10.1007/s003940170011. [DOI] [PubMed] [Google Scholar]
- Cervenak J, Andrikovics H, Ozvegy-Laczka C, Tordai A, Nemet K, Varadi A, et al. The role of the human ABCG2 multidrug transporter and its variants in cancer therapy and toxicology. Cancer Lett. 2006;234:62–72. doi: 10.1016/j.canlet.2005.01.061. [DOI] [PubMed] [Google Scholar]
- Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA. 1998;95:9831–9836. doi: 10.1073/pnas.95.17.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun. 2004;317:269–275. doi: 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Critchfield JW, Welsh CJ, Phang JM, Yeh GC. Modulation of adriamycin accumulation and efflux by flavonoids in HCT-15 colon cells. Activation of P-glycoprotein as a putative mechanism. Biochem Pharmacol. 1994;48:1437–1445. doi: 10.1016/0006-2952(94)90568-1. [DOI] [PubMed] [Google Scholar]
- Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem. 2008;107:1518–1528. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- Duffy KB, Spangler EL, Devan BD, Guo Z, Bowker JL, Janas AM, et al. A blueberry-enriched diet provides cellular protection against oxidative stress and reduces a kainate-induced learning impairment in rats. Neurobiol Aging. 2008;29:1680–1689. doi: 10.1016/j.neurobiolaging.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Dupuy J, Lespine A, Sutra JF, Alvinerie M. The interaction between moxidectin and MDR transporters in primary cultures of rat hepatocytes. J Vet Pharmacol Ther. 2006;29:107–111. doi: 10.1111/j.1365-2885.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- Eisenblatter T, Huwel S, Galla HJ. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res. 2003;971:221–231. doi: 10.1016/s0006-8993(03)02401-6. [DOI] [PubMed] [Google Scholar]
- El Mohsen MA, Marks J, Kuhnle G, Moore K, Debnam E, Kaila Srai S, et al. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br J Nutr. 2006;95:51–58. doi: 10.1079/bjn20051596. [DOI] [PubMed] [Google Scholar]
- Felgines C, Texier O, Besson C, Fraisse D, Lamaison JL, Remesy C. Blackberry anthocyanins are slightly bioavailable in rats. J Nutr. 2002;132:1249–1253. doi: 10.1093/jn/132.6.1249. [DOI] [PubMed] [Google Scholar]
- Felgines C, Talavera S, Texier O, Gil-Izquierdo A, Lamaison JL, Remesy C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J Agric Food Chem. 2005;53:7721–7727. doi: 10.1021/jf051092k. [DOI] [PubMed] [Google Scholar]
- Fricker G. Drug interactions with natural products at the blood brain barrier. Curr Drug Metab. 2008;9:1018–1025. doi: 10.2174/138920008786927758. [DOI] [PubMed] [Google Scholar]
- Fromm MF. Importance of P-glycoprotein for drug disposition in humans. Eur J Clin Invest. 2003;33:6–9. doi: 10.1046/j.1365-2362.33.s2.4.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso M, Rimbach G, Sasai M, Nakahara M, Matsugo S, Uchida Y, et al. Electron spin resonance spectroscopy studies on the free radical scavenging activity of wine anthocyanins and pyranoanthocyanins. Mol Nutr Food Res. 2005;49:1112–1119. doi: 10.1002/mnfr.200500100. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I, Ambudkar SV. P-glycoprotein and multidrug resistance. Curr Opin Genet Dev. 1996;6:610–617. doi: 10.1016/s0959-437x(96)80091-8. [DOI] [PubMed] [Google Scholar]
- Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2004;310:334–341. doi: 10.1124/jpet.104.065342. [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Wagenaar E, Karnekamp B, Merino G, Jonker JW, Schinkel AH. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis. 2006;27:123–130. doi: 10.1093/carcin/bgi176. [DOI] [PubMed] [Google Scholar]
- Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35:1333–1340. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle K, Kraus M, Scheppach W, Ackermann M, Ridder F, Richling E. Studies on apple and blueberry fruit constituents: do the polyphenols reach the colon after ingestion? Mol Nutr Food Res. 2006;50:418–423. doi: 10.1002/mnfr.200500211. [DOI] [PubMed] [Google Scholar]
- Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, Fillmore SA, Graf BA, et al. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- Katayama K, Masuyama K, Yoshioka S, Hasegawa H, Mitsuhashi J, Sugimoto Y. Flavonoids inhibit breast cancer resistance protein-mediated drug resistance: transporter specificity and structure-activity relationship. Cancer Chemother Pharmacol. 2007;60:789–797. doi: 10.1007/s00280-007-0426-7. [DOI] [PubMed] [Google Scholar]
- Kim JM, Kim JS, Yoo H, Choung MG, Sung MK. Effects of black soybean [Glycine max (L.) Merr.] seed coats and its anthocyanidins on colonic inflammation and cell proliferation in vitro and in vivo. J Agric Food Chem. 2008;56:8427–8433. doi: 10.1021/jf801342p. [DOI] [PubMed] [Google Scholar]
- Kitagawa S. Inhibitory effects of polyphenols on p-glycoprotein-mediated transport. Biol Pharm Bull. 2006;29:1–6. doi: 10.1248/bpb.29.1. [DOI] [PubMed] [Google Scholar]
- Kuhnle M, Egger M, Muller C, Mahringer A, Bernhardt G, Fricker G, et al. Potent and Selective Inhibitors of Breast Cancer Resistance Protein (ABCG2) Derived from the p-Glycoprotein (ABCB1) Modulator Tariquidar. J Med Chem. 2009;52:1190–1197. doi: 10.1021/jm8013822. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. Role of transporters in the tissue-selective distribution and elimination of drugs: transporters in the liver, small intestine, brain and kidney. J Control Release. 2002;78:43–54. doi: 10.1016/s0168-3659(01)00480-1. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. ATP-binding cassette, subfamily G (ABCG family) Pflugers Arch. 2007;453:735–744. doi: 10.1007/s00424-006-0134-x. [DOI] [PubMed] [Google Scholar]
- Lazarowski A, Czornyj L, Lubienieki F, Girardi E, Vazquez S, D'Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48:140–149. doi: 10.1111/j.1528-1167.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther. 2005;312:44–52. doi: 10.1124/jpet.104.073320. [DOI] [PubMed] [Google Scholar]
- Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, et al. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113:2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- Mahar Doan KM, Humphreys JE, Webster LO, Wring SA, Shampine LJ, Serabjit-Singh CJ, et al. Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther. 2002;303:1029–1037. doi: 10.1124/jpet.102.039255. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7:E118–E133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Inaba H, Kishi M, Tominaga S, Hirayama M, Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J Agric Food Chem. 2001;49:1546–1551. doi: 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- Matsunaga N, Imai S, Inokuchi Y, Shimazawa M, Yokota S, Araki Y, et al. Bilberry and its main constituents have neuroprotective effects against retinal neuronal damage in vitro and in vivo. Mol Nutr Food Res. 2009;53:869–877. doi: 10.1002/mnfr.200800394. [DOI] [PubMed] [Google Scholar]
- Mitsunaga Y, Takanaga H, Matsuo H, Naito M, Tsuruo T, Ohtani H, et al. Effect of bioflavonoids on vincristine transport across blood-brain barrier. Eur J Pharmacol. 2000;395:193–201. doi: 10.1016/s0014-2999(00)00180-1. [DOI] [PubMed] [Google Scholar]
- del Moral RG, Olmo A, Aguilar M, O'Valle F. P glycoprotein: a new mechanism to control drug-induced nephrotoxicity. Exp Nephrol. 1998;6:89–97. doi: 10.1159/000020510. [DOI] [PubMed] [Google Scholar]
- Mullen W, Edwards CA, Serafini M, Crozier A. Bioavailability of pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J Agric Food Chem. 2008;56:713–719. doi: 10.1021/jf072000p. [DOI] [PubMed] [Google Scholar]
- Murkovic M, Toplak H, Adam U, Pfannhauser W. Analysis of Anthocyanins in Plasma for Determination of their Bioavailability. J Food Compos Anal. 2000;13:291–296. [Google Scholar]
- Nabekura T, Kamiyama S, Kitagawa S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem Biophys Res Commun. 2005;327:866–870. doi: 10.1016/j.bbrc.2004.12.081. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Karp JE, Tan M, Doyle LA, Peters T, Yang W, et al. Quantitative analysis of breast cancer resistance protein and cellular resistance to flavopiridol in acute leukemia patients. Clin Cancer Res. 2003;9:3320–3328. [PubMed] [Google Scholar]
- Ozvegy C, Varadi A, Sarkadi B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. J Biol Chem. 2002;277:47980–47990. doi: 10.1074/jbc.M207857200. [DOI] [PubMed] [Google Scholar]
- Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. Fast access of some grape pigments to the brain. J Agric Food Chem. 2005;53:7029–7034. doi: 10.1021/jf050565k. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Kroetz DL. Functional implications of genetic polymorphisms in the multidrug resistance gene MDR1 (ABCB1) Pharm Res. 2004;21:904–913. doi: 10.1023/b:pham.0000029276.21063.0b. [DOI] [PubMed] [Google Scholar]
- Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, et al. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–628. [PubMed] [Google Scholar]
- Rahman MM, Ichiyanagi T, Komiyama T, Sato S, Konishi T. Effects of anthocyanins on psychological stress-induced oxidative stress and neurotransmitter status. J Agric Food Chem. 2008;56:7545–7550. doi: 10.1021/jf800930s. [DOI] [PubMed] [Google Scholar]
- Ramirez MR, Izquierdo I, do Carmo Bassols Raseira M, Zuanazzi JA, Barros D, Henriques AT. Effect of lyophilised Vaccinium berries on memory, anxiety and locomotion in adult rats. Pharmacol Res. 2005;52:457–462. doi: 10.1016/j.phrs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- Salminen H, Heinonen M. Plant phenolics affect oxidation of tryptophan. J Agric Food Chem. 2008;56:7472–7481. doi: 10.1021/jf800708t. [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992;267:4854–4858. [PubMed] [Google Scholar]
- Sarkadi B, Ozvegy-Laczka C, Nemet K, Varadi A. ABCG2 – a transporter for all seasons. FEBS Lett. 2004;567:116–120. doi: 10.1016/j.febslet.2004.03.123. [DOI] [PubMed] [Google Scholar]
- Satué-Gracia MT, Heinonen M, Frankel EN. Anthocyanins as antioxidants on human low-density lipoprotein and lecithin-liposome systems. J Agric Food Chem. 1997;45:3362–3367. [Google Scholar]
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Sato H, Sugiyama Y. Evaluation of drug-drug interaction in the hepatobiliary and renal transport of drugs. Annu Rev Pharmacol Toxicol. 2005;45:689–723. doi: 10.1146/annurev.pharmtox.44.101802.121444. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Lau FC, Joseph JA. Berry fruit supplementation and the aging brain. J Agric Food Chem. 2008;56:636–641. doi: 10.1021/jf072505f. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348:1442–1448. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Tsukahara S, Ishikawa E, Mitsuhashi J. Breast cancer resistance protein: molecular target for anticancer drug resistance and pharmacokinetics/pharmacodynamics. Cancer Sci. 2005;96:457–465. doi: 10.1111/j.1349-7006.2005.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Dai H, Shaik N, Elmquist WF. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 2003;55:83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
- Talavera S, Felgines C, Texier O, Besson C, Gil-Izquierdo A, Lamaison JL, et al. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J Agric Food Chem. 2005;53:3902–3908. doi: 10.1021/jf050145v. [DOI] [PubMed] [Google Scholar]
- Tarozzi A, Morroni F, Hrelia S, Angeloni C, Marchesi A, Cantelli-Forti G, et al. Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neurosci Lett. 2007;424:36–40. doi: 10.1016/j.neulet.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Thomasset S, Berry DP, Cai H, West K, Marczylo TH, Marsden D, et al. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev Res (Phila Pa) 2009;2:625–633. doi: 10.1158/1940-6207.CAPR-08-0201. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Horio F, Osawa T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999;449:179–182. doi: 10.1016/s0014-5793(99)00407-x. [DOI] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- Wang EJ, Barecki-Roach M, Johnson WW. Elevation of P-glycoprotein function by a catechin in green tea. Biochem Biophys Res Commun. 2002;297:412–418. doi: 10.1016/s0006-291x(02)02219-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Mazza G. Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-gamma-activated RAW 264.7 macrophages. J Agric Food Chem. 2002;50:850–857. doi: 10.1021/jf010976a. [DOI] [PubMed] [Google Scholar]
- Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Wu X, Gu L, Prior RL, McKay S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J Agric Food Chem. 2004;52:7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- Yoo HH, Lee M, Chung HJ, Lee SK, Kim DH. Effects of diosmin, a flavonoid glycoside in citrus fruits, on P-glycoprotein-mediated drug efflux in human intestinal Caco-2 cells. J Agric Food Chem. 2007;55:7620–7625. doi: 10.1021/jf070893f. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Shukitt-Hale B, Joseph JA. Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med. 2004;37:1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004a;21:1263–1273. doi: 10.1023/b:pham.0000033015.84146.4c. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004b;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- Zhao R, Raub TJ, Sawada GA, Kasper SC, Bacon JA, Bridges AS, et al. Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood-brain barrier. Drug Metab Dispos. 2009;37:1251–1258. doi: 10.1124/dmd.108.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Lim LY, Chowbay B. Herbal modulation of P-glycoprotein. Drug Metab Rev. 2004;36:57–104. doi: 10.1081/dmr-120028427. [DOI] [PubMed] [Google Scholar]


