Abstract
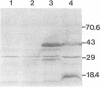
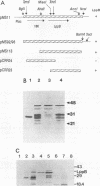
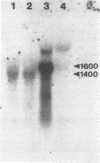
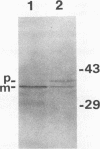
Haemophilus somnus is a facultative intracellular pathogen which causes a wide range of diseases in cattle. To identify putative virulence determinants, a genomic library of H. somnus in Escherichia coli was screened for Congo red binding, a property associated with virulence in pathogenic bacteria, and subsequently with bovine hyperimmune sera raised against H. somnus HS25. A Congo red-binding clone carrying a 1.8-kb DNA insert was found to encode a strongly seroreactive LppB protein with an apparent molecular weight of 40,000. The nucleotide sequence of the entire DNA insert was determined. Two open reading frames coding for polypeptides with calculated molecular weights of 21,893 and 30,721 were identified. The larger open reading frame encoded LppB, while the smaller reading frame encoded a nonseroreactive protein with a relative molecular mass of approximately 18 kDa. The 16 amino-terminal amino acids of the deduced LppB polypeptide showed strong sequence homology to the signal peptide of secreted bacterial proteins, and the sequence Leu-Ala-Ala-Cys at the putative cleavage site corresponds to the consensus cleavage sequence of bacterial lipoproteins. Synthesis of the mature LppB lipoprotein in H. somnus was inhibited by globomycin, a specific inhibitor of signal peptidase II. LppB was localized to the outer membrane of H. somnus.
Full text
PDF

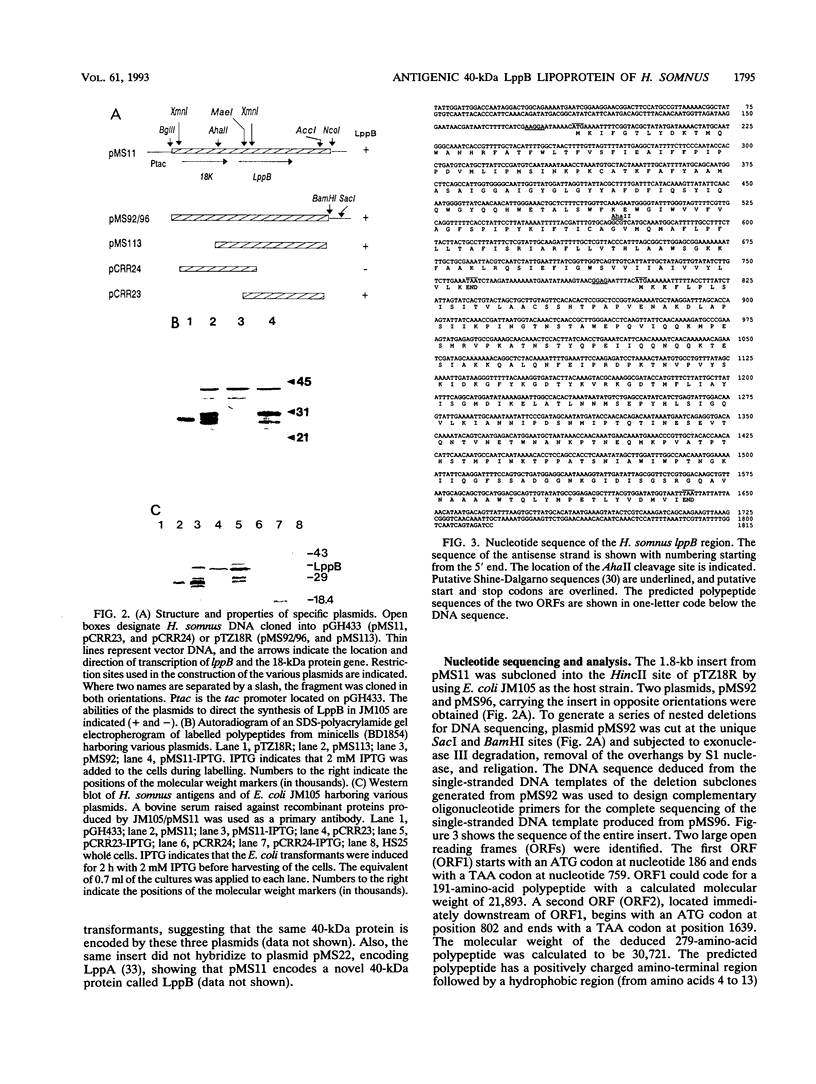



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee-Bhatnagar N., Frasch C. E. Expression of Neisseria meningitidis iron-regulated outer membrane proteins, including a 70-kilodalton transferrin receptor, and their potential for use as vaccines. Infect Immun. 1990 Sep;58(9):2875–2881. doi: 10.1128/iai.58.9.2875-2881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J., Pugsley A. P., Stragier P. A gene for a new lipoprotein in the dapA-purC interval of the Escherichia coli chromosome. J Bacteriol. 1991 Sep;173(17):5523–5531. doi: 10.1128/jb.173.17.5523-5531.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Chikami G., Yarnall M., Smith J., Guiney D. G. Cloning and expression of genes encoding Haemophilus somnus antigens. Infect Immun. 1988 Oct;56(10):2736–2742. doi: 10.1128/iai.56.10.2736-2742.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Kania S. A., Gogolewski R. P. Characterization of immunodominant surface antigens of Haemophilus somnus. Infect Immun. 1991 Dec;59(12):4295–4301. doi: 10.1128/iai.59.12.4295-4301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B. Molecular aspects of some virulence factors of Haemophilus somnus. Can J Vet Res. 1990 Apr;54 (Suppl):S57–S62. [PubMed] [Google Scholar]
- Daskaleros P. A., Payne S. M. Congo red binding phenotype is associated with hemin binding and increased infectivity of Shigella flexneri in the HeLa cell model. Infect Immun. 1987 Jun;55(6):1393–1398. doi: 10.1128/iai.55.6.1393-1398.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Effect of iron restriction on the outer membrane proteins of Actinobacillus (Haemophilus) pleuropneumoniae. Infect Immun. 1989 Mar;57(3):798–804. doi: 10.1128/iai.57.3.798-804.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J., Ray P. H. Inhibition of prolipoprotein signal peptidase by globomycin. J Biol Chem. 1985 May 25;260(10):5891–5894. [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French B. T., Maul H. M., Maul G. G. Screening cDNA expression libraries with monoclonal and polyclonal antibodies using an amplified biotin-avidin-peroxidase technique. Anal Biochem. 1986 Aug 1;156(2):417–423. doi: 10.1016/0003-2697(86)90275-7. [DOI] [PubMed] [Google Scholar]
- Gerlach G. F., Anderson C., Potter A. A., Klashinsky S., Willson P. J. Cloning and expression of a transferrin-binding protein from Actinobacillus pleuropneumoniae. Infect Immun. 1992 Mar;60(3):892–898. doi: 10.1128/iai.60.3.892-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolewski R. P., Kania S. A., Liggitt H. D., Corbeil L. B. Protective ability of antibodies against 78- and 40-kilodalton outer membrane antigens of Haemophilus somnus. Infect Immun. 1988 Sep;56(9):2307–2316. doi: 10.1128/iai.56.9.2307-2316.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris F. W., Janzen E. D. The Haemophilus somnus disease complex (Hemophilosis): A review. Can Vet J. 1989 Oct;30(10):816–822. [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Trust T. J. Porphyrin binding by the surface array virulence protein of Aeromonas salmonicida. J Bacteriol. 1985 Dec;164(3):1332–1336. doi: 10.1128/jb.164.3.1332-1336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mulvey M. R., Loewen P. C. Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel sigma transcription factor. Nucleic Acids Res. 1989 Dec 11;17(23):9979–9991. doi: 10.1093/nar/17.23.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunnariwo J. A., Cheng C., Ford J., Schryvers A. B. Response of Haemophilus somnus to iron limitation: expression and identification of a bovine-specific transferrin receptor. Microb Pathog. 1990 Dec;9(6):397–406. doi: 10.1016/0882-4010(90)90058-x. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Pendrak M. L., Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990 Oct;172(10):5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prpic J. K., Robins-Browne R. M., Davey R. B. Differentiation between virulent and avirulent Yersinia enterocolitica isolates by using Congo red agar. J Clin Microbiol. 1983 Sep;18(3):486–490. doi: 10.1128/jcm.18.3.486-490.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux C. R., Bergeron H., Lin L., Grothe S., O'Connor-McCourt M., Lau P. C. A fusion plasmid for the synthesis of lipopeptide-antigen chimeras in Escherichia coli. Gene. 1992 Jul 1;116(1):13–20. doi: 10.1016/0378-1119(92)90623-w. [DOI] [PubMed] [Google Scholar]
- Sak B. D., Eisenstark A., Touati D. Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the katF gene product. Proc Natl Acad Sci U S A. 1989 May;86(9):3271–3275. doi: 10.1073/pnas.86.9.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample A. K., Czuprynski C. J. Elimination of hydrogen peroxide by Haemophilus somnus, a catalase-negative pathogen of cattle. Infect Immun. 1991 Jul;59(7):2239–2244. doi: 10.1128/iai.59.7.2239-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Potter A. A. Cloning, sequencing, expression, and functional studies of a 15,000-molecular-weight Haemophilus somnus antigen similar to Escherichia coli ribosomal protein S9. J Bacteriol. 1992 Jan;174(1):17–23. doi: 10.1128/jb.174.1.17-23.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Rioux C. R., Potter A. A. Molecular cloning, nucleotide sequence, and characterization of a 40,000-molecular-weight lipoprotein of Haemophilus somnus. Infect Immun. 1992 Mar;60(3):826–831. doi: 10.1128/iai.60.3.826-831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widders P. R., Dorrance L. A., Yarnall M., Corbeil L. B. Immunoglobulin-binding activity among pathogenic and carrier isolates of Haemophilus somnus. Infect Immun. 1989 Feb;57(2):639–642. doi: 10.1128/iai.57.2.639-642.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Zagaglia C., Casalino M., Colonna B., Conti C., Calconi A., Nicoletti M. Virulence plasmids of enteroinvasive Escherichia coli and Shigella flexneri integrate into a specific site on the host chromosome: integration greatly reduces expression of plasmid-carried virulence genes. Infect Immun. 1991 Mar;59(3):792–799. doi: 10.1128/iai.59.3.792-799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]