Abstract
Background and purpose:
The risk for cardiovascular events including venous and arterial disease and stroke is elevated after treatment with estrogen and medroxyprogesterone acetate (MPA) in postmenopausal women. Here, we have investigated the effect of MPA on arterial thrombosis and atherosclerosis in a murine model of atherosclerosis.
Experimental approach:
Apolipoprotein E (ApoE)−/− mice were bilaterally ovariectomized and treated with placebo, MPA (27.7 µg·day−1) and MPA + 17-β-oestradiol (E2; 1.1 µg·day−1) for 90 days, on a Western-type diet. Thrombotic response was measured in a photothrombosis model, platelet activation by fluorescence activated cell sorting (FACS) analysis (CD62P) and thrombin generation by the endogenous thrombin potential (ETP). Furthermore, aortic plaque burden and aortic root plaque composition were determined.
Key results:
MPA and MPA + E2-treated animals showed an aggravated thrombotic response shown by significantly reduced time to stable occlusion. The pro-thrombotic effect of MPA was paralleled by increased ETP whereas platelet activation was not affected. Furthermore, MPA + E2 reduced the number of cells positive for α-smooth muscle actin and increased hyaluronan in the plaque matrix. Interestingly, total plaque burden was reduced by MPA but unchanged by MPA + E2.
Conclusion and implications:
Long-term treatment with MPA and MPA + E2 increased arterial thrombosis despite inhibitory effects of MPA on atherosclerosis in ApoE-deficient mice. Increased thrombin formation, reduced smooth muscle content and remodelling of non-collagenous plaque matrix may be involved in the pro-thrombotic effects. Thus, MPA exhibits differential effects on arterial thrombosis and on atherosclerosis.
Keywords: progestins, hyaluronan, atherosclerosis, thrombosis, hormone replacement therapy
Introduction
Hormone replacement therapy (HRT) was expected to prevent cardiovascular events in postmenopausal women. This assumption was based on several epidemiological and observational studies suggesting that postmenopausal oestrogen therapy would reduce mortality from coronary heart disease (CHD) (Stampfer et al., 1985; Grodstein et al., 2000). Furthermore, animal studies in monkeys and mice also suggested anti-atherosclerotic effects of oestrogen substitution (Adams et al., 1990; Bourassa et al., 1996). However, recently large prospective randomized clinical trials questioned this concept and revealed increased thromboembolic event rates, including stroke (Rossouw et al., 2002; Manson et al., 2003; Wassertheil-Smoller et al., 2003; Cushman et al., 2004). Therefore, the concept that oestrogens are generally protective against CHD in women was abandoned and long-term HRT is no longer recommended (Rossouw et al., 2002). However, it is considered that oestrogens might protect from atherosclerosis only in young women if initiated early after menopause, whereas treatment of women with advanced atherosclerosis, years or decades after menopause might lead to worsening of clinical outcomes (‘window of opportunity’) (Dubey et al., 2005; Rossouw et al., 2007).
A trend towards inhibition of CHD was observed in the arm of the Women's Health Initiative (WHI) trial with conjugated equine oestrogens (CEEs) alone (Anderson et al., 2004). In contrast in the arm receiving CEE plus medroxyprogesterone acetate (MPA) a trend towards increased CHD was recorded, which might be an indication of an effect of MPA on cardiovascular disease and perhaps atherothrombosis (Rossouw et al., 2002). Comprehending the role of progestin therapy is, however, complicated by the fact that many progestin derivatives have been developed that differ with respect to the concomitant activation or antagonism of other steroid receptors. MPA activates glucocorticoid receptors, which could mimic part of the anti-atherosclerotic effects of glucocorticoids (Asai et al., 1993), and has, on the other hand, also anti-androgenic activity, which might diminish protective oestrogen effects. In monkeys, MPA interferes with anti-atherosclerotic oestrogen effects whereas progesterone does not (Adams et al., 1990; Adams et al., 1997). Furthermore, the effect on endothelial cells is variable between the progestins; for instance, progesterone increases endothelial nitric oxide (NO) release whereas MPA does not, a finding attributed to differences in the downstream signalling in endothelial cells (Simoncini et al., 2004).
MPA is currently used in addition to HRT also in premenopausal women as a contraceptive and to treat various gynaecological conditions such as endometriosis, polycystic ovarian syndrome and irregular uterine bleeding (Cullins, 1996). Further research on the effects of MPA on atherothrombosis in animal models will contribute to a better understanding of its effects on CHD and the underlying mechanisms of this frequently used progestin. Therefore, the aim of the present study was to evaluate the effect of MPA alone or in combination with oestradiol on arterial thrombosis and atherosclerosis in apolipoprotein E (ApoE)-deficient mice.
Methods
Animals
All animal care and experimental procedures complied with the guidelines for the use of experimental animals as outlined in the ‘Deutsches Tierschutzgesetz’ and according to the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication 85–23, revised 1985). Homozygous ApoE-deficient mice (strain: Maeda) were obtained from Taconic M&B (Ejby, Denmark). Mice were kept in a 12 h light/dark cycle with access to food and water ad libitum. At weaning (age 28 days) mice were bilaterally ovariectomized (OVX). Anaesthesia was performed using a mixture of ketamine (100 mg·kg−1) and xylazine (5 mg·kg−1). Mice were randomly assigned to three treatment groups, namely, placebo, MPA and MPA + 17-β-oestradiol (E2). At 42 days of age, placebo pellets or slow-release hormone pellets (Innovative Research of America), prepared to dispense 27.7 µg·day−1 MPA or 1.1 µg·day−1 E2 for 90 days, were implanted subcutaneously. The dose of oestrogen used in our study was based on earlier studies by Elhage et al. (1997) who observed maximal inhibition of fatty streak formation with E2 at doses between 0.83 µg·day−1 and 1.6 µg·day−1. The dose of MPA used in our study was based on earlier studies by Shultz et al. (2004) and Hanke et al. (1996) who used progestin doses equalling a progestin/oestrogen ratio between 10:1 and 100:1. The treatment period was based on earlier studies performed by Bourassa et al., (1996) and Marsh et al. (1999) who described anti-atherosclerotic actions of E2. Starting at the day of pellet-implantation mice were fed a Western-type diet containing 21% butter fat and 0.15% cholesterol by weight (Ssniff, Soest, Germany). Animal weights were determined after 132 days. The experimental design is summarized in Figure 1A. The drug/molecular target nomenclature used here follows Alexander et al. (2008).
Figure 1.
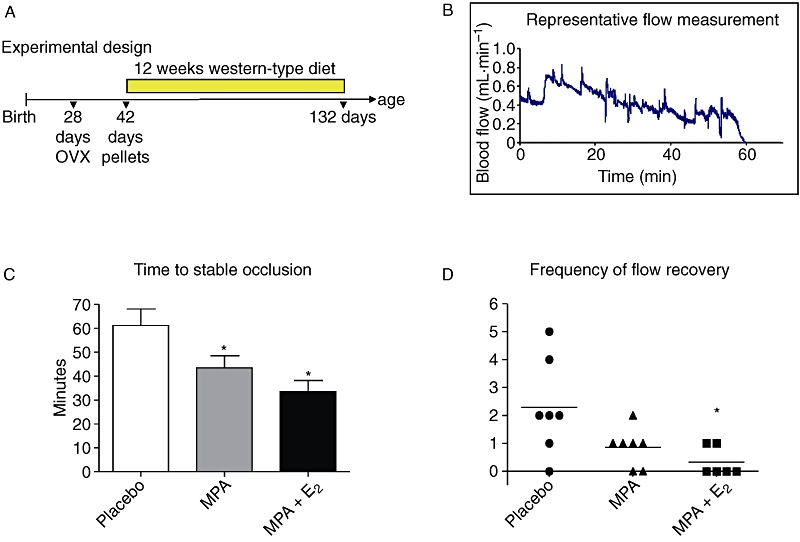
Thrombotic response. (A) Experimental design. (B) Representative blood-flow measurement after initiation of photochemical injury at time 0. (C) Times to stable occlusion from mice receiving placebo, medroxyprogesterone acetate (MPA; 27.7 µg·day−1) and MPA + E2 (1.1 µg·day−1). (D) Graph showing the frequency of flow-recovery as a measure of thrombus stability in mice treated with placebo, MPA and MPA + E2. Data represent means ± standard error of the mean; n= 6–8, *, P < 0.05 versus placebo.
Determination of aortic plaque burden
Animals were perfusion fixed with 4% paraformaldehyde, aortas were carefully removed and postfixed with 4% paraformaldehyde (4°C, overnight). The next day aortas were transferred into 1× phosphate-buffered saline (PBS), freed from adventitia and subsequently equilibrated in 78% methanol for 5 min. Subsequently, Oil-Red-O staining was performed for 90 min at room temperature and aortas washed in 78% methanol for 10 min afterwards. Finally en face pictures were taken and the percentage of Oil-Red-O stained area of total surface area was determined by ImageJ 1.37v software (National Institutes of Health, Bethesda MD, USA) and defined as plaque burden. Eighteen to 21 mice were analysed in each treatment group.
Measurement of lipoprotein profiles
Total cholesterol and triglycerides were determined after feeding by the department of Clinical Chemistry of the University Clinics Düsseldorf according to standard procedures (enzymatic colour-reaction kits; Chol and TG, cobas, Roche, Mannheim, Germany). Ten to 13 mice were analysed in each treatment group.
Photochemical induction of thrombosis
Thrombosis of the right carotid artery was induced by injection of Rose Bengal and subsequent irradiation using a green light laser as previously described (Wilson et al., 2003). Time to first occlusion was defined as the time when blood-flow first ceased to zero. Time to stable occlusion was defined as the time at which blood-flow stayed at zero for ≥10 min. The frequency of flow recovery was determined in the interval between first and stable occlusion. Every time when the blood-flow increased to more than 0.09 mL/min after an intermediate occlusion was defined as one event of flow-recovery. The sum of these events was set as frequency of flow recovery. Two out of 10 placebo mice and one out of nine MPA + E2 animals died before stable occlusion and were excluded. Finally, 6–7 mice were analysed in each treatment group.
Analysis of platelet activation and endogenous thrombin potential
Whole blood was obtained by cardiac puncture of mice anaesthetized with a mixture of ketamine (100 mg·kg−1) and xylazine (5 mg·kg−1). Sodium citrate (0.02 M, final concentration) was used as anticoagulant. Platelet-rich plasma (PRP) was prepared by centrifugation at 850×g for 45 s and diluted 1:10 with PBS. The percentage of platelets in PRP was determined after incubation of 25 µL PRP with 5 µL phycoerythrin (PE)-conjugated rat anti-mouse anti-CD41 antibody (BD Pharmingen, Heidelberg, Germany) for 30 min and diluted with 500 µL isotone for analysis on a Cytomics FC 500 Cytometer (Beckman Coulter, Krefeld, Germany). PE-conjugated mouse IgG1-PE (Beckman Coulter) was used as isotypic control. PRP was characterized by 98–99% CD41 positive particles. Platelet P-selectin (CD62P) expression as read out for platelet activation was determined by FACS analysis using CD62P-antibody (FITC-conjugated rat-anti-mouse anti-CD62P antibody, BD Pharmingen) and the respective FITC-conjugated isotypic control (mouse IgG1-FITC, Beckman Coulter) as previously described (Zimmermann et al., 2003). To detect differences in the magnitude of maximal platelet activation, PRP was preincubated with convulxin (5 µg/mL, Alexis, Lörrach, Germany) for 5 min and subsequently CD62P surface expression was detected as described above. Analysis was performed using CXP Analysis Software 2.2. Platelet-poor plasma (PPP) was prepared from the PRP and used for the measurement of endogenous thrombin potential (ETP) using a modified thrombinoscope method (Stampfuss et al., 2005). Briefly, 15 µL PPP, 10 µL platelet membranes, 1 pmol innovin as tissue factor source (final concentration), 55 µL PBS and 20 µL recalcification buffer [20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.35, 60 mg/mL bovine serum albumin (BSA) and 100 mM Ca2+ (final concentration)] containing the fluorogenic substrate (Z-Gly-Gly-Arg-7-amino-4-methylcoumarin; Bachem, Weil am Rhein, Germany) were mixed and thrombin generation was monitored for 60 min using a Fluoroscan Ascent plate reader (Fluoroskan Ascent, Thermo Electron Corporation, Vantaa, Finland). Resulting curves were analysed using Thrombinoscope Analysis 3.0 software. For these mechanistic studies on platelet activation and thrombin generation in response to hormone treatment a separate group of OVX mice was treated as described earlier but for only 2 weeks.
Contraction-relaxation experiments
Endothelial function was examined in 5 mm thoracic aortic ring segments as previously described (Suvorava et al., 2005). Briefly, mice were killed by CO2, aortas were cautiously removed and aortic rings prepared. Subsequently aortic rings were repeatedly depolarised with 80 mM KCl. Aortic rings were precontracted submaximally with 200 nM phenylephrine and endothelium-dependent relaxation induced by cumulative addition of acetylcholine (10−9–10−5 M). Endothelium-independent relaxation to increasing concentrations of the NO-donor S-nitroso-N-acetyl-D,L-penicillamine (SNAP) (10−10–10−5 M) was examined after precontraction with increasing concentrations of phenylephrine (10−9–10−5 M). Two aortic rings from each animal were measured and the resulting data averaged. Five to seven mice were analysed in each treatment group.
Histochemistry and immunohistochemistry
Animals were perfusion fixed with 4% paraformaldehyde, tissues were excised and post-fixed with 4% paraformaldehyde (4 h) and transferred to 20% sucrose (overnight) before embedding at −40°C in Tissue Tek® medium according to routine procedures. Aortic root sections (14 µm) were fixed in ice-cold acetone for smooth muscle α-actin (α-SM-actin staining), in 96% ethanol for hyaluronic acid (HA) and Mac2 double staining or in 10% formalin for Oil-Red-O staining, for 15 min at 4°C. Primary antibody against α-SM-actin, (1:50; Abcam, Cambridge, UK) was used and α-SM-actin was detected by a sheep anti-rabbit Cy3 conjugate (1:200, Sigma, Steinheim, Germany). The accumulation of HA and the retention of macrophages in aortic root plaques was analysed by HA/Mac2 double-staining. Slides were incubated with biotinylated hyaluronic acid-binding protein (HAbP; 2 µg·mL−1; Seikagaku, Tokyo, Japan) at 4°C overnight. After three washes with PBS sections were incubated with streptavidin-FITC (1:200, Dako, Glostrup, Denmark). Subsequently, retention of macrophages was analysed by staining macrophage-antigen Mac2 with a rat-anti-mouse Mac2 first antibody and a rhodamineX-coupled goat-anti-rat IgG as secondary antibody on the same sections. Nuclear staining was performed by using ProLong® Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen, Eugene, OR, USA). The extent of lipid deposition in aortic root plaques was evaluated by Oil-Red-O staining. 5–9 mice were analysed in each treatment group.
Image analysis
Images of all stained samples were captured at 40x magnification using a BX-50 microscope (Olympus, Hamburg, Germany) and ColorView II camera (Soft Imaging System) and AnalySIS 3.2 software (Soft Imaging System, Münster, Germany). For the quantification of α-SM actin, macrophages, hyaluronan and lipid deposits, ImageJ 1.37v software (National Institutes of Health) was used. A colour deconvolution tool was applied to 8 bit converted images to filter out the positively stained areas. Choice of threshold values and quantification of threshold-marked areas were performed as previously described (Dai et al., 2007). Areas of plaque deposition in the aortic root were identified morphologically and measurements were only performed there. Measurements from at least five sections were averaged.
Statistical analysis
Data are presented as mean ± standard error of the mean of the number of (n) mice. Statistical significance among the concentration-response curves was analysed by two-way analysis of variance and one-way analysis of variance was used for plaque score, quantitative image analysis, thrombosis, CD62P-expression analysis and ETPs. Frequency of flow recovery was analysed using the Kruskal–Wallis test. P values < 0.05 were considered as statistically significant.
Results
Arterial thrombosis
The experimental design is depicted in Figure 1A. OVX caused significant uterine atrophy, demonstrating that the procedure was effective (data not shown). Total plasma cholesterol and triglyceride levels (Table 1) were not affected by either form of hormone treatment. However, the combined treatment with MPA and E2 significantly decreased body weight of mice compared with placebo, whereas animals treated with MPA alone showed no effects on body weight (Table 1). After photochemical induction of thrombus formation, the time to stable occlusion of the right carotid artery was determined. Figure 1B shows a representative chart of the decrease of blood-flow after photochemical injury. MPA significantly shortened times to occlusion of the right carotid artery (Figure 1C) suggesting an aggravated thrombotic response. In addition MPA caused a trend towards reduced frequency of flow-recovery (Figure 1D) as compared with placebo-treated animals suggesting increased stability of newly formed platelet aggregates or increased adhesion of the thrombus to the vessel wall. Both parameters, time to occlusion and frequency of flow recovery were also affected by MPA + E2 (Figure 1C,D).
Table 1.
Body weight, total cholesterol and triglycerides
| Body weights (g) | Total cholesterol (mg·mL−1) | Triglycerides (mg·mL−1) | |
|---|---|---|---|
| Placebo | 35.0 ± 0.5 | 8.1 ± 0.8 | 0.9 ± 0.1 |
| MPA | 34.2 ± 0.6 | 8.9 ± 0.8 | 1.1 ± 0.1 |
| MPA + E2 | 28.7 ± 0.9* | 7.1 ± 0.7 | 1.0 ± 0.2 |
Body weight and lipid parameters after feeding were determined at the end of the experimental period at the age of 132 days
P < 0.05 versus placebo and medroxyprogesterone acetate (MPA).
Platelet activity and endogenous thrombin potential (ETP)
To address the mechanisms that might be responsible for the pro-thrombotic effect of MPA and MPA + E2, platelet activation and thrombin generation were determined. FACS analysis of CD62P revealed no differences in the basal expression levels on platelets derived from placebo versus MPA and MPA + E2 (Figure 2A,B). Activation of platelets with the snake venom convulxin resulted in a four-fold increase of CD62P expression and was not affected by MPA. In the group receiving MPA + E2, the platelet activation in response to convulxin was even reduced. Taken together the results do not support increased platelet activation as the reason for the shortened time to occlusion in the photothrombosis model. However, thrombin generation was affected by the hormone treatment. MPA treatment caused a significant increase of the ETP that reflects the area under the curve of the thrombin generation time course. In contrast MPA + E2 did not alter the ETP (Figure 2C,D). Taken together, these experiments suggest that increased thrombin formation as detected by the ETP measurements may explain partially the aggravated thrombotic response in the photothrombosis model after MPA treatment.
Figure 2.
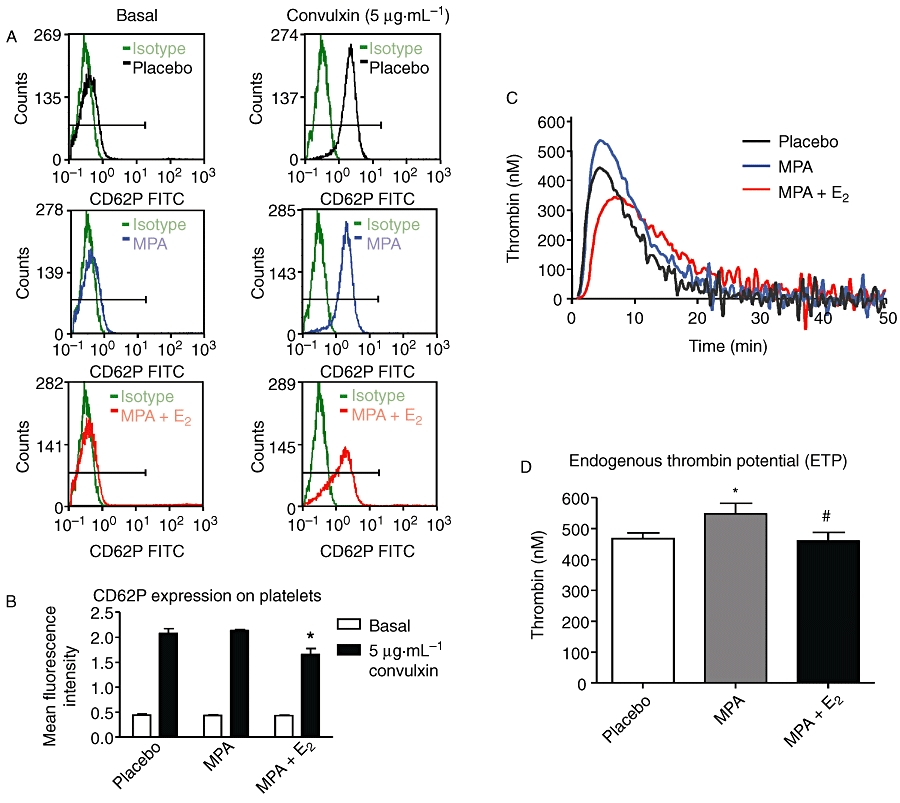
Platelet activation and endogenous thrombin potential (ETP) (A) Representative plots of ex vivo FACS analysis of CD62P-expression on platelets in platelet-rich plasma from mice treated with placebo, medroxyprogesterone acetate (MPA; 27.7 µg·day−1ay) and MPA + E2 (1.1 µg·day−1). Left panel: basal CD62P expression; right panel: CD62P expression after stimulation with 5 µg/mL convulxin (B) Quantitative analysis of CD62P expression. (C) Representative curves for thrombin generation over time (ETP) in mice treated with placebo, MPA and MPA + E2. (D) Quantitative analysis of ETP. Data represent means ± standard error of the mean; n= 6–7, *P < 0.05 versus placebo, #P < 0.05 versus MPA.
Aortic plaque burden
Next, the extent of plaque burden was determined in order to search for the mechanisms that might underlie the increased thrombotic response in atherosclerotic mice chronically treated with MPA. Of note, MPA decreased atherosclerotic plaque burden in the thoracic and abdominal aorta by about 20% (Figure 3A,B). In mice receiving MPA + E2 no effect on atherosclerosis compared with placebo was detected (Figure 3A,B). Therefore, effects on atherogenesis are unlikely to be responsible for the aggravated thrombogenic response to MPA treatment.
Figure 3.
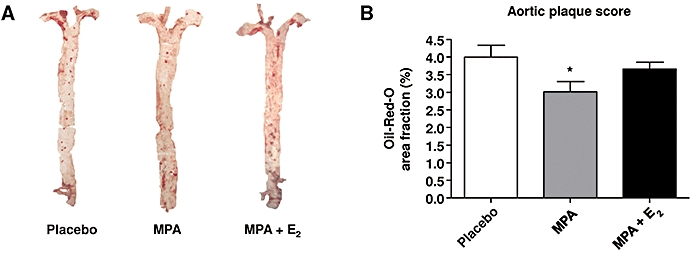
Plaque burden. (A) Oil-Red-O stained aortas from mice substituted with medroxyprogesterone acetate (MPA; 27.7 µg·day−1) and MPA + E2 (1.1 µg·day−1). (B) Aortic plaque scores from mice treated with placebo, MPA and MPA + E2. Data represent means ± standard error of the mean; n= 18–21; *P < 0.05 versus placebo.
Endothelium-dependent vasorelaxation
Endothelial function was considered as an underlying mechanism affecting both thrombotic responses and atherosclerosis in MPA treated mice. Thus, endothelium-dependent ACh-induced vasorelaxation of thoracic aortic rings of the chronically treated animals was determined in organ bath experiments and found to be unchanged in rings derived from both treatment groups, MPA or MPA + E2 (Figure 4A,B), excluding the possibility that effects on endothelial function are involved in the pro-thrombotic or anti-atherosclerotic actions of MPA in this model. To evaluate the endothelium-independent effects of MPA and MPA + E2 on vasorelaxation the response to the exogenous NO-donor SNAP was analysed. Remarkably, thoracic aortic rings from MPA-treated animals showed a significantly impaired relaxation in response to SNAP whereas aortic rings from mice receiving combined treatment did not differ from placebo (Half maximal effective concentration values SNAP: placebo, 0.34 ± 0.07 mM; MPA, 1.20 ± 0.31 mM; MPA + E2: 0.56 ± 0.08 mM, n= 6–8, Figure 4C,D).
Figure 4.
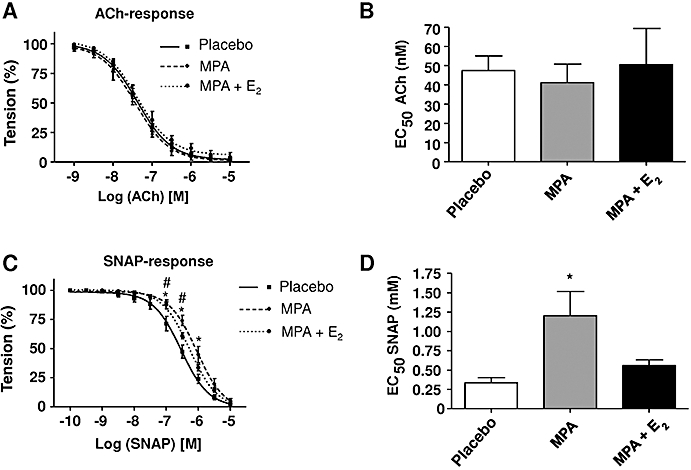
ACh-dependent vasorelaxation of aortic rings. (A) Concentration-response curves in response to ACh of aortic rings derived from mice treated with placebo, medroxyprogesterone acetate (MPA; 27.7 µg·day−1) and MPA + E2 (1.1 µg·day−1). (B) Half maximal effective concentration (EC50) values for ACh-induced vasorelaxation. (C) Concentration-response curves in response to S-nitroso-N-acetyl-D,L-penicillamine (SNAP) in response to treatment with placebo, MPA and MPA + E2. (D) EC50 values for SNAP. EC50 values for ACh calculated from concentration-response curves in (A). EC50 values for SNAP calculated from concentration-response curves in (C). Data represent means ± standard error of the mean; n= 5–7, *P < 0.05 for placebo versus MPA, #P < 0.05 for placebo versus MPA + E2.
Plaque composition at the aortic root
In addition the composition of atherosclerotic plaques with respect to extracellular matrix (ECM), smooth muscle cells (SMCs) and macrophages was measured. Differentiated SMC in plaques at the aortic root were detected by α-SM-actin staining. Interestingly, MPA caused a trend to reduced α-SM-actin staining that became significant after MPA + E2 treatment (Figure 5A–C,G). In contrast macrophage content of plaques, as determined by Mac2 immunostaining, was not affected (Figure 5D–F,H). Of note, HA content was significantly elevated after MPA + E2 (Figure 5D–F,I). Lipid deposits in aortic root plaques (Figure 6A–D), collagen content and collagen fibril packing as evidenced by picro-sirius red staining and birefringence analysis were not affected (not shown). These data on plaque composition clearly indicated remodelling of the non collagenous ECM, namely, HA, and decreased amounts of differentiated SMC in response to MPA and MPA + E2.
Figure 5.
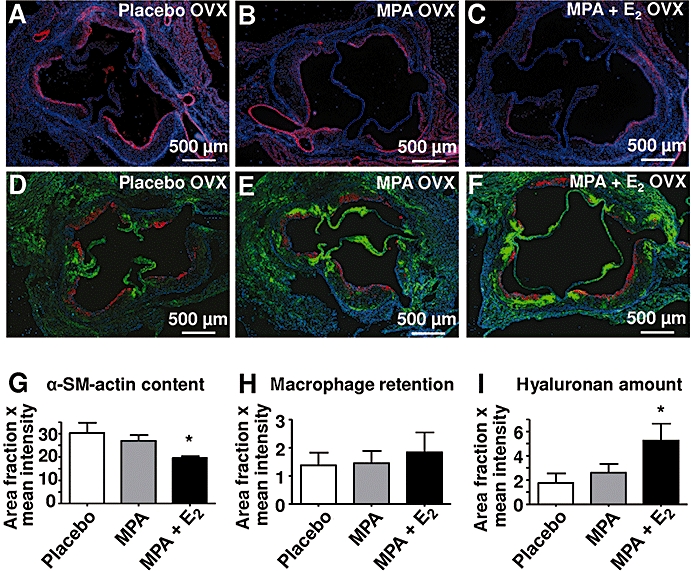
Plaque composition. (A–C, G) Aortic root sections stained for α-SM-actin (red) from mice treated with placebo, medroxyprogesterone acetate (MPA; 27.7 µg·day−1) and MPA + E2 (1.1 µg·day−1). (D–F, H–I). Aortic root sections stained for hyaluronan and the macrophage antigen Mac2. (G–I) Data represent area fractions of positively stained antigens as determined by digital image analysis; means ± standard error of the mean; n= 6–10; *P < 0.05 versus placebo.
Figure 6.
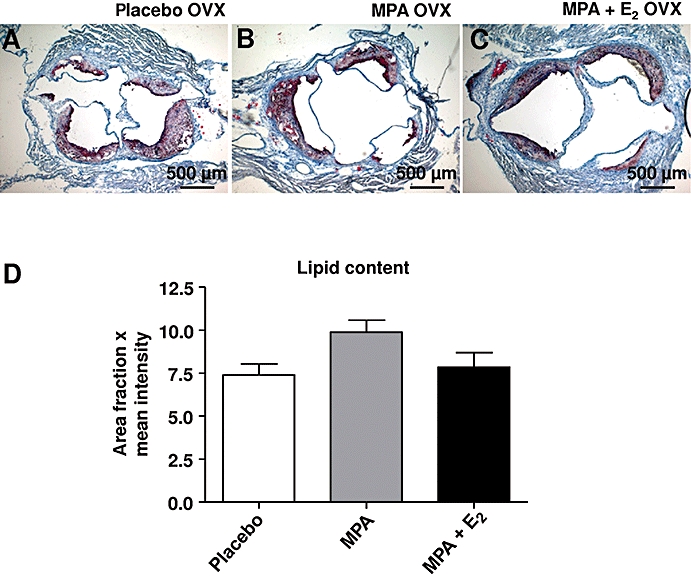
Lipid content of aortic root plaques. (A–C) Aortic root sections stained for lipid deposits from mice treated with placebo, medroxyprogesterone acetate (MPA; 27.7 µg·day−1) and MPA + E2 (1.1 µg·day−1). (D) Data represent fractions of positively stained areas as determined by digital image analysis; means ± standard error of the mean; n= 5–9.
Discussion
The main findings of the present work were that, on the one hand, chronic treatment of ApoE−/− mice with MPA aggravated the response in an experimental model of arterial thrombosis and, on the other hand, slightly reduced plaque burden. The model of photochemically induced thrombosis was chosen, because it is regarded as the most sensitive in vivo thrombosis model and one that will detect even subtle changes in the thrombotic response, with the highest sensitivity and accuracy (Westrick et al., 2007). In this model, oxidative damage to the endothelium is caused by photochemical activation of Rose Bengal and subsequent adhesion of platelets to the subendothelial matrix and initiation of coagulation (Westrick et al., 2007). Both MPA and MPA + E2 enhanced the thrombotic response compared with the placebo-treated animals. In addition, the frequency of flow-recovery was reduced by MPA and MPA + E2, which suggests that either the platelet aggregate was more stable or that the adhesion of the platelet thrombus to the carotid artery wall was stronger in MPA-treated OVX mice. A potential explanation of how MPA treatment might cause the increased response to photochemical induced thrombosis would be increased activation of platelets. However, mechanistic experiments revealed that basal platelet activation was not affected by any treatment and that the convulxin-induced P-selectin expression (a marker of platelet activation) was not increased by MPA or MPA + E2. Thus, platelet activation can be excluded as the underlying cause of the pro-thrombotic state. This is in line with the finding that platelet function in postmenopausal women with CHD is not affected and that ex vivo platelet aggregation in response to ADP is even inhibited by both oestrogens and by MPA (Bar et al., 2000). Interestingly, the ETP as a readout for the extent of thrombin generation after addition of exogenous tissue factor was increased in response to MPA, whereas no effect on the ETP was observed after MPA + E2. Increased thrombin actions on both platelets and fibrinogen in response to MPA might therefore contribute to both increased thrombus formation and thrombus stability. This is in line with clinical results showing that oestrogens + progestins decrease anticoagulant factors such as antithrombin III and protein C (Scarabin et al., 1997; Hoibraaten et al., 2000; Sumino et al., 2005), and increase haemostatic factors in some studies (Scarabin et al., 1997). The result that MPA + E2 treatment did not affect ETP suggests that E2 reversed the MPA effect on ETP. Furthermore, it suggests that additional mechanisms are responsible for the observed pro-thrombotic effect of MPA + E2 treatment.
In search of these additional mechanisms underlying the anti-atherogenic effects of MPA and the prothrombotic effect of MPA and MPA + E2, plaque composition was determined. MPA + E2 decreased α-SM-actin accumulation in plaques of the aortic root, which reflects either reduced SMC content and/or reduced differentiation of SMC (Yoshida et al., 2007). These effects on SMC could be of significance, because less SMC accumulation is considered to weaken fibrous caps and de-differentiated SMC are synthetically more active and might therefore support neointimal expansion and luminal narrowing. In addition, HA content was increased in response to MPA + E2 whereas collagen remained unaffected. HA is a carbohydrate component of the ECM that is thought to support SMC proliferation and migration (Evanko et al., 1999) as well as intimal ECM expansion and is thus likely to be a promoter of luminal narrowing during the course of atherosclerosis (Toole et al., 2002). In addition, HA has been proposed as a pro-thrombogenic matrix component, because dramatic accumulation of HA occurs at the luminal surface of human eroded plaques that caused fatal coronary thrombosis (Kolodgie et al., 2002). Therefore, increased HA content of atherosclerotic plaques might be involved in the increased thrombotic response in the present model especially in response to MPA + E2. The increase of HA and the decrease of SMC became significant only in the combination group suggesting additive effects of MPA and E2 on these parameters and possibly providing a mechanism for the increased thrombotic response in the combination group.
In addition to the increased ETP and increased HA accumulation other mechanisms may contribute to the pro-thrombogenic effect of MPA and MPA + E2 as well. For instance, changes in gene expression within the vascular wall such as increased expression of thrombin receptors in vascular SMC have been described before (Herkert et al., 2001). In addition, down-regulation of endothelial NO synthase in endothelial cells (Zerr-Fouineau et al., 2007) or up-regulation of NADPH oxidase activity (Wassmann et al., 2005) have been reported in response to MPA. Interestingly, MPA has been shown to favour coronary vasospasm in monkeys, which might also confer increased cardiovascular risk (Miyagawa et al., 1997). Taken together, the prothrombotic effect of MPA might be related to increased thrombin formation and/or in the case of the combination treatment due to the remodelling of the arterial wall including increased levels of HA. Future studies are needed to address the role of HA-rich ECM in the arterial responses to MPA and MPA + E2.
Despite the pro-thrombotic effect of MPA discussed earlier, the present results demonstrated that MPA reduced the extent of atherosclerosis in the present ApoE−/−-model by about 20%. It is known that ApoE deficient mice can develop atherosclerosis prior to the development of endothelial dysfunction (Fransen et al., 2008) and that endothelial dysfunction is associated with atherosclerotic plaques (Crauwels et al., 2003). Here we report that endothelium-dependent relaxation in response to ACh was not affected in either treatment group, thereby excluding improvement of endothelial function as a mechanism underlying the anti-atherosclerotic MPA effect. In contrast, vasorelaxation in response to SNAP was significantly impaired after MPA treatment suggesting increased degradation of exogenously formed NO, e.g. through oxidation (d'Uscio et al., 2001). A potential mechanism of the anti-atherosclerotic action of MPA may be that MPA does not only activate progesterone receptors, but also has partial agonistic effects on androgen receptors and glucocorticoid receptors (Poulin et al., 1989; Poulin et al., 1991). The androgenic effect of MPA has been suggested to mediate anti-inflammatory actions during atherosclerosis in postmenopausal women (Wakatsuki et al., 2002). Koubovec et al. (2004) showed that MPA reduces cytokine expression in mouse fibroblasts and mediates anti-inflammatory effects through the glucocorticoid receptor. Thus the anti-atherosclerotic effect of MPA detected in the present study might be attributable to both its androgenic and/or glucocorticosteroid properties.
Taken together, the present results on arterial thrombosis in response to MPA in mice are compatible with the clinical findings that HRT with oestrogens and MPA is associated with increased risk of MI and stroke (Hulley et al., 1998; Rossouw et al., 2002; Anderson et al., 2004; Rossouw et al., 2007; Vickers et al., 2007). The present study demonstrated that MPA was strongly pro-thrombotic either alone and in combination with E2, although atherosclerosis was inhibited. Possible mechanisms include increased thrombin formation and changes in vascular gene expression resulting in altered plaque matrix and SMC phenotype. It might be of clinical interest to evaluate in future studies whether the pro-thrombotic effect can be differentiated from the anti-atherosclerotic effect by use of alternative progestins that differ from MPA with respect to agonism and antagonism on other steroid receptors.
Acknowledgments
This work was funded by the Bundesinstitut für Arzneimittel und Medizinprodukte, Deutsche Forschungsgemeinschaft, SFB 612 (Teilprojekt B9).
Glossary
Abbreviations:
- BSA
bovine serum albumin
- CEE
conjugated equine estrogens
- CHD
coronary heart disease
- E2
17-β-oestradiol
- ECM
extracelluar matrix
- ETP
endogenous thrombin potential
- FITC
fluorescein-isothiocyanate
- HA
hyaluronan
- HAbP
hyaluronan binding protein
- HRT
hormone replacement therapy
- MPA
medroxyprogesterone acetate
- OVX
ovariectomized
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PPP
platelet-poor plasma
- PRP
platelet-rich plasma
- SMC
smooth muscle cells
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
- WHI
World Health Initiative
Conflicts of Interest
None.
References
- Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, et al. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10(6):1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17(1):217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd) 2008;153(Suppl. 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Asai K, Funaki C, Hayashi T, Yamada K, Naito M, Kuzuya M, et al. Dexamethasone-induced suppression of aortic atherosclerosis in cholesterol-fed rabbits. Possible mechanisms. Arterioscler Thromb. 1993;13(6):892–899. doi: 10.1161/01.atv.13.6.892. [DOI] [PubMed] [Google Scholar]
- Bar J, Lahav J, Hod M, Ben-Rafael Z, Weinberger I, Brosens J. Regulation of platelet aggregation and adenosine triphosphate release in vitro by 17beta-estradiol and medroxyprogesterone acetate in postmenopausal women. Thromb Haemost. 2000;84(4):695–700. [PubMed] [Google Scholar]
- Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1996;93(19):10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crauwels HM, Van Hove CE, Holvoet P, Herman AG, Bult H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc Res. 2003;59(1):189–199. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- Cullins VE. Noncontraceptive benefits and therapeutic uses of depot medroxyprogesterone acetate. J Reprod Med. 1996;41(Suppl. 5):428–433. [PubMed] [Google Scholar]
- Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292(13):1573–1580. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21(6):1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- Dai G, Freudenberger T, Zipper P, Melchior A, Grether-Beck S, Rabausch B, et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am J Pathol. 2007;171(5):1451–1461. doi: 10.2353/ajpath.2007.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res. 2005;66(2):295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Elhage R, Arnal JF, Pieraggi MT, Duverger N, Fievet C, Faye JC, et al. 17 beta-estradiol prevents fatty streak formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17(11):2679–2684. doi: 10.1161/01.atv.17.11.2679. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19(4):1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Fransen P, Van Assche T, Guns PJ, Van Hove CE, De Keulenaer GW, Herman AG, et al. Endothelial function in aorta segments of apolipoprotein E-deficient mice before development of atherosclerotic lesions. Pflugers Arch. 2008;455(5):811–818. doi: 10.1007/s00424-007-0337-9. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133(12):933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- Hanke H, Hanke S, Bruck B, Brehme U, Gugel N, Finking G, et al. Inhibition of the protective effect of estrogen by progesterone in experimental atherosclerosis. Atherosclerosis. 1996;121(1):129–138. doi: 10.1016/0021-9150(95)05710-2. [DOI] [PubMed] [Google Scholar]
- Herkert O, Kuhl H, Sandow J, Busse R, Schini-Kerth VB. Sex steroids used in hormonal treatment increase vascular procoagulant activity by inducing thrombin receptor (PAR-1) expression: role of the glucocorticoid receptor. Circulation. 2001;104(23):2826–2831. doi: 10.1161/hc4801.099737. [DOI] [PubMed] [Google Scholar]
- Hoibraaten E, Os I, Seljeflot I, Andersen TO, Hofstad A, Sandset PM. The effects of hormone replacement therapy on hemostatic variables in women with angiographically verified coronary artery disease: results from the estrogen in women with atherosclerosis study. Thromb Res. 2000;98(1):19–27. doi: 10.1016/s0049-3848(99)00233-9. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, et al. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol. 2002;22(10):1642–1648. doi: 10.1161/01.atv.0000034021.92658.4c. [DOI] [PubMed] [Google Scholar]
- Koubovec D, Berghe WV, Vermeulen L, Haegeman G, Hapgood JP. Medroxyprogesterone acetate downregulates cytokine gene expression in mouse fibroblast cells. Mol Cell Endocrinol. 2004;221(1–2):75–85. doi: 10.1016/j.mce.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Marsh MM, Walker VR, Curtiss LK, Banka CL. Protection against atherosclerosis by estrogen is independent of plasma cholesterol levels in LDL receptor-deficient mice. J Lipid Res. 1999;40(5):893–900. [PubMed] [Google Scholar]
- Miyagawa K, Rosch J, Stanczyk F, Hermsmeyer K. Medroxyprogesterone interferes with ovarian steroid protection against coronary vasospasm. Nat Med. 1997;3(3):324–327. doi: 10.1038/nm0397-324. [DOI] [PubMed] [Google Scholar]
- Poulin R, Baker D, Poirier D, Labrie F. Androgen and glucocorticoid receptor-mediated inhibition of cell proliferation by medroxyprogesterone acetate in ZR-75-1 human breast cancer cells. Breast Cancer Res Treat. 1989;13(2):161–172. doi: 10.1007/BF01806528. [DOI] [PubMed] [Google Scholar]
- Poulin R, Baker D, Poirier D, Labrie F. Multiple actions of synthetic ‘progestins’ on the growth of ZR-75-1 human breast cancer cells: an in vitro model for the simultaneous assay of androgen, progestin, estrogen, and glucocorticoid agonistic and antagonistic activities of steroids. Breast Cancer Res Treat. 1991;17(3):197–210. doi: 10.1007/BF01806369. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17(11):3071–3078. doi: 10.1161/01.atv.17.11.3071. [DOI] [PubMed] [Google Scholar]
- Shultz JM, Zhu XD, Knopp RH, Leboeuf RC, Rosenfeld ME. Norgestimate and medroxyprogesterone acetate do not attenuate the atheroprotective effects of 17beta-estradiol in ovariectomized, apolipoprotein E-deficient mice. Fertil Steril. 2004;82(Suppl. 3):1133–1139. doi: 10.1016/j.fertnstert.2004.05.069. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Mannella P, Fornari L, Caruso A, Willis MY, Garibaldi S, et al. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology. 2004;145(12):5745–5756. doi: 10.1210/en.2004-0510. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 1985;313(17):1044–1049. doi: 10.1056/NEJM198510243131703. [DOI] [PubMed] [Google Scholar]
- Stampfuss JJ, Schror K, Weber AA. Green tea catechins containing a galloyl group in the 3′ position inhibit tissue factor-induced thrombin generation. Thromb Haemost. 2005;93(6):1200–1201. [PubMed] [Google Scholar]
- Sumino H, Ichikawa S, Sawada Y, Sakamoto H, Kumakura H, Takayama Y, et al. Effects of hormone replacement therapy on blood coagulation and fibrinolysis in hypertensive and normotensive postmenopausal women. Thromb Res. 2005;115(5):359–366. doi: 10.1016/j.thromres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Suvorava T, Lauer N, Kumpf S, Jacob R, Meyer W, Kojda G. Endogenous vascular hydrogen peroxide regulates arteriolar tension in vivo. Circulation. 2005;112(16):2487–2495. doi: 10.1161/CIRCULATIONAHA.105.543157. [DOI] [PubMed] [Google Scholar]
- Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277(7):4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- Vickers MR, MacLennan AH, Lawton B, Ford D, Martin J, Meredith SK, et al. Main morbidities recorded in the women's international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ. 2007;335(7613):239. doi: 10.1136/bmj.39266.425069.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on vascular inflammatory markers in postmenopausal women receiving estrogen. Circulation. 2002;105(12):1436–1439. doi: 10.1161/hc1202.105945. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Wassmann K, Wassmann S, Nickenig G. Progesterone antagonizes the vasoprotective effect of estrogen on antioxidant enzyme expression and function. Circ Res. 2005;97(10):1046–1054. doi: 10.1161/01.RES.0000188212.57180.55. [DOI] [PubMed] [Google Scholar]
- Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series) Arterioscler Thromb Vasc Biol. 2007;27(10):2079–2093. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- Wilson KM, Lynch CM, Faraci FM, Lentz SR. Effect of mechanical ventilation on carotid artery thrombosis induced by photochemical injury in mice. J Thromb Haemost. 2003;1(12):2669–2674. doi: 10.1111/j.1538-7836.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Gan Q, Shang Y, Owens GK. Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am J Physiol Cell Physiol. 2007;292(2):C886–C895. doi: 10.1152/ajpcell.00449.2006. [DOI] [PubMed] [Google Scholar]
- Zerr-Fouineau M, Chataigneau M, Blot C, Schini-Kerth VB. Progestins overcome inhibition of platelet aggregation by endothelial cells by down-regulating endothelial NO synthase via glucocorticoid receptors. FASEB J. 2007;21(1):265–273. doi: 10.1096/fj.06-6840com. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Wenk A, Kim U, Kienzle P, Weber AA, Gams E, et al. Functional and biochemical evaluation of platelet aspirin resistance after coronary artery bypass surgery. Circulation. 2003;108(5):542–547. doi: 10.1161/01.CIR.0000081770.51929.5A. [DOI] [PubMed] [Google Scholar]


