Abstract
Background and purpose:
Rhythmical transient constrictions of the lymphatic vessels provide the means for efficient lymph drainage and interstitial tissue fluid balance. This activity is critical during inflammation, to avoid or limit oedema resulting from increased vascular permeability, mediated by the release of various inflammatory mediators. In this study, we investigated the mechanisms by which prostaglandin E2 (PGE2) and prostacyclin modulate lymphatic contractility in isolated guinea pig mesenteric lymphatic vessels.
Experimental approach:
Quantitative RT-PCR was used to assess the expression of mRNA for enzymes and receptors involved in the production and action of PGE2 and prostacyclin in mesenteric collecting lymphatic vessels. Frequency and amplitude of lymphatic vessel constriction were measured in the presence of these prostaglandins and the role of their respective EP and IP receptors assessed.
Key results:
Prostaglandin E2 and prostacyclin decreased concentration-dependently the frequency, without affecting the amplitude, of lymphatic constriction. Data obtained in the presence of the EP4 receptor antagonists, GW627368x (1 µM) and AH23848B (30 µM) and the IP receptor antagonist CAY10441 (0.1 µM) suggest that PGE2 predominantly activates EP4, whereas prostacyclin mainly stimulates IP receptors. Inhibition of responses to either prostaglandin with H89 (10 µM) or glibenclamide (1 µM) suggested a role for the activation of protein kinase A and ATP-sensitive K+ channels.
Conclusions and implications:
Our findings characterized the inhibition of lymphatic pumping induced by PGE2 or prostacyclin in guinea pig mesenteric lymphatics. This action is likely to impair oedema resolution and to contribute to the pro-inflammatory actions of these prostaglandins.
Keywords: lymphatic pumping, prostanoids, EP4 receptor, EP2 receptor, IP receptor
Introduction
Lymphatic vessels are present at densities similar to blood vessels throughout most of the body. One of their main physiological functions is to maintain tissue fluid balance by removing excess fluid from the interstitium, propelling lymph along the lymphatic vessel tree and returning it to the blood stream, thereby avoiding swelling and oedema. Efficient lymph drainage in many body regions is achieved by rhythmical transient constrictions of a succession of lymphatic chambers connected by unidirectional valves, which constitute the collecting lymphatic vessels. This contractile activity is intrinsic to the smooth muscle constituting the media layer of the lymphatic vessel wall and is triggered by L-type Ca2+ channel-mediated action potential-like spikes (Kirkpatrick and McHale, 1977; Allen et al., 1983; van Helden, 1993). Lymphatic contractile activity adapts to changes in fluid load, strengthening with increases in interstitial fluid volume or pressure, as can occur during acute oedemagenic stress due to haemodilution (Benoit et al., 1989). Indeed, elevation in transmural fluid pressure is one of the most important means to activate lymphatic pumping. Consequently, impairment of the lymphatic pumping function can lead to profound swelling and oedema. During inflammation, oedema occurs as a result of increased vascular permeability, consequent to the release of inflammatory mediators. Although the elevation in interstitial fluid pressure is critical in setting lymphatic pumping rate and can be altered by it, many of the inflammatory mediators present in the oedematous extracellular space have been shown to modulate lymphatic contractility (see Johnston, 1987; von der Weid, 2001).
Among these inflammatory mediators, arachidonic acid metabolites are important and very effective modulators of lymphatic pumping. In isolated mesenteric lymphatic vessels, both arachidonic acid and its metabolites induced powerful excitatory and inhibitory responses with inhibition of pumping in the presence of prostaglandin E2 (PGE2) and prostacyclin and an increase in pumping caused by leukotrienes B4, C4 and D4, PGF2α and the thromboxane A2 (TXA2) mimetic U46619 (Johnston and Gordon, 1981; Johnston and Feuer, 1983; Johnston et al., 1983; Elias and Johnston, 1988; Sjoberg and Steen, 1991; von der Weid et al., 2001; von der Weid, 2001; Chan et al., 2004). Other reports have demonstrated the ability of the lymphatic endothelium itself to generate prostanoids, such as PGH2/TXA2, PGE2 or prostacyclin (Oguogho et al., 2000), which play a role in the modulation of the contractile activity under agonist (Rayner and van Helden, 1997; Gao et al., 1999; Chan et al., 2004) or intraluminal flow stimulation (Mizuno et al., 1998; Koller et al., 1999). Studies have also reported synthesis of PGE2 and prostacylin from arachidonic acid in human lymphatic vessels (Mannheimer et al., 1980; Sinzinger et al., 1984a). In those vessels, prostacyclin formation was increased during pulsatile perfusion (Sinzinger et al., 1986b) and during application of leukotrienes C4 and D4 (Sinzinger et al., 1986a), but prostacyclin was not demonstrated to have a direct effect on pumping, other than counteracting the positive contractile effect of TXA2 (Sinzinger et al., 1984b).
Prostanoids are produced by the catalytic action of cyclooxygenases (COX-1 and COX-2) and specific prostaglandin synthases. They mediate their biological effects by acting on members of a family of G-protein coupled membrane receptors, to activate distinct signal transduction pathways (Hata and Breyer, 2004). In lymphatic vessels, two main pathways are involved in the modulation of lymphatic pumping by prostanoids: an increase in cyclic nucleotide (i.e. cAMP) production, which causes pumping inhibition, and an increase in InsP3/[Ca2+]i, mediating pumping activation (von der Weid et al., 1996; Fox and von der Weid, 2002; Chan and von der Weid, 2003). We have recently demonstrated using an experimental model of inflammatory bowel disease in guinea pigs, that the contractile function of mesenteric lymphatic vessels was impaired in highly inflamed animals. This dysfunction was markedly reversed in vitro and in vivo upon application of indomethacin, a COX inhibitor, suggesting a role for prostanoids in the inflammation-induced lymphatic contractile dysfunction (Wu et al., 2006). Although the responsible metabolite(s) have not been identified, previous findings revealing that PGE2 and prostacyclin decreased lymphatic pumping in the same preparation (Chan et al., 2004) make them likely candidates. However, the receptors involved in such prostaglandin-induced lymphatic pumping inhibition have yet to be determined and the signalling pathways activated by the stimulated receptors to be examined. The important contribution of PGE2 and prostacyclin to lymphatic physiology and the potential role(s) they may play in inflammation-induced lymphatic contractile dysfunction prompted us to examine in the present study the expression profile of EP and IP receptors in guinea pig mesenteric lymphatic vessels and to determine which receptors and which downstream signalling pathways PGE2 and prostacyclin activated to decrease lymphatic contractile response.
Methods
Tissue preparation
All animal care and experimental procedures were approved by the University of Calgary Animal Care and Ethics Committee and conforms to the guidelines established by the Canadian Council on Animal Care. Male guinea pigs (7–15 days of age) were sacrificed by decapitation during deep anesthesia induced by inhalation of halothane. The small intestine with its attached mesentery was rapidly dissected and placed in a physiological saline solution (PSS) of the following composition (mM): CaCl2, 2.5; KCl, 5; MgCl2, 2; NaCl, 120; NaHCO3, 25; NaH2PO4, 1; glucose, 11. The pH was maintained at 7.4 by constant bubbling with 95% O2/5% CO2.
Real-time PCR
Mesenteric arcades were isolated from the ileal region of the small intestine. Mesenteric lymphatic vessels, arteries and veins were microdissected, cleaned of surrounding tissue and immediately immersed into RNase-and DNase-free collection tubes containing the RNA stabilization reagent, RNAlater (Qiagen, Mississauga, ON, Canada). The RNA-stabilized, microdissected tissues were disrupted and homogenized using sonication and QIAshredder (Qiagen), and underwent RNA extraction using the Qiagen Micro RNeasy Kit (Qiagen). cDNA was subsequently synthesized using Superscript II Reverse Transcriptase enzyme (Invitrogen) with oligo (dT) primers. cDNA integrity and quality was confirmed by RT-PCR using β-actin primers. Real-time amplification of target genes was performed using Quanti Tect Syber Green (Qiagen) in an iCycler iQ Real-Time PCR Instrument (Bio-Rad). Guinea pig real-time primer sequences for COX-1, COX-2, prostacylin synthase (PGIS), prostacyclin receptor (IP), prostaglandin E synthase 1 and 2 (PGES-1, -2), EP1, EP2, EP3 and EP4 receptors were designed using conserved homologies of human and rat sequences. Real-time primer sequences, as well as the associated human and rat gene accession numbers are listed in Table 1. To confirm we had the gene of interest, real-time samples for each gene underwent PCR purification using the QIAquick PCR Purification Kit (Qiagen) and were subsequently sequenced. Gene expression was normalized to β-actin expression. RT-PCR results were analysed using the ΔΔCT method.
Table 1.
Real-time PCR primer sequences (all 5′–3′)
| Gene | Human AN | Rat AN | Forward | Reverse | Size (bp) |
|---|---|---|---|---|---|
| β-actin | NM_001101 | NM_031144 | AGGGTGTAACGCAGCAAAGT | ACTCTCCACCTTCCAGCAGA | 118 |
| COX-1 | NM_000962 | NM_017043 | CCTGCTCAGGAGGAAATTCA | GTGAAGCCAGGACCCATCT | 123 |
| COX-2 | NM_000963 | NM_017232 | TCCTGTCACTGCGTTTAATCTC | GTGGGGCTGAGGTCTTCC | 128 |
| PGIS | NM_000961 | NM_031557 | AGGGAAACCCATCTTCACG | GAGGCAGCTGAGGTTGCTAT | 134 |
| IP | NM_000960 | NM_001077644 | ATGGGAGACCTCCTTGCTTT | GGACACAGACAGCAGAACCA | 144 |
| PGES-1 | NM_004878 | NM_021583 | CACTTCCTGCTCTTCCTGCT | GCTTCCCACAGGATCTGC | 109 |
| PGES-2 | NM_025072 | NM_001107832 | GGCCTCTTTCGACTACATCG | CCGCTTCCCACAGGATCT | 112 |
| EP1 | NM_000955 | NM_013100 | CTACCCATCTTCTCCATGACG | CGAGGAACAGCAGGAAGGT | 117 |
| EP2 | NM_000956 | NM_031088 | CTGCTCGGGACCTGCCTC | TGAGCATCGTGGCCAGGC | 130 |
| EP3 | NM_000957 | NM_012704 | CTCACCATGACGGTCTTTGA | GTCTTCATGTGGCTCGCATA | 110 |
| EP4 | NM_000958 | NM_032076 | CACCTACATGAAGGGCCAGT | TGGCACAGATGATGCTAAGG | 101 |
Real-time PCR primer sequences for guinea pig COX-1, COX-2, prostacylin synthase (PGIS), prostacyclin receptor (IP), prostaglandin E synthase 1 and 2 (PGES-1, PGES-2), EP1, EP2, EP3 and EP4 receptors.
COX, cyclooxygenase.
Vessel constriction measurements
Lymphatic tissue was prepared as previously described (von der Weid et al., 1996; Fox and von der Weid, 2002). In brief, small collecting lymphatic vessels (diameter 170–250 µm) from the ileal region were dissected together with their associated artery and vein and left intact within the surrounding mesentery. The mesentery was used to pin out the tissues on the Sylgard-coated base of a 2 mL organ bath. The bath was mounted on the stage of an inverted microscope (CK40, Olympus) and continuously superfused at a flow rate of 3 mL·min−1 with PSS heated to 36°C. To induce a consistent rate of vessel constrictions, the vessel lumen was perfused through a fine glass micropipette inserted into a cut opening of the vessel. The cannula was connected to an infusion pump via Teflon tubing allowing the vessel lumen to be perfused in the direction of the valves at a flow rate of 2.5 µL·min−1. This flow rate was selected from preliminary experiments as the most reliable rate that induced a regular rhythmical contractile activity in lymphatic vessels in the range of diameter used and for the duration of the experiment (typically 3–4 h). Lymphatic vessel chambers or lymphangions were observed by video-microscopy, with diameter changes and constriction frequency continuously measured with a video-dimension analyser (Model V94, Living Systems Instrumentation, Burlington, VT, USA). This device, designed to sense the optically denser wall of the vessel, at a chosen scan line seen on the monitor, followed any change in vessel diameter with a rapid (<20 ms) time resolution. Data were then recorded on a computer via an analogue-to-digital converter (PowerLab/4SP, ADInstruments, Mountain View, CA, USA). Preparations were allowed a 30 min equilibration period prior to the first drug application. Treatments were only performed on vessels with a consistent pumping frequency of at least five contractions per min during the minimum 30 min equilibrium period. A 5 min control period of contractile activity was recorded prior to the application for 1 min of increasing concentrations of PGE2 or iloprost. Prostaglandin applications were separated with 10 min superfusion with PSS, or the time necessary for the vessel to recover from the previous application. When investigating the effect of antagonists or inhibitors, these agents were present in the superfusion solution 15 min before application of the first agonist concentration and for the duration of the experiment.
Statistical analysis
Data are expressed as means ± one standard error of the mean (SEM). Statistical significance was assessed using two-tailed unpaired Student's t-test or anova followed by Tukey's post hoc test, with a P value of 0.05 or less considered significant.
Materials
The following drugs were used in the present study: PGE2, iloprost, butaprost, CAY10441, GW627368x were obtained from Cayman Chemicals; GR32191B, SC19220, glibenclamide, AH6809, AH23848 from Sigma-Aldrich and H89 from Alexis Corp. They were prepared as 10 mM stock solution in EtOH or DMSO and diluted to their final concentration in PSS. The final concentration of the vehicles never exceeded 0.1%, which at this maximal concentration did not alter lymphatic pumping.
The nomenclature for receptors used in this study conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Results
Expression of EP and IP receptor mRNA in guinea pig mesenteric lymphatic vessels
Quantitative RT-PCR, using the β-actin signal as a denominator revealed that mRNA transcripts for the EP1, EP2 and EP4 receptors, as well as for the IP receptor were all expressed in lymphatic vessels (Figure 1). As also shown in Figure 1, the lymphatic vessel expression profile was similar to that obtained from mesenteric veins and arteries, with EP4 receptor expression dominating in the arterial tissue (P < 0.05 vs. vein). In the three vessel types investigated, mRNA transcripts for EP3 receptors were only marginally expressed or entirely absent.
Figure 1.

Expression of mRNA for prostaglandin E2 and I2 receptors in guinea pig mesenteric lymphatic vessels. Quantitative real-time PCR analysis of EP1, 2, 3 and 4 and IP receptors mRNA expression in mesenteric lymphatic vessels, arteries and veins. Data are mean ± SEM of eight animals, expressed relative to the number of copies of β-actin. *P < 0.05 (anova with Tukey's post hoc test).
Effect of PGE2 and iloprost on lymphatic vessel contractile activity
Prostaglandin E2 and iloprost decreased the contractile activity of lymphatic vessels in a concentration-dependent manner (Figure 2). In the presence of the prostaglandins, contraction frequency of the vessels was markedly decreased to the point that pumping stopped, with the duration of inhibition increasing with agonist concentration. The amplitude of any persisting contractions during the treatment was minimally affected. For reasons we were not able to explain, a few vessels did not respond to the inhibitory action of PGE2 (3 of 15, designated ‘non-responders’). For the 12 responding tissues, the PGE2-induced inhibition was noticeable at 30 nM and reached its maximum around 30 µM (17 ± 8% of control), with a calculated pEC50 of 5.9 ± 0.2. We used this ‘responders’ concentration–response curve, illustrated in Figure 2B, as control for comparison with curves in the presence of antagonists in the following figures.
Figure 2.

Concentration-dependent effects of PGE2 and iloprost on the contractile activity of guinea pig mesenteric lymphatic vessels. (A) Original traces of vessel diameter changes (downward deflections represent constrictions) in response to increasing concentrations of PGE2 (top traces) and iloprost (bottom traces), in perfused lymphatics. (B) Concentration–response relationships of the effect of PGE2 (top graph) and iloprost (bottom graph) on lymphatic pumping. The response to PGE2 was minimal in three vessels (non-responders). In these and following concentration–response curves, data are mean ± SEM, with the number of experiments performed with each treatment indicated in brackets. PGE2, prostaglandin E2.
Iloprost also inhibited mesenteric lymphatic vessel contractile activity in a similar fashion. These effects were observed in the concentration range of 30 nM to 10 µM (max inhibition 15 ± 9% of control, pEC50 6.2 ± 0.2, n= 9; Figure 2B).
EP4 receptors play a major role in PGE2-induced decrease in lymphatic vessel contraction frequency
We assessed the contribution of EP2, EP3 and EP4 receptors in the PGE2-induced inhibition of lymphatic constriction rate. The response to PGE2 was markedly decreased, in the concentration range tested, in the presence of the EP4 receptor antagonists, AH23848B (30 µM) and GW627368x (1 µM; Figure 3A) and pEC50 of 5.0 ± 0.4 and 5.0 ± 0.3, respectively, were significantly different from control (P < 0.05). The involvement of EP2 receptors was shown to be minimal, as the selective EP2 receptor agonist, butaprost (10 nM–50 µM), was without significant effect (Figure 3B). Similarly, lymphatic contractile activity was not altered by sulprostone (10 nM–50 µM), an EP3 receptor selective agonist (Figure 3B). Administration of GW627368x (1 µM) together with the IP receptor antagonist CAY10441 (0.1 µM) did not inhibit the PGE2-induced response further than with GW627368x alone (data not shown).
Figure 3.

Contribution of EP2, EP3 and EP4 receptors to the PGE2-induced decrease in contractile activity in mesenteric lymphatic vessels. (A) Concentration–response relationships of the effect of PGE2 on lymphatic pumping in control conditions and in the presence of the EP4 receptor antagonists AH23848B or GW627368x. (B) Concentration–response relationships of the effect of the EP2 receptor agonist butaprost and the EP3 receptor agonist sulprostone on lymphatic pumping. PGE2, prostaglandin E2.
PGE2-induced reduction of lymphatic vessel contraction frequency is modulated by EP1 and TP receptor stimulation
Prostaglandin E2 has been shown to also activate two excitatory receptors, TP and EP1 (see Narumiya et al., 1999). To evaluate the possible involvement of these two receptors in the PGE2-induced inhibition of lymphatic constriction frequency, we used the TP receptor antagonist GR32191B and the EP1 receptor antagonist AH6809. PGE2-induced lymphatic pumping inhibition was significantly enhanced during TP receptor inhibition by GR32191B (1 µM) in the low concentration range (P < 0.05 vs. control at 10 and 30 nM). However, the pEC50 of 6.2 ± 0.4 was not different from control (Figure 4A). In the presence of AH6809 (3 µM), the response to PGE2 was particularly marked in two of the seven vessels examined (population 2, gray diamonds, Figure 4B), with almost total inhibition of contractions, at concentrations as low as 30 nM. This result suggests an important contribution of EP1 receptors in this subset of vessels, which would counteract the inhibitory action of EP4 receptor activation. However, in five of the seven preparations evaluated the PGE2 concentration–response curve was not affected by AH6809 (Figure 4B). We were not able to rationalize this variation in sensitivity to AH6809 in terms of ongoing disease, dietary changes or other factors that might have affected the animals.
Figure 4.

Contribution of EP1 and TP receptors to the PGE2-induced decrease in contractile activity in mesenteric lymphatic vessels. Concentration–response relationships of the inhibitory of PGE2 on lymphatic pumping in control conditions and in the presence of the TP antagonist GR32191B (A) and the EP1 antagonist AH6809 (B). In two vessels, inhibition of EP1 markedly increased the inhibitory action of PGE2. PGE2, prostaglandin E2.
Role of IP, EP1 and EP4 receptors in iloprost-induced decrease in frequency of lymphatic vessel contraction
To evaluate which receptors were stimulated by iloprost, we first determined the concentration–response relationship in the presence of the IP receptor antagonist CAY10441 (0.1 µM). Application of this compound caused an inhibition of the iloprost-induced response and the agonist concentration–response curve was significantly shifted to the right (pEC50 5.1 ± 0.3, P < 0.05 vs. control; Figure 5A). Vessels treated with the selective EP4 receptor antagonist, GW627368x (1 µM) had their response to iloprost slightly reduced, and the combined application of GW627368x and CAY10441 further shifted the iloprost concentration–response curve to the right (Figure 5A). However, differences did not reach significance for the four vessels tested. On the other hand, the inhibitory response to iloprost was significantly enhanced in the presence of the EP1 receptor antagonist, AH6809 (3 µM) and the concentration–response curve was shifted to the left (pEC50 6.9 ± 0.2, P < 0.05 vs. control Figure 5B).
Figure 5.

Contribution of IP, EP1 and EP4 receptors to the iloprost-induced decrease in contractile activity in mesenteric lymphatic vessels. Concentration–response relationships of the effect of iloprost on lymphatic pumping in control conditions and in the presence of the IP receptor antagonist CAY10441 and the EP4 antagonist GW627368x (A) and the EP1 antagonist AH6809 (B).
Signal transduction of PGE2 and iloprost-induced responses
EP4 and IP receptor stimulation with PGE2 and iloprost, respectively, have been shown to be coupled to the cAMP/protein kinase A (PKA) signalling pathway in many smooth muscle preparations. Thus, we assessed the effects of PGE2 or iloprost in the presence of the PKA inhibitor H89. At a concentration of 10 µM, H89 consistently reduced the inhibitory effects of PGE2-and iloprost on constriction frequency in the concentration range tested (Figure 6). Various agonists shown to stimulate the cAMP/PKA signal transduction pathway in lymphatic vessels also activate ATP-sensitive K+ (KATP) channels (von der Weid and van Helden, 1996; von der Weid, 1998; Chan and von der Weid, 2003; Chan et al., 2004; Hosaka et al., 2006). In the presence of the KATP channel blocker, glibenclamide (1 µM), the inhibitory actions of PGE2 and iloprost were strongly attenuated (Figure 6), indicating an involvement of KATP channels in diminishing pumping activity. It is interesting to note the positive increase in constriction frequency at low concentrations of PGE2 in the presence of either H89 or glibenclamide, probably reflecting the activation of TP, and possibly EP1, receptors by PGE2, an action not affected by inhibition of PKA and KATP channels.
Figure 6.
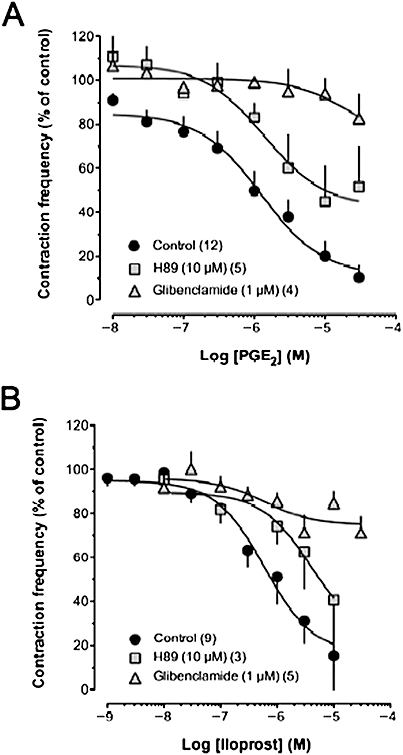
Role of PKA and KATP channel activation in the inhibition of mesenteric lymphatic vessel contractile activity by iloprost and PGE2. Concentration–response relationships of the inhibitory effect of PGE2 (A) and iloprost (B) in the presence of the PKA inhibitor H89 and the KATP channel blocker glibenclamide. PGE2, prostaglandin E2; PKA, protein kinase A.
Lymphatic vessels possess the biochemical machinery to produce PGE2 and prostacyclin
Prostaglandins are produced in many tissues, and evidence strongly suggests that this synthesis occurs in lymphatic vessels as well. We investigated the ability of lymphatic vessels to express message for the enzymes necessary to synthesize these prostaglandins. Our RT-PCR data, presented in Figure 7 reveal that lymphatic vessels express mRNA transcripts for PGIS and the microsomal forms of PGES (mPGES-1 and mPGES-2) at a level similar to that of mesenteric veins and arteries (P > 0.05 between all groups). Expression of the mRNA transcript for the cytosolic isoform of PGES (cPGES) was very low in lymphatic vessels, veins and arteries from two animals and absent in tissues from two others (data not shown). To assess metabolic steps upstream of PGE2 and PGI2 synthesis, we also investigated the mRNA transcript for the enzymes responsible for synthesis of the intermediary molecule prostaglandin H2 (PGH2), specifically COX-1 and COX-2. In lymphatics, veins and arteries, mRNA transcript for COX-2, an inducible marker of inflammation, was only marginally detected, consistent with the non-inflamed state, whereas COX-1 mRNA was well expressed in all lymphatic vessels as well as veins and arteries (Figure 7).
Figure 7.

Expression of mRNA for prostaglandin E2 and I2 synthases in guinea pig mesenteric lymphatic vessels. Quantitative real-time PCR analysis of COX-1, COX-2, PGIS, PGES-1 and PGES-2 mRNA expression in mesenteric lymphatic vessels, arteries and veins. Data are mean ± SEM of eight animals, expressed relative to the number of copies of β-actin. COX, cyclooxygenase; PGES, prostaglandin E synthase.
Discussion and conclusions
Prostaglandins play critical roles in physiological and pathophysiological events. They are heavily involved in the inflammatory process, where they are produced and released by inflamed tissues as well as by immune cells at the site of inflammation. Consequently, these prostaglandins are present in the inflamed interstitial space and can be detected in the lymph drained from these areas. Studies have shown that compounds such as TXA2, PGH2 and substantial amounts of 8-epi-PGF2α are present in lymph draining human and porcine legs (Oguogho et al., 1998). Furthermore, Johnston et al. (1980) carried out studies on cannulated lymphatic vessels from the sheep hind leg and noticed that the prostaglandin content in afferent lymph and the lymph that drained regional lymph nodes was increased by subcutaneous injection of E. coli. Their findings also showed that the major product of arachidonate metabolism present in the lymph nodes and connective tissue was PGE2. Thromboxane, prostacyclin (both measured as their stable metabolites) and PGF2α were also present, although in smaller amounts. A possible source of prostaglandins in the lymph draining inflamed sites could be the direct release from dendritic cells, macrophages and lymphocytes en route to the lymph nodes via lymphatic vessels (Cavanagh and Von Andrian, 2002; Angeli and Randolph, 2006). More importantly, lymphatic vessels, and in particular the lymphatic endothelium, have been shown to directly produce these prostaglandins (Rayner and van Helden, 1997; Mizuno et al., 1998; Gao et al., 1999; Koller et al., 1999; Chan et al., 2004) and convert exogenously added arachidonic acid to PGE2, prostacyclin and PGF2α in lymphatic vessels isolated from human legs and bovine mesentery (Johnston and Gordon, 1981; Mannheimer et al., 1980; Sinzinger et al., 1984a).
Prostaglandin E2 and prostacyclin are potent inhibitors of lymphatic vessel contractility/pumping, a function critical to lymph flow and drainage. Inhibition of lymphatic pumping could thereby lead to lymph stasis and, in inflammatory settings, impairment of oedema resolution. Indeed, PGE2 and prostacyclin have been proposed as mediators of the compromised contractility of mesenteric lymphatic vessels observed in an animal model of intestinal inflammation, where oedema is a typical feature, as contractile function was partially restored following treatment with COX inhibitors (Wu et al., 2006). In order to further understand the role and effect of PGE2 and prostacyclin on lymphatic contractile activity, we established in the present study the expression profile of EP and IP receptor mRNA transcripts in mesenteric lymphatics and characterized the roles of these receptors in the pumping inhibitory responses to PGE2 and the stable prostacyclin mimetic iloprost.
Our data demonstrate the concentration-dependent decrease in lymphatic constriction frequency, induced by PGE2 and iloprost. The potencies of these two prostanoids appear lower than that reported in other smooth muscle preparations. For example, in piglet saphenous vein, human middle cerebral artery and pulmonary vein, they are 100- to 1000-fold more potent (Coleman et al., 1994; Davis et al., 2004; Foudi et al., 2008). Although these discrepancies could result from species and/or tissue differences, the confounding and variable involvement of other receptors, such as EP1 and TP receptors (see below), in the responses induced by PGE2 and iloprost should also be considered.
Our data suggest EP4 as the main receptor involved in PGE2-induced decrease in lymphatic pumping. This proposal is substantiated by the strong inhibition of PGE2-induced response in the presence of the EP4/TP receptor antagonist AH23848B (Coleman et al., 1994) and the more selective EP4 receptor antagonist GW627368x (Wilson et al., 2006). Together with the significant level of mRNA transcripts for EP4 receptors found in lymphatic vessels, our pharmacological data suggest the functional expression of EP4 receptor proteins in guinea pig lymphatic vessels. The effect of PGE2 via EP4 receptors was shown to be quite variable from vessel to vessel. In a small number of vessels, we observed no or little response to PGE2, suggesting the existence of different vessel populations or a differential expression of the receptors among/along lymphatic vessels.
We also observed in some vessels that the PGE2-induced inhibitory response was counterbalanced by an increase in constriction frequency caused by activation of TP receptors and, in a sub-population of vessels, by a strong stimulation of EP1 receptors. Involvement of these receptors in the PGE2 response could represent another cause of the small effect observed in the ‘non-responders’. The EP1 receptor antagonist we used, AH6809, is also described as an EP2 and DP receptor antagonist. However, the limited expression of EP2 receptor message in these lymphatic vessels we report here and the absence of effect of PGD2 on the lymphatic contractile response (S. Roizes, unpublished data) support a main action of AH6809 on EP1 receptors. Involvement of several receptors, with antagonistic action, in the PGE2-induced contractile response is also consistent with our previously reported observation (Chan et al., 2004) that PGE2 induced a biphasic change in the lymphatic smooth muscle membrane potential, first increasing the occurrence of spontaneous transient depolarizations, electrical events involved in the pacemaking of lymphatic phasic contractions (van Helden, 1993; von der Weid et al., 2008), and then decreasing them during a marked hyperpolarization. The time-dependence of such biphasic response to PGE2 was more rarely observed during vessel pumping measurements.
The present findings also suggest a negligible contribution of EP2 receptors in the PGE2 response, the EP2 receptor agonist butaprost being without significant effect. This statement is further supported by the low level of EP2 receptor mRNA detected in the lymphatics. Similarly, EP3 receptors are not thought to be significantly expressed in guinea pig mesenteric lymphatics. This was demonstrated by the absence of EP3 receptor mRNA in lymphatics and the lack of contractile response to the EP3 receptor agonist sulprostone. The classical EP3 receptor agonist, sulprostone, has also been described as an EP1 or TP receptor agonist (Coleman et al., 1987; Coleman and Sheldrick, 1989); this could explain the tendency of sulprostone to increase lymphatic pumping at the highest concentrations tested (300 µM, Figure 3B).
Decreases in lymphatic pumping in response to various agonists, such as isoprenaline, forskolin, 5-HT and calcitonin-gene-related peptide have been associated with an inhibition of spontaneous transient depolarizations, and a hyperpolarization of the smooth muscle membrane potential (von der Weid and van Helden, 1996; von der Weid, 1998; Chan and von der Weid, 2003; Chan et al., 2004; Hosaka et al., 2006). Pharmacological assessment performed in these studies suggested that the hyperpolarization, decreases of spontaneous transient depolarizations and pumping, were sensitive to inhibition of PKA and blockade of KATP channels. Importantly, involvement of this transduction pathway was also demonstrated during activation of the proteinase-activated receptor PAR-2, which inhibits pumping via prostanoids synthesized and released by the lymphatic endothelium (Chan et al., 2004). Involvement of cAMP and PKA in the PGE2-induced decrease in lymphatic pumping is also well supported by the consistent observation that EP4 receptors are coupled to Gs proteins (see Hata and Breyer, 2004). We showed here that inhibition of PKA activity with H89 strongly affected PGE2-induced decrease in lymphatic pumping, confirming activation of this signalling pathway. Furthermore, abolition of PGE2 action in the presence of glibenclamide also suggests the involvement of KATP channels.
The action of iloprost to modulate lymphatic pumping is mainly mediated by stimulation of IP receptors, as the response was strongly inhibited by CAY10441. Our findings showing an inhibition of iloprost-induced decrease in pumping frequency with GW627368x suggest that EP4 receptors might also be involved. Moreover, an enhanced iloprost-induced response in the presence of the antagonist AH6809 seems to also implicate EP1 receptor activation. Like the action of PGE2, the effect of iloprost is mediated by activation of the PKA/KATP signalling pathway, as demonstrated by inhibition of iloprost response with H89 and glibenclamide. These findings further confirm the involvement of IP and EP4 receptors in iloprost and PGE2-induced responses and may also explain the almost identical biphasic effect of iloprost and PGE2 on the lymphatic muscle membrane potential (Chan et al., 2004). The alteration of iloprost responses in the presence of GW627368x may suggest that this agonist could also act through EP4 receptors in mesenteric lymphatic vessels. Examples of the IP receptor agonist iloprost activating EP4 receptors have been reported in HEK293 cells expressing human recombinant EP4 receptors (Wilson et al., 2004) and in piglet saphenous veins (Wilson and Giles, 2005), where the iloprost responses were sensitive to EP4 receptor antagonism. Iloprost has also been shown to mediate vasodilatory functions via EP4 receptors in the case of low IP receptor expression associated with pulmonary arterial hypertension (Lai et al., 2008). Functional assays have shown that GW627368x is devoid of both agonist and antagonist affinity for IP receptors, but also for other prostanoid receptors, such as CRTH2/DP2, EP2, EP3, and FP, and could at most increase the maximum effect of iloprost on IP receptors by 55% (Wilson et al., 2006).
The origin of the EPs and IP receptor mRNA transcripts and whether they are endothelial or smooth muscle-derived could not be precisely determined in our study. This is mainly due to the small size of the vessels making difficult to obtain pure cell-type populations. Pharmacological evidence suggests that the lymphatic endothelium is the main source of prostanoids and that it possesses the enzymatic equipment to synthesize these molecules. Indeed, inhibition of lymphatic pumping following activation of PAR-2 receptors was shown to be dependent on a functional endothelium, significantly reduced by indomethacin and mimicked by application of PGE2 or prostacyclin (Chan et al., 2004). Moreover, stimulation of guinea pig lymphatic vessels with substance P or ATP was also shown to be mediated by an endothelium release of TXA2, leading to an increase in lymphatic pumping (Rayner and van Helden, 1997; Gao et al., 1999). It is thus very likely that the dominant pool of prostanoids is produced in the endothelium and that lymphatic vessels possess the biosynthetic machinery to produce prostaglandins in physiological conditions. Our study clearly shows that this is the case by demonstrating expression at the mRNA level of COX-1 and the prostaglandin synthases, PGIS and mPGES-1 and -2, involved in prostacyclin and PGE2 synthesis. Message for COX-2, on the other hand was not detected, as might be expected in a non-inflamed state.
Lymphatic pumping is a major determinant in draining fluid away from an inflammation site to minimize oedema. Prostaglandins are critical players in inflammatory reactions and in modulation of lymphatic contractile activity. In light of our findings, we suggest that the inhibitory effect of PGE2/prostacyclin on lymphatic pumping could impair oedema resolution and so to contribute to the pro-inflammatory actions of these prostaglandins in the pathophysiology of inflammation. Thus, extrapolating from our work with the guinea pig lymphatic preparation, our data would support the therapeutic use of cyclo-oxygenase inhibitors or non-steroid anti-inflammatory drugs to diminish inflammation-induced lymphatic stasis in humans.
Acknowledgments
This study was supported by grants from the Crohn's and Colitis Foundation of Canada and the Canadian Institutes of Health Research. S. Rehal was the recipient of a studentship from the Canadian Lymphedema Foundation. The authors would like to thank R. Tao for technical assistance and Dr M. Hollenberg for his valuable comments on the manuscript.
Glossary
Abbreviations:
- COX
cyclooxygenase
- KATP
ATP-sensitive K+
- PG
prostaglandin
- PKA
protein kinase A
- TX
thromboxane
Conflicts of interest
None.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JM, McHale NG, Rooney BM. Effect of norepinephrine on contractility of isolated mesenteric lymphatics. Am J Physiol. 1983;244(4):H479–H486. doi: 10.1152/ajpheart.1983.244.4.H479. [DOI] [PubMed] [Google Scholar]
- Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol. 2006;4(4):217–228. doi: 10.1089/lrb.2006.4406. [DOI] [PubMed] [Google Scholar]
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol. 1989;257:H2059–2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80(5):448–462. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- Chan AK, von der Weid P-Y. 5-HT decreases contractile and electrical activities in lymphatic vessels of the guinea-pig mesentery: role of 5-HT 7-receptors. Br J Pharmacol. 2003;139(2):243–254. doi: 10.1038/sj.bjp.0705264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AK, Vergnolle N, Hollenberg MD, von der Weid P-Y. Proteinase-activated receptor 2 modulates guinea-pig mesenteric lymphatic vessel pacemaker potential and contractile activity. J Physiology. 2004;560:563–576. doi: 10.1113/jphysiol.2004.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Sheldrick RL. Prostanoid-induced contraction of human bronchial smooth muscle is mediated by TP-receptors. Br J Pharmacol. 1989;96(3):688–692. doi: 10.1111/j.1476-5381.1989.tb11869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Kennedy I, Sheldrick RLG. Evidence for the existence of three subtypes of PGE2 sensitive (EP) receptors in smooth muscle. Br J Pharmacol. 1987;91:323P. [Google Scholar]
- Coleman RA, Grix SP, Head SA, Louttit JB, Mallett A, Sheldrick RL. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994;47(2):151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Murdoch CE, Ali M, Purbrick S, Ravid R, Baxter GS, et al. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br J Pharmacol. 2004;141(4):580–585. doi: 10.1038/sj.bjp.0705645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias RM, Johnston MG. Modulation of fluid pumping in isolated bovine mesenteric lymphatics by a thromboxane/endoperoxide analogue. Prostaglandins. 1988;36(1):97–106. doi: 10.1016/0090-6980(88)90105-0. [DOI] [PubMed] [Google Scholar]
- Foudi N, Kotelevets L, Louedec L, Leseche G, Henin D, Chastre E, et al. Vasorelaxation induced by prostaglandin E2 in human pulmonary vein: role of the EP4 receptor subtype. Br J Pharmacol. 2008;154(8):1631–1639. doi: 10.1038/bjp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JL, von der Weid P-Y. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol. 2002;136(8):1210–1218. doi: 10.1038/sj.bjp.0704820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zhao J, Rayner SE, van Helden DF. Evidence that the ATP-induced increase in vasomotion of guinea-pig mesenteric lymphatics involves an endothelium-dependent release of thromboxane A2. Br J Pharmacol. 1999;127(7):1597–1602. doi: 10.1038/sj.bjp.0702710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103(2):147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol. 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K, Rayner SE, von der Weid P-Y, Zhao J, Imtiaz MS, van Helden DF. Calcitonin gene-related peptide activates different signaling pathways in mesenteric lymphatics of guinea pigs. Am J Physiol Heart Circ Physiol. 2006;290(2):H813–H822. doi: 10.1152/ajpheart.00543.2005. [DOI] [PubMed] [Google Scholar]
- Johnston MG. Interaction of inflammatory mediators with the lymphatic vessel. Pathol Immunopathol Res. 1987;6(3):177–189. doi: 10.1159/000157044. [DOI] [PubMed] [Google Scholar]
- Johnston MG, Feuer C. Suppression of lymphatic vessel contractility with inhibitors of arachidonic acid metabolism. J Pharmacol Exp Ther. 1983;226(2):603–607. [PubMed] [Google Scholar]
- Johnston MG, Gordon JL. Regulation of lymphatic contractility by arachidonate metabolites. Nature. 1981;293(5830):294–297. doi: 10.1038/293294a0. [DOI] [PubMed] [Google Scholar]
- Johnston MG, Hay JB, Movat HZ. The distribution of prostaglandins in afferent and efferent lymph from inflammatory sites. Am J Pathol. 1980;99(3):695–714. [PMC free article] [PubMed] [Google Scholar]
- Johnston MG, Kanalec A, Gordon JL. Effects of arachidonic acid and its cyclo-oxygenase and lipoxygenase products on lymphatic vessel contractility in vitro. Prostaglandins. 1983;25(1):85–98. doi: 10.1016/0090-6980(83)90138-7. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CT, McHale NG. Electrical and mechanical activity of isolated lymphatic vessels [proceedings] J Physiol. 1977;272(1):33P–34P. [PubMed] [Google Scholar]
- Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol. 1999;277(6):R1683–R1689. doi: 10.1152/ajpregu.1999.277.6.R1683. Pt 2. [DOI] [PubMed] [Google Scholar]
- Lai YJ, Pullamsetti SS, Dony E, Weissmann N, Butrous G, Banat GA, et al. Role of the prostanoid EP4 receptor in iloprost-mediated vasodilatation in pulmonary hypertension. Am J Respir Crit Care Med. 2008;178(2):188–196. doi: 10.1164/rccm.200710-1519OC. [DOI] [PubMed] [Google Scholar]
- Mannheimer E, Sinzinger H, Oppolzer R, Silberbauer K. Prostacyclin synthesis in human lymphatics. Lymphology. 1980;13(1):44–46. [PubMed] [Google Scholar]
- Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol. 1998;274:R790–R796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Oguogho A, Kaliman J, Sinzinger H. Levels of eicosanoids (6-oxo-PGF1 alpha and 8-epi-PGF2 alpha) in human and porcine lymphatics and lymph. Lymphology. 1998;31(4):186–189. [PubMed] [Google Scholar]
- Oguogho A, Aghajanian AA, Sinzinger H. Prostaglandin synthesis in human lymphatics from precursor fatty acids. Lymphology. 2000;33(2):62–66. [PubMed] [Google Scholar]
- Rayner SE, van Helden DF. Evidence that the substance P-induced enhancement of pacemaking in lymphatics of the guinea-pig mesentery occurs through endothelial release of thromboxane A2. Br J Pharmacol. 1997;121(8):1589–1596. doi: 10.1038/sj.bjp.0701306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzinger H, Kaliman J, Mannheimer E. Arachidonic acid metabolites of human lymphatics. Lymphology. 1984a;17(2):39–42. [PubMed] [Google Scholar]
- Sinzinger H, Kaliman J, Mannheimer E. Regulation of human lymph contractility by prostaglandins and thromboxane. Lymphology. 1984b;17(2):43–45. [PubMed] [Google Scholar]
- Sinzinger H, Kaliman J, Mannheimer E. Effect of leukotrienes C4 and D4 on prostaglandin I2-liberation from human lymphatics. Lymphology. 1986a;19(2):79–81. [PubMed] [Google Scholar]
- Sinzinger H, Kaliman J, Mannheimer E. Enhanced prostaglandin I2-formation of human lymphatics during pulsatile perfusion. Lymphology. 1986b;19(4):153–156. [PubMed] [Google Scholar]
- Sjoberg T, Steen S. Contractile properties of lymphatics from the human lower leg. Lymphology. 1991;24(1):16–21. [PubMed] [Google Scholar]
- von der Weid P-Y. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and beta-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol. 1998;125(1):17–22. doi: 10.1038/sj.bjp.0702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P-Y. Lymphatic vessel pumping and inflammation-the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment Pharmacol Ther. 2001;15(8):1115–1129. doi: 10.1046/j.1365-2036.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- von der Weid P-Y, van Helden DF. b-Adrenoceptor-mediated hyperpolarization in lymphatic smooth muscle of guinea pig mesentery. Am J Physiol. 1996;270(5):H1687–H1695. doi: 10.1152/ajpheart.1996.270.5.H1687. [DOI] [PubMed] [Google Scholar]
- von der Weid P-Y, Crowe MJ, van Helden DF. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol. 1996;493:563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P-Y, Zhao J, van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol. 2001;280(6):H2707–2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- von der Weid P-Y, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol. 2008;295(5):H1989–2000. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Giles H. Piglet saphenous vein contains multiple relaxatory prostanoid receptors: evidence for EP4, EP2, DP and IP receptor subtypes. Br J Pharmacol. 2005;144(3):405–415. doi: 10.1038/sj.bjp.0706088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Rhodes SA, Wood RL, Shield VJ, Noel LS, Gray DW, et al. Functional pharmacology of human prostanoid EP2 and EP4 receptors. Eur J Pharmacol. 2004;501(1–3):49–58. doi: 10.1016/j.ejphar.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Giblin GM, Roomans S, Rhodes SA, Cartwright KA, Shield VJ, et al. GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl] acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006;148(3):326–339. doi: 10.1038/sj.bjp.0706726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TF, Carati CJ, Macnaughton WK, von der Weid P-Y. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G566–574. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]


