Abstract
Background and purpose:
The endocannabinoid, anandamide, has anti-arrhythmic effects. The aim of the present study was to explore the electrophysiological effects of anandamide on rat myocardium.
Experimental approach:
Evoked action potentials (APs) were recorded using intracellular recording technique in rat cardiac papillary muscles. In addition, L-type Ca2+ current was measured and analysed using whole-cell patch-clamp recording technique in isolated rat cardiac ventricular myocytes.
Key results:
In cardiac papillary muscles, anandamide (1, 10, 100 nM) decreased AP duration in a concentration-dependent manner. Furthermore, 100 nM anandamide decreased AP amplitude, overshoot and Vmax in partially depolarized papillary muscles. These effects were abolished by AM251 (100 nM), a selective antagonist for CB1 receptors, but not AM630 (100 nM), a CB2 receptor antagonist. Furthermore, an agonist of L-type Ca2+ channels, Bay K 8644 (0.5 µM), a K+ channel blocker tetraethylammonium chloride (20 mM) and the nitric oxide synthase inhibitor l-NAME (1 mM) had no effect on anandamide-induced decrease in AP duration. In isolated ventricular myocytes, anandamide (1, 10, 100 nM) decreased L-type Ca2+ current concentration-dependently, and shifted the current–voltage relationship curve of the Ca2+ current. Anandamide (100 nM) shifted the steady-state inactivation curve to the left and the recovery curve to the right. Blockade of CB1 receptors with AM251 (100 nM), but not CB2 receptors with AM630 (100 nM), eliminated the effect of anandamide on L-type Ca2+ currents.
Conclusions and implications:
These data suggest that anandamide suppressed AP and L-type Ca2+ current in cardiac myocytes through CB1 receptors.
Keywords: anandamide, action potential, L-type Ca2+ channel, cardiomyocyte, electrophysiology
Introduction
Life-threatening ventricular arrhythmias are often associated with sudden cardiac death in patients with coronary artery disease (Wit and Janse, 2001). Cardioventricular arrhythmias may be caused by myocardial ischaemia when the myocardium experences an imbalance between oxygen supply and metabolic demand. Arrhythmias may result from disturbance of transmembrane currents in the myocytes (Lopshire and Zipes, 2006) and, in this regard, ion channels play a crucial role in regulating the electrical activity of cardiac myocytes. Many types of arrhythmias are due to abnormalities of ion channel expression or function (Nattel and Carlsson, 2006). For example, the L-type Ca2+ channel plays an important role in action potential (AP) generation, morphology and duration (APD), and its mutations can lead to severe arrhythmia disorders (Splawski et al., 2005).
Anandamide is one of the endocannabinoids, which are involved in many physiological and patho-physiological processes such as neurobehaviour, gastrointestinal function, stress and anxiety and cardiovascular functions (Ralevic et al., 2002; Hiley and Ford, 2004; Grant and Cahn, 2005; Hiley, 2009). At least two types of cannabinoid receptors, CB1 and CB2, have been found and cloned depending on their pharmacological effect and gene sequences (Matsuda et al., 1990; Munro et al., 1993); nomenclature follows (Alexander et al., 2008) and these two types of receptors are found in many tissues including cardiac myocytes (Pertwee, 1997; Pacher and Hasko, 2008). The endocannabinoids are importantly involved in regulating cardiovascular function. For example, endocannabinoids tonically suppress cardiac contractility in spontaneously hypertensive rats (Batkai et al., 2004), and anandamide can limit the damage induced by ischaemia–reperfusion in rat isolated hearts through different mechanisms (Underdown et al., 2005; Lépicier et al., 2007). Similarly, anandamide protects the heart from adrenaline-induced arrhythmias (Ugdyzhekova et al., 2001) or arrhythmias induced by ischaemia/reperfusion (Krylatov et al., 2002a). However, the cellular mechanism underlying for the anti-arrhythmic effects of anandamide is not clear. Therefore, in this study, we determined the electrophysiological effect of anandamide on rat cardiac myocytes, using intracellular and whole-cell patch clamp recording techniques.
Methods
Animals
All animal care and experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council, 1996). Adult male Sprague–Dawley rats, weighing 230–280 g, were provided by the Experimental Animal Center of Hebei Province.
Intracellular recording in papillary muscles
Preparation of papillary muscle
The rats were anaesthetized with sodium pentobarbital (50 mg·kg−1, i.p.), and the heart was quickly removed and placed in cold (0–4°C) modified Tyrode's solution containing (in mM): NaCl, 136.8; KCl, 5.4; MgCl2, 1.05; CaCl2, 1.08; NaHCO3, 1.2; glucose, 11.0; and Tris 5.0 (pH 7.35–7.45, gassed with 100% oxygen). The papillary muscle was then excised from the right ventricle and fixed by stainless steel needles on a thin silicon rubber disc placed on the bottom of a perfusion chamber. The tissue was perfused (4 mL·min−1) with modified Tyrode's solution (35.5–36.5°C) gassed with 100% O2.
Intracellular recording
The papillary muscle was paced by square pulse (1 Hz, 1 ms, 1.5 times threshold) delivered through a stimulation electrode placed on the silicon rubber disc. The pacing pulses were generated by an electronic stimulator (YC-2, Chengdu Instrument Factory, Chengdu, China). The APs were recorded with a glass microelectrode (resistance of 10–20 MΩ when filled with 3 M KCl) coupled to the electrical signals were first amplified by a high input impedance amplifier (SWF-2W, Chengdu Instrument Factory) and then processed by multiple channel physiological signal collecting and processing system (RM 6240, Chengdu Instrument Factory). Resting membrane potential (RP), AP overshoot (OS) and AP amplitude (APA); maximal rising velocity of depolarization in phase 0 (Vmax); duration of 50% repolarization (APD50); and duration of 90% repolarization (APD90) were measured.
Experimental protocols
The preparations were equilibrated for 60 min in modified Tyrode's solution before beginning an experiment. The APs were recorded before and after bath application of drugs at 1, 5, 10, 15, 20, 25 and 30 min. There were five experimental groups: (i) To determine the effects of anandamide on AP in normal papillary muscles, APs were recorded before and during application of anandamide (1, 10 and 100 nM). (ii) To determine the effect of anandamide on partially depolarized papillary muscles, the tissue was first perfused for 60 min in normal modified Tyrode's solution. Slow response APs were induced by exposing the papillary muscles to modified Tyrode's solution containing KCl (18 mM) and isoprenaline (1.5 mM). Then, the effect of anandamide (100 nM) on AP was determined. (iii) To determine the receptor subtypes that mediated the effect of anandamide on APs, the effect of 100 nM anandamide on APs in papillary muscles was tested after perfusion of a CB1 receptor antagonist AM251 (100 nM) or CB2 receptor antagonist AM630 (100 nM) for 15 min. (iv) To determine if Ca2+ current is involved in the effect of anandamide on APs, the effect of anandamide 100 nM on the APs was tested after pretreatment of tissue with an agonist of L-type Ca2+ channels, Bay K 8644 (0.5 µM), or a blocker of K+ channels, tetraethylammonium chloride (TEA; 20 mM). (v) To determine if nitric oxide is involved in the effect of anandamide on the APs, the effect of anandamide 100 nM on the APs was tested after pretreatment of tissue with 1 mM NG-nitro-l-arginine methyl ester (l-NAME).
Experiments with cardiac myocytes
Isolation of ventricular myocytes
Single ventricular myocytes were isolated from the hearts of adult rats by enzymatic dissociation as described previously (Chang et al., 2002). Briefly, the rats were anaesthetized by intraperitoneal injection of sodium pentobarbital (50 mg·kg−1) and heparin (300 U·kg−1). The rat heart was excised and retrogradely perfused on a Langendorff apparatus with oxygenated ice-cold Ca2+-free Tyrode's solution via the aorta at a perfusion rate of 4 mL·min−1 for 5 min. The Ca2+-free Tyrode's solution contained (in mM) NaCl, 135; KCl, 5.4; MgCl2, 1.0; NaH2PO4, 0.33; glucose 5; and HEPES 10 (pH was adjusted to 7.4 with NaOH). Then, the heart was perfused with Ca2+-free Tyrode's solution with the addition of CaCl2 (34 µM) and collagenase II (300 mg·L−1) at 37°C for 12 min. Finally, the left ventricle was removed and teased into smaller pieces in Krebs' solution containing (in mM) KOH, 80; KCl, 40; NaH2PO4, 25; MgSO4, 3; glutamic acid, 50; taurine, 20; EGTA, 1; HEPES, 10; and glucose, 10 (pH was adjusted to 7.4 with KOH). Single myocytes were harvested after filtration through a nylon mesh (pore size 200 µm) and stored in Krebs' solution (at room temperature) for at least 1 h, then the concentration of Ca2+ in Kreb's solution was gradually increased to 1.0 mM before the experiment.
Measurement of Ca2+ current
Whole-cell recordings were performed in isolated ventricular myocytes. The myocytes were placed in the recording chamber (0.4 mL) mounted on the stage of an inverted microscope (CK2, Olympus, Tokyo, Japan). After settling to the bottom of the chamber, cells were superfused with external solution for 10 min at a rate of 2–3 mL·min−1 at room temperature. The external solution contained (in mM) TEA–Cl, 140; MgCl2, 2.0; CaCl2, 1.5; glucose, 10; HEPES, 10; and tetrodotoxin (TTX) 10 µM (pH was adjusted with TEA–OH to 7.3–7.4; gassed with 100% O2). Transmembrane currents were recorded with an Axopatch amplifier (200 B, Axon Instruments, Inc., Foster City, CA, USA). Glass microelectrodes were made using a microelectrode puller (model P-97, Sutter Instrument Co., Novato, CA, USA) by two-stage pulling, and had a resistance of 2.0–4.0 MΩ when filled with electrode internal solution containing (in mM) Mg–ATP, 3; CsCl, 140; HEPES, 10; and EGTA, 10 (pH 7.2 adjusted with CsOH). Only rod-type shape cells with clear cross-striations were used for the experiments. After gigaseal formation, the membrane was ruptured with a gentle suction to obtain the whole-cell voltage clamp configuration. Membrane capacitance and series resistance were compensated after membrane rupture to minimize the duration of capacitive current. To record the L-type Ca2+ currents, the external solution was changed to the Na+-free solution in which Na+ was replaced by equimolar TEA (TEA–Cl). Na+ current was also inactivated at the holding potential −50 mV and blocked by TTX (10 µM). K+ currents were suppressed by substituting K+ by Cs+. Computer-generated voltage pulses were programmed using the pCLAMP 10.0 software (Axon Instruments).
Statistics
Data were expressed as mean ± SEM. Differences in parameters before and after drug application were analysed by paired Student's t-test. Differences between groups were evaluated by one-way analysis of variance followed by Dunnet's post hoc test. Statistical significance was accepted at P < 0.05.
Materials
Anandamide, AM251 and AM630 were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). BayK-8644, TEA, l-NAME were purchased from Sigma-Aldrich Co (St Louis, MO, USA). Collagenase II was purchased from Invitrogen·Gibco (Grand Island, NY, USA). Anandamide was initially dissolved in dimethylsulphoxide (DMSO) and the final concentration of DMSO during the experiment was less than 0.1%. In our pilot study, we found that DMSO (up to 0.1%) alone had no significant effect on the electrophysiological characteristics of myocytes.
Results
Effect of anandamide on AP in rat papillary muscle
Effect of anandamide on AP
Anandamide (1 nM) had no effect on the AP in cardiac papillary muscle, but at higher concentrations (10 and 100 nM) decreased APD50 and APD90 in a concentration-dependent manner (Figure 1). Anandamide (100 nM) decreased APD50 by 29% and APD90 by 30%, respectively (Table 1 and Figure 1A), but had no effect on RP, APA, and OS and Vmax (P > 0.05, Table 1). We also determined the effect of anandamide on APs in partially depolarized papillary muscles induced by high K+. Anandamide (100 nM) decreased APD50 by 31%, and APD90 by 24%. In these tissues, anandamide (100 nM) clearly reduced APA by 20%, OS by 91% and Vmax by 42% (P < 0.01, Table 2 and Figure 1B).
Figure 1.
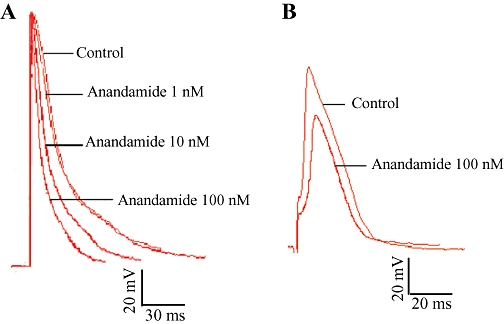
Effects of anandamide on action potential in normal papillary muscle (A) and partially depolarized papillary muscle (B). Results are shown for control conditions and with anandamide (1, 10, 100 nM).
Table 1.
Effects of anandamide on action potential parameters in normal rat papillary muscles (n= 6)
| RP (mV) | OS (mV) | APA (mV) | Vmax (Vs−1) | APD50 (ms) | APD90 (ms) | |
|---|---|---|---|---|---|---|
| Control | −82.3 ± 3.9 | 12.0 ± 2.7 | 94.5 ± 4.8 | 70.2 ± 5.8 | 24.6 ± 2.7 | 98.8 ± 5.2 |
| Anandamide (nM) | ||||||
| 1 | −84.6 ± 1.8 | 12.1 ± 3.8 | 96.7 ± 3.1 | 73.3 ± 7.1 | 23.9 ± 3.9 | 96.2 ± 6.6 |
| 10 | −84.5 ± 3.4 | 10.7 ± 2.7 | 95.2 ± 2.0 | 73.3 ± 7.1 | 19.1 ± 2.8**# | 80.7 ± 5.2***## |
| 100 | −86.7 ± 4.3 | 9.2 ± 2.7 | 95.9 ± 4.8 | 70.5 ± 5.9 | 17.5 ± 2.1**##++ | 68.9 ± 5.2***###+++ |
P < 0.01,
P < 0.001 versus control.
P < 0.05,
P < 0.01,
P < 0.001 versus 1 nM anandamide.
P < 0.01,
P < 0.001 versus 10 nM anandamide.
APA, amplitude of action potential; APD50, duration of 50% repolarization; APD90, duration of 90% repolarization; OS, overshoot; RP, resting membrane potential; Vmax, maximal rising velocity of depolarization in phase 0.
Table 2.
Effects of anandamide (100 nM) on action potential in partially depolarized papillary muscles (n= 6)
| RP (mV) | OS (mV) | APA (mV) | Vmax (Vs−1) | APD50 (ms) | APD90 (ms) | |
|---|---|---|---|---|---|---|
| Control | −53.3 ± 3.0 | 5.3 ± 1.0 | 58.6 ± 4.0 | 29.3 ± 5.5 | 33.9 ± 3.3 | 60.2 ± 4.3 |
| Anandamide | −46.3 ± 6.2 | 0.5 ± 0.7** | 46.7 ± 6.6** | 17.1 ± 3.4* | 23.3 ± 2.2** | 45.7 ± 2.7* |
P < 0.05,
P < 0.01 versus control.
Blockade of CB1 receptors eliminated the effect of anandamide on APs
To determine the CB receptor subtypes that mediated anandamide-induced suppression of APs, we used the selective CB1 receptor antagonist AM251, and the CB2 receptor antagonist AM630. By themselves, AM251 (100 nM) or AM630 (100 nM) had no significant effect on APD50, APD90, APA, OS and Vmax. However, pretreatment with AM251 (100 nM) for 15 min completely abolished the effects of anandamide (100 nM) on APs. In a separate group of cells, anandamide (100 nM) still decreased the APD50, APD90, APA, OS and Vmax after pretreatment of the cells with AM630 (100 nM) (Table 3).
Table 3.
Influences of AM251 (100 nM) and AM630 (100 nM) on the anandamide (100 nM)-induced changes on action potential in rat papillary muscles (n= 6)
| RP (mV) | OS (mV) | APA (mv) | Vmax (Vs−1) | APD50 (ms) | APD90 (ms) | |
|---|---|---|---|---|---|---|
| Control | −67.2 ± 0.6 | 0.5 ± 0.4 | 67.7 ± 0.5 | 56.9 ± 3.5 | 25.1 ± 0.6 | 74.5 ± 2.7 |
| Anandamide | −66.1 ± 0.9 | 1.4 ± 0.9 | 67.4 ± 0.4 | 59.9 ± 0.8 | 20.1 ± 0.7** | 64.2 ± 2.6* |
| AM251 | −68.8 ± 1.4 | 1.3 ± 0.5 | 70.1 ± 3.7 | 52.9 ± 3.5 | 25.6 ± 0.6### | 78.4 ± 1.9# |
| AM251+Anandamide | −68.2 ± 1.9 | 1.7 ± 0.4 | 70.3 ± 3.7 | 56.9 ± 3.5 | 25.0 ± 1.5## | 78.2 ± 1.5## |
| Control | −69.8 ± 2.0 | 5.4 ± 0.8 | 75.2 ± 2.9 | 48.5 ± 0.5 | 19.5 ± 1.4 | 70.8 ± 2.1 |
| Anandamide | −71.0 ± 2.8 | 3.0 ± 1.8 | 74.1 ± 2.8 | 49.9 ± 0.8 | 17.3 ± 1.5** | 54.2 ± 2.6*** |
| AM630 | −70.5 ± 1.9 | 2.6 ± 1.4 | 75.7 ± 3.0 | 48.6 ± 0.4 | 18.9 ± 0.6# | 66.2 ± 3.6# |
| AM630+Anandamide | −70.9 ± 1.7 | 3.69 ± 0.7 | 74.6 ± 0.6 | 48.1 ± 0.7 | 16.3 ± 0.5*+ | 56.6 ± 3.4**+++ |
P < 0.05,
P < 0.01,
P < 0.001 versus control.
P < 0.05,
P < 0.05,
P < 0.001 versus anandamide.
P < 0.05,
P < 0.001 versus AM630.
Effects of Bay K-8644, TEA and l-NAME on the anandamide-induced changes of AP
To determine if Ca2+ currents were involved in the effect of anandamide on APs in cardiac papillary muscle, we assessed the effects of the L-type Ca2+ channel opener Bay K-8644 (0.5 µM) and K+ channel blocker TEA (20 mM) in our system. Given alone, Bay K-8644 or TEA prolonged APD50 and APD90 (P < 0.05), but had no significant effect on RP, OS, APA and Vmax (P > 0.05). After exposure to either Bay K 8644 or TEA, this prolonged APD was still reduced by anandamide (100 nM) (Table 4).
Table 4.
Influences of Bay K 8644 (0.5 µM), TEA (20 mM) and l-NAME (1 mM) on the anandamide (100 nM)-induced changes on action potential in rat papillary muscles (n= 6)
| RP (mV) | OS (mV) | APA (mv) | Vmax (Vs−1) | APD50 (ms) | APD90 (ms) | |
|---|---|---|---|---|---|---|
| Control | −78.1 ± 3.2 | 8.8 ± 2.1 | 86.8 ± 4.9 | 79.1 ± 4.5 | 32.1 ± 2.7 | 106.5 ± 4.3 |
| Anandamide | −77.3 ± 3.7 | 8.8 ± 2.5 | 87.6 ± 5.2 | 78.9 ± 4.4 | 27.5 ± 2.7** | 95.7 ± 4.3** |
| Bay K 8644 | −73.4 ± 3.6 | 9.6 ± 2.5 | 83.2 ± 3.9 | 75.4 ± 4.6 | 35.8 ± 3.0*## | 121.6 ± 7.1*## |
| Bay K 8644 + Anandamide | −76.0 ± 3.2 | 8.4 ± 3.3 | 84.4 ± 4.7 | 70.7 ± 4.0 | 32.3 ± 2.7#++ | 106.2 ± 3.2#+ |
| Control | −75.1 ± 10.7 | 17.8 ± 8.6 | 92.9 ± 8.2 | 106.6 ± 4.7 | 38.2 ± 1.4 | 115.7 ± 4.7 |
| Anandamide | −78.7 ± 9.8 | 18.3 ± 9.5 | 97.0 ± 9.6 | 104.5 ± 5.6 | 28.1 ± 1.9** | 96.8 ± 5.4** |
| TEA | −77.9 ± 8.8 | 20.6 ± 9.1 | 98.5 ± 8.2 | 110.0 ± 6.1 | 58.8 ± 2.9**## | 156.1 ± 3.7**## |
| TEA + Anandamide | −79.6 ± 11.3 | 19.9 ± 7.9 | 99.5 ± 9.7 | 105.6 ± 8.5 | 45.9 ± 1.4**##• | 130.2 ± 2.9*##• |
| Control | −71.1 ± 11.0 | 23.2 ± 9.3 | 94.3 ± 5.0 | 105.8 ± 9.4 | 23.1 ± 5.9 | 110.7 ± 9.5 |
| Anandamide | −67.8 ± 11.1 | 23.9 ± 10.5 | 91.7 ± 5.4 | 108.1 ± 13.9 | 16.5 ± 1.7* | 84.0 ± 13.5** |
| l-NAME | −73.0 ± 3.9 | 18.6 ± 3.2 | 93.6 ± 2.5 | 108.1 ± 1.2 | 22.8 ± 5.8# | 112.8 ± 9.7## |
| l-NAME + Anandamide | −68.4 ± 4.7 | 22.4 ± 3.3 | 90.8 ± 2.5 | 106.9 ± 10.4 | 14.4 ± 4.4*▴ | 87.5 ± 8.8**▴ |
P < 0.05,
P < 0.01 versus control.
P < 0.05,
P < 0.05 versus anandamide.
P < 0.05,
P < 0.01 versus Bay K 8644.
P < 0.05 versus TEA.
P < 0.05 versus l-NAME.
We also determined if nitric oxide is involved in the effect of anandamide on APs in cardiac papillary muscle. In a separate group of cells, pretreatment with 1 mM l-NAME had no significant effect on the electrophysiological parameters of APs measured, and anandamide (100 nM) still decreased the APD50 and APD90 after pretreatment of the cells with 1 mM l-NAME (Table 4).
Effect of anandamide on L-type Ca2+ current in ventricular myocyte
Effect of anandamide on peak L-type Ca2+ current and current–voltage (I–V) relationship
L-type Ca2+ current was recorded by depolarizing the myocytes from a holding potential (−40 mV) to 0 mV for a duration of 300 ms (frequency, 0.1 Hz). Application of nisoldipine (0.5 µM), a selective L-type Ca2+ channel antagonist, completely blocked this inward current (data not shown). The rundown of L-type Ca2+ current was minimized by adding Mg–ATP (3 mM) and EGTA (10 mM) in the electrode internal solution (Belles et al., 1998). Anandamide (1, 10, 100 nM) decreased the peak amplitude of L-type Ca2+ current in a concentration-dependent manner (Figure 2A).
Figure 2.
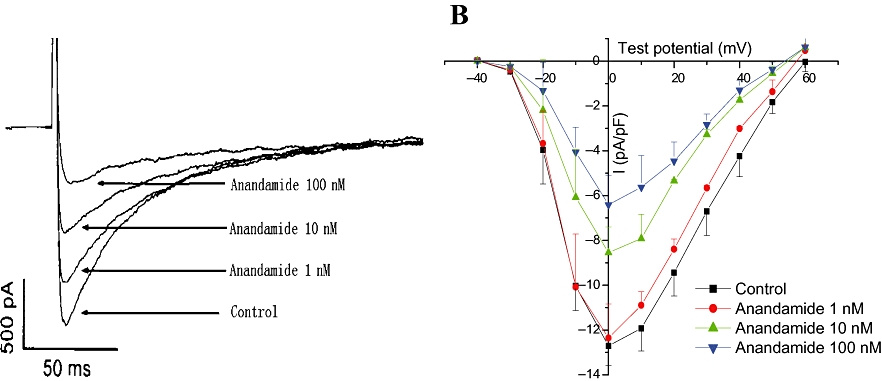
Effect of anandamide on L-type Ca2+ currents in rat isolated ventricular myocytes. (A) Anandamide concentration-dependently decreased L-type Ca2+ currents recorded during 300 ms depolarization from a holding potential of −40 to 0 mV in isolated myocytes. (B) Effects of anandamide on current–voltage curve of L-type Ca2+ currents in rat isolated ventricular myocytes (n= 6).
We determined the I–V relationship of L-type calcium current by applying a series of step depolarizing pulses from −40 mV to +60 mV in 10 mV increments for a duration of 1000 ms. The I–V curve was plotted according to the currents at different holding potentials. L-type Ca2+ current was activated at a holding potential of −30 mV, and the peak amplitude occurred at a holding potential of 0 mV. Anandamide (1, 10, 100 nM) shifted the I–V curve upward (Figure 2B). However, the current density at a holding potential of 0 mV was reduced (n= 6, P < 0.01), as shown in Figure 2B.
Effect of anandamide on kinetics of L-type Ca2+ channels
To investigate the effects of anandamide on L-type Ca2+ channel activation and inactivation, steady-state activation and inactivation curves were obtained before and after anandamide application. The activation curves were derived from the I–V relationships (Figure 2B). The activation curves were fitted according to the Boltzmann equation: G/Gmax= 1/{1 + EXP [−(V−V0.5)/κ]}. Anandamide 100 nM shifted half activation potential from −16.3 ± 0.44 mV to −11.9 ± 0.42 mV, slope parameter (κ) from 4.6 ± 0.35 mV to 4.4 ± 0.38 mV. These values of the activation were not significantly different between the two groups (n= 6, P > 0.05) (Figure 3).
Figure 3.
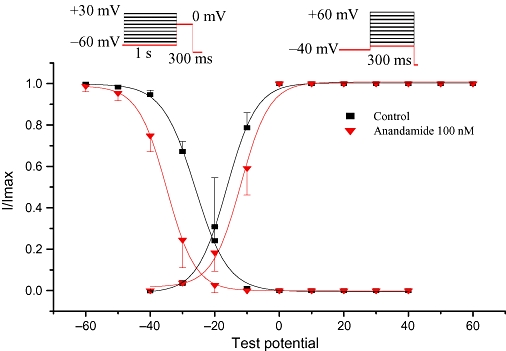
Effects of anandamide on steady-state activation (right) and inactivation (left) of L-type Ca2+ currents in rat isolated ventricular myocytes (n= 6).
Steady-state inactivation curve of the L-type Ca2+ channel was determined by using the following protocol: a series of pre-pulses (from −60 mV to +30 mV in 10 mV increments for a duration of 1000 ms) followed by test pulses (holding potential at +0 mV for 300 ms) were applied to the myocyte. Then, the holding potentials were set to −80 mV (at the pulse frequency was 0.1 Hz). The inactivation curves were plotted as voltages in the pre-pulses and currents in the test pulses. In addition, the inactivation curves were fitted according to the Boltzmann equation (Figure 3). Anandamide (100 nM) significantly changed the half inactivation potential from −26.1 ± 0.20 mV to −34.9 ± 0.20 mV (n= 6, P < 0.05). However, slope parameter (κ) was not significantly changed (5.1 ± 0.16 mV vs. 4.8 ± 0.15 mV, n= 6, P > 0.05).
Effects of anandamide on recovery of L-type Ca2+ channels from inactivation
The recovery of L-type Ca2+ channels was determined by using double-pulse protocol consisting of two identical pulses (holding potential from −80 to +10 mV for 1000 ms) in a variable intervals from 0 to 2500 ms in 10 ms increments. Double-pulse stimulation was repeated every 6 s. Plots of the normalized steady-state currents as a function of the interval between the two test pulses were built and fitted to a monoexponential function. Anandamide (100 nM) shifted the curve to the right (Figure 4) and increased the half-recovery time of Ca2+ channel from inactivation from 80.7 ± 20.09 ms to 330.0 ± 120.31 ms (n= 6, P < 0.01).
Figure 4.
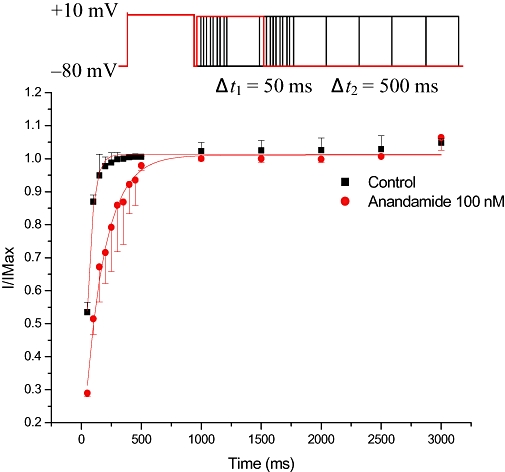
Recovery of L-type Ca2+ currents from the steady-state inactivation. Anandamide 100 nM markedly slowed the recovery time course of L-type Ca2+ currents from inactivation (n= 6).
Role of CB1 and CB2 receptors in the effect of anandamide on L-type Ca2+ channels
Blockade of CB1 receptors with AM251 (100 nM) or CB2 receptors with AM630 (100 nM) had no effect on L-type Ca2+ currents in cardiac myocytes. However, pretreatment of cells with AM251 (100 nM) for 15 min abolished the inhibitory effect of anandamide (100 nM) on L-type Ca2+ channels, whereas pretreatment of the myocytes with AM630 (100 nM) did not affect their response to anandamide (100 nM) (Figure 5).
Figure 5.
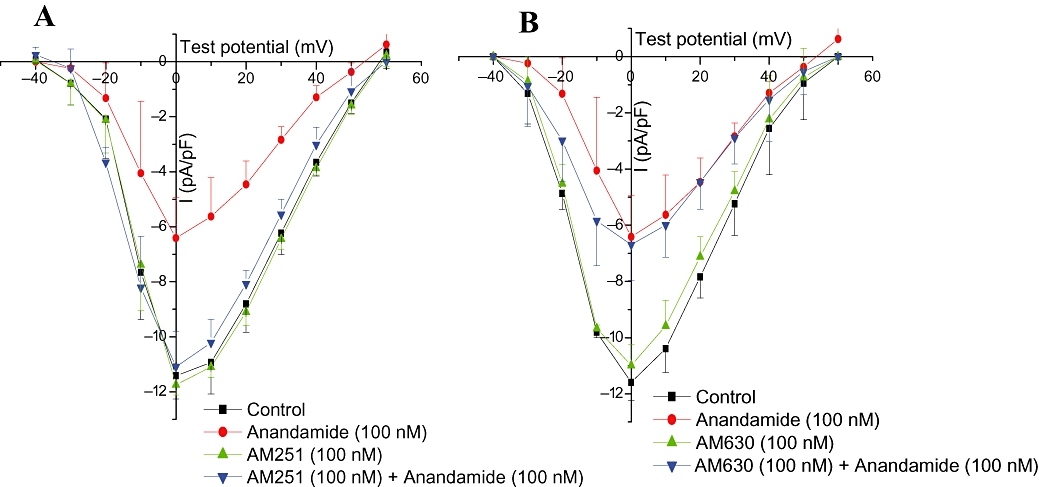
Effects of AM251 (100 nM) (A) and AM630 (100 nM) (B) on anandamide-induced L-type Ca2+ current change in rat isolated ventricular myocytes (n= 6).
Discussion
In the present study, we explored the effect of anandamide on the electrophysiological properties of rat cardiac myocytes. We found that anandamide concentration-dependently shortened the AP duration in cardiac papillary muscle, and decreased L-type Ca2+ current in ventricular myocytes. These effects were mediated by CB1, but not CB2 receptors. Furthermore, anandamide facilitated the inactivation of L-type Ca2+ current and inhibited its recovery from inactivation. These results suggested that anandamide suppresses of AP and L-type Ca2+ current through CB1 receptors in myocytes. This novel mechanism may be involved in the anti-arrhythmic action of anandamide.
Distinct transmembrane ion currents are involved in the depolarization and repolarization of AP in excitable cells. In cardiac myocytes, the depolarizing phase of AP results from Na+ influx through the fast Na+ channels. Although K+ efflux predominantly drives membrane repolarization, Ca2+ influx through L-type Ca2+ channels delays the repolarization phase of AP in cardiac myocytes. Therefore, inhibition of Ca2+ influx or/and facilitation of K+ efflux shorten(s) AP duration (Robert et al., 2001). We found that anandamide reduced AP duration (both APD50 and APD90) without significantly changing the AP depolarization phase in cardiac papillary muscle. These data suggested that anandamide suppressed the Ca2+ influx or/and facilitated K+ efflux, but had no effects on Na+ currents, in the AP polarization phase. To further determine the effect of anandamide on Ca2+ currents in the papillary muscle, we induced slow response APs in partially depolarized papillary muscle by exposing the tissue to KCl (18 mM) and isoprenaline (1.5 mM). Under this condition, Ca2+ currents play an important role in both depolarization and repolarization phases of APs (Morad and Tung, 1982). We found that anandamide significantly decreased APD, APA, OS and Vmax in slow response APs. These data suggested that anandamide suppressed Ca2+ influx. In addition, the increase of APD by Bay K 8644 (an L-type Ca2+ channel agonist) was totally reversed by anandamide, but the increase of APD by TEA (a K+ channel blocker) was only partially reversed by anandamide. These data provide further evidence that anandamide suppresses Ca2+ influx.
L-type Ca2+ channels in cardiac myocytes play an important role in the generation of AP in physiological and patho-physiological conditions such as arrhythmia. Furthermore, the L-type Ca2+ channels are the predominant mechanism of Ca2+ influx in cardiac cells (Faber et al., 2007). Blockade of L-type Ca2+ channels provides anti-arrhythmic actions (Triggle, 2007), and we, therefore, determined the effect of anandamide on L-type Ca2+ currents in ventricular cardiomyocytes, using the whole-cell patch clamp technique. Our results showed that anandamide decreased L-type Ca2+ currents and shifted I–V relationship curves upward. These data suggested that anandamide inhibited L-type Ca2+ channels in rat ventricular myocytes. We further analysed the kinetics of L-type Ca2+ currents in response to anandamide. Anandamide had no effect on the maximal activation, but shifted steady-state inactivation curve to the left, suggesting that the voltage-dependent steady-state inactivation of the L-type Ca2+ channel was accelerated. In addition, anandamide markedly shifted the recovery curve of L-type Ca2+ currents to the right, suggesting that anandamide attenuated the recovery of L-type Ca2+ channels from inactivation. Taken together, these results provide electrophysiological evidence that anandamide inhibited L-type Ca2+ channels through facilitation of steady-state inactivation and attenuation of recovery from inactivation.
We also determined the receptor subtypes responsible for the effect of anandamide on the AP and L-type Ca2+ currents. Two types of cannabinoid receptors, CB1 and CB2, have been cloned (Matsuda et al., 1990; Munro et al., 1993) and widely expressed in the cardiovascular system such as blood vessels and cardiac tissue (Pertwee, 1997). Anandamide is a natural constituent of the plasma membrane and is considered to be a CB1 and CB2 receptor agonist because it exhibits pharmacological activities comparable to the plant cannabinoids (Felder and Glass, 1998). Activation of CB1 receptor reduced the infarct size induced by low-flow ischemia through production of nitric oxide in rat isolated hearts (Lépicier et al., 2007). Furthermore, blockade of CB2 receptors eliminated the cardiac protective effect of endocannabinoids in rat isolated hearts exposed to low-flow ischaemia and reperfusion (Krylatov et al., 2002b; Lépicier et al., 2003). In this study, we found that blockade of CB1 receptors with the selective antagonist AM251 completely blocked the inhibitory effect of anandamide on AP duration in papillary muscles. In addition, AM251 abolished the inhibitory effects of anandamide on L-type Ca2+ current in cadiomyocytes. However, a CB2 receptor antagonist AM630 failed to change the inhibitory effects of anandamide on AP in papillary muscles and L-type Ca2+ current in cardiac myocytes. The results indicated the inhibitory effects of anandamide on AP and L-type Ca2+ current were mediated by CB1 receptors, but not CB2 receptors. However, the signal transduction pathways linking CB1 receptors and voltage-dependent L-type Ca2+ channel in the cardiac cell are not known. Future studies are warranted to investigate these signal pathways.
Previous studies have shown that activation of CB1 receptors in the arterial and capillary endothelial cells in the heart limits infarct size through NO formation in isolated rat hearts (Lépicier et al., 2007). Furthermore, activation of CB1 cannabinoid receptors by another endocannabinoid, 2-arachidonylglycerol, mediates nitric oxide-induced preconditioning in isolated rat hearts (Wagner et al., 2006). We also examined the role of nitric oxide in the action of anandamide on AP in papillary muscle. A nitric oxide synthase inhibitor l-NAME failed to alter the effect of anandamide on APs in papillary muscle, suggesting that the inhibitory effect of anandamide on APs in papillary muscle was independent of nitric oxide production. On the other hand, earlier work in a rat model of nitric oxide-mediated delayed preconditioning showed that the endocannabinoid concentration in heart tissue increased (Wagner et al., 2006). One can speculate that the concentration of endocannabinoids increases during patho-physiological processes such as cardiac ischaemia and arrhythmia. Then, the endocannabinoids may act as cardiac protective factors to exert anti-ischaemic and anti-arrhythmic actions through suppression of Ca2+ influx.
In summary, this study provides new ionic mechanisms underlying the cardiac protective action of anandamide in isolated rat heart. This mechanism may be importantly involved in the anti-arrhythmic effects of the endocannabinoids, such as anandamide.
Glossary
Abbreviations:
- AP
action potential
- APA
action potential amplitude
- APD50
duration of 50% repolarization
- APD90
duration of 90% repolarization
- CB
cannabinoid
- DMSO
dimethylsulphoxide
- l-NAME
NG-nitro-l-arginine methyl ester
- OS
action potential overshoot
- RP
resting membrane potential
- TEA
tetraethylammonium chloride
- TTX
tetrodotoxin
- Vmax
maximal rising velocity of depolarization in phase 0
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, et al. Endocannabinoids acting at CB1R's regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belles B, Malecot CO, Hescheler J, Trautwein W. ‘Rundown’ of the Ca2+ current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflugers Arch. 1998;411:353–560. doi: 10.1007/BF00587713. [DOI] [PubMed] [Google Scholar]
- Chang GJ, Su MJ, Hung LM, Lee SS. Cardiac electrophysiologic and antiarrhythmic actions of a pavine alkaloid derivative, O-methylneocaryachine, in rat heart. Br J Pharmacol. 2002;136:459–471. doi: 10.1038/sj.bjp.0704736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber GM, Silva J, Livshitz L, Rudy Y. Kinetic properties of the cardiac L-type Ca2+ channel and its role in myocyte electrophysiology: a theoretical investigation. Biophys J. 2007;92:1522–1543. doi: 10.1529/biophysj.106.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- Grant I, Cahn BR. Cannabis and endocannabinoid modulators: therapeutic promises and challenges. Clin Neurosci Res. 2005;5:185–199. doi: 10.1016/j.cnr.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR. Endocannabinoids and the heart. J Cardiovasc Pharmacol. 2009;53:267–276. doi: 10.1097/FJC.0b013e318192671d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signaling. Biol Rev. 2004;79:187–205. doi: 10.1017/s1464793103006201. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Krylatov AV, Uzhachenko RV, Maslov LN, Bernatatskaya NA, Markriyannis A, Mekhoulam R, et al. Endogenous cannabinoids improve myocardial resistance to arrhythmogenic effects of coronary occlusion and reperfusion: a possible mechanism. Bull Exp Biol Med. 2002a;33:122–124. doi: 10.1023/a:1015574100494. [DOI] [PubMed] [Google Scholar]
- Krylatov AV, Uzhachenko RV, Maslov LN, Ugdyzhekova DS, Bernatskaia NA, Pertwee R, et al. Anandamide and R-(+)-methanandamide prevent development of ischemic and reperfusion arrhythmia in rats by stimulation of CB2-receptors. Eksp Klin Farmakol. 2002b;65:6–9. [PubMed] [Google Scholar]
- Lépicier P, Bouchard JF, Lagneux C, Lamontagne D. Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol. 2003;139:805–815. doi: 10.1038/sj.bjp.0705313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lépicier P, Lagneux C, Sirois MG, Lamontagne D. Endothelial CB1-receptors limit infarct size through NO formation in rat isolated hearts. Life Sci. 2007;81:1373–1380. doi: 10.1016/j.lfs.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Zipes DP. Sudden cardiac death: better understanding of risks, mechanisms and treatment. Circulation. 2006;114:1134–1136. doi: 10.1161/CIRCULATIONAHA.106.647933. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AG, Bonner TI. Structure of cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Morad M, Tung L. Ionic events responsible for the cardiac resting and action potential. Am J Cardiol. 1982;49:584–594. doi: 10.1016/s0002-9149(82)80016-7. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;65:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nature reviews. Drug Discov. 2006;5:1034–1049. doi: 10.1038/nrd2112. [DOI] [PubMed] [Google Scholar]
- Pacher P, Hasko G. Endocannabinoids and cannabinoid receptors in ischaemia–reperfusion injury and preconditioning. Br J Pharmacol. 2008;153:252–256. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Smart D. Cannabinoid modulation of sensory neuron transmission via cannabinoid and vanilloid receptors: roles in regulation of cardiovascular function. Life Sci. 2002;71:2577–2594. doi: 10.1016/s0024-3205(02)02086-6. [DOI] [PubMed] [Google Scholar]
- Robert SK, Abriel H, Rivolta I. Ion channels in the heart. In: Sperelakis N, Kurachi Y, Terzic A, Cohen MV, editors. Heart Physiology and Pathophysiology. 4th. San Diego, CA, USA: Academic Press; 2001. pp. 1137–1151. [Google Scholar]
- Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle DJ. Calcium channel antagonists: clinical uses – past, present and future. Biochem Pharmacol. 2007;74:1–9. doi: 10.1016/j.bcp.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Ugdyzhekova DS, Bernatskaya NA, Stefano JB, Graier VF, Tam SW, Mekhoulam R. Endogenous cannabinoid anandamide increases heart resistance to arrhythmogenic effects of epinephrine: role of CB1 and CB2 receptors. Bull Exp Biol Med. 2001;131:251–253. doi: 10.1023/a:1017651432193. [DOI] [PubMed] [Google Scholar]
- Underdown NJ, Hiley CR, Ford WR. Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia–reperfusion by a novel cannabinoid mechanism. Br J Pharmacol. 2005;146:809–816. doi: 10.1038/sj.bjp.0706391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Abesser M, Harvey-White J, Ertl G. 2-Arachidonylglycerol acting on CB1 cannabinoid receptors mediates delayed cardioprotection induced by nitric oxide in rat isolated hearts. J Cardiovasc Pharmacol. 2006;47:650–655. doi: 10.1097/01.fjc.0000211752.08949.eb. [DOI] [PubMed] [Google Scholar]
- Wit AL, Janse MJ. Reperfusion arrhythmia and sudden cardiac death: a century of progress toward an understanding of the mechanisms. Circ Res. 2001;89:741–743. [PubMed] [Google Scholar]


