Abstract
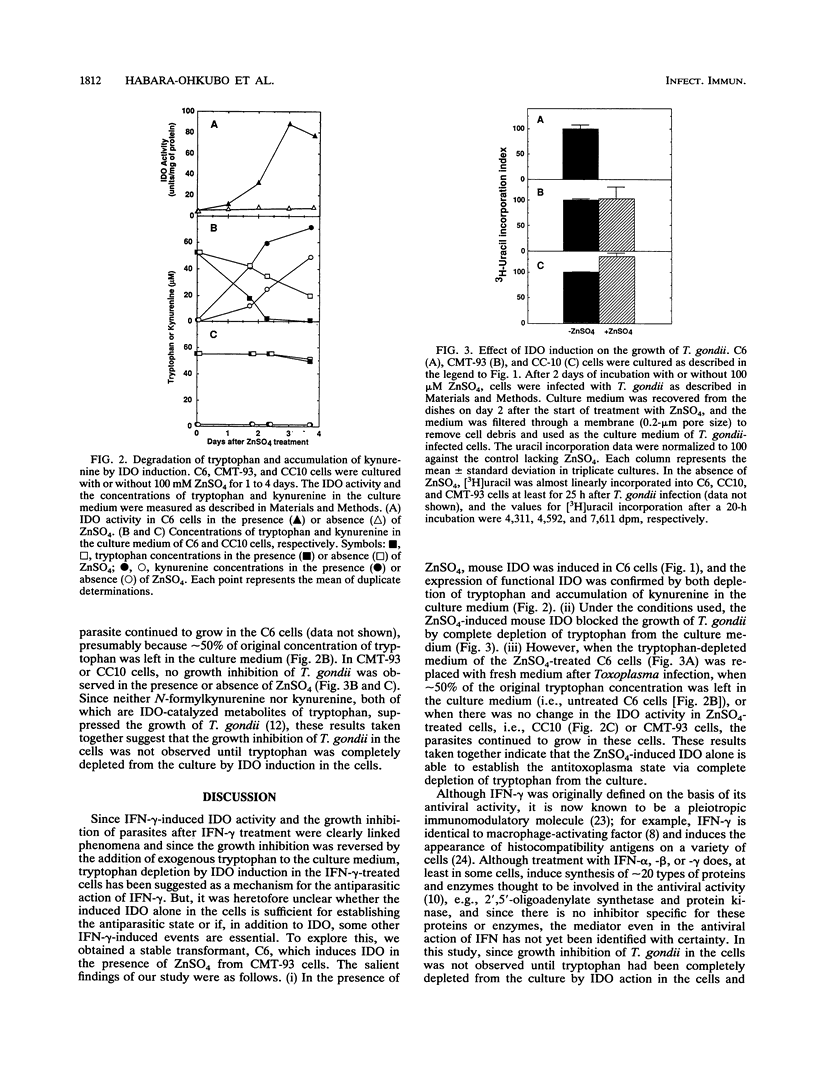
Indoleamine 2,3-dioxygenase (IDO), a tryptophan-degrading enzyme, is inducible by various interferons (IFNs). IDO-mediated tryptophan degradation, but not the formation of IDO-catalyzed tryptophan metabolites, has been suggested as a mechanism for the antiparasitic action of IFN-gamma. To determine whether the IFN-gamma-induced IDO alone is sufficient for establishing the antiparasitic state, we constructed a mouse IDO expression plasmid containing a heavy metal-responsive metallothionein promoter and obtained a stable transformant (C6) by transfection of this plasmid into mouse rectal cancer (CMT-93) cells. In the presence of 100 microM ZnSO4, C6 cells yielded a high level of IDO; and after a 2-day culture period, the enzyme induction resulted in complete depletion of tryptophan from the culture medium. Under these conditions, the growth of Toxoplasma gondii in C6 cells infected with the organisms on day 3 after enzyme induction was completely blocked. In the absence of ZnSO4, however, IDO induction was negligible in C6 cells, and T. gondii continued to grow. Furthermore, in a transformant (CC10) carrying an antisense mouse IDO plasmid or in parental CMT-93 cells, IDO was not induced at all even in the presence of 100 microM ZnSO4, and T. gondii continued to grow in these cells as well. These results taken together indicate that complete depletion of tryptophan from the culture by IDO alone is sufficient to establish the antitoxoplasma state in mouse cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne G. I., Lehmann L. K., Landry G. J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986 Aug;53(2):347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Sondel P. M., Byrne G. I. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987 Oct 1;139(7):2414–2418. [PubMed] [Google Scholar]
- Fukunaga R., Sokawa Y., Nagata S. Constitutive production of human interferons by mouse cells with bovine papillomavirus as a vector. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5086–5090. doi: 10.1073/pnas.81.16.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habara-Ohkubo A., Takikawa O., Yoshida R. Cloning and expression of a cDNA encoding mouse indoleamine 2,3-dioxygenase. Gene. 1991 Sep 15;105(2):221–227. doi: 10.1016/0378-1119(91)90154-4. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Katoh K., Takahashi Y., Hayashi S., Kondoh H. Improved mammalian vectors for high expression of G418 resistance. Cell Struct Funct. 1987 Dec;12(6):575–580. doi: 10.1247/csf.12.575. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki Y., Edelstein M. P., Duch D. S. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon gamma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1242–1246. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Eckel M., Rebhun S. Interferon-gamma suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol. 1986 Sep;20(3):215–224. doi: 10.1016/0166-6851(86)90101-5. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977 Aug;24(3):449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- Schmitz J. L., Carlin J. M., Borden E. C., Byrne G. I. Beta interferon inhibits Toxoplasma gondii growth in human monocyte-derived macrophages. Infect Immun. 1989 Oct;57(10):3254–3256. doi: 10.1128/iai.57.10.3254-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman J. D., Gonias S. L., Pfefferkorn E. R. Murine gamma interferon fails to inhibit Toxoplasma gondii growth in murine fibroblasts. Infect Immun. 1990 Mar;58(3):833–834. doi: 10.1128/iai.58.3.833-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer-Avni Y., Wallach D., Sarov I. Reversion of the antichlamydial effect of tumor necrosis factor by tryptophan and antibodies to beta interferon. Infect Immun. 1989 Nov;57(11):3484–3490. doi: 10.1128/iai.57.11.3484-3490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Growth inhibition of Toxoplasma gondii in cell cultures treated with murine type II interferon. Nihon Juigaku Zasshi. 1982 Dec;44(6):865–871. doi: 10.1292/jvms1939.44.865. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Takikawa O., Kuroiwa T., Yamazaki F., Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988 Feb 5;263(4):2041–2048. [PubMed] [Google Scholar]
- Takikawa O., Yoshida R., Kido R., Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem. 1986 Mar 15;261(8):3648–3653. [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Imanishi J., Oku T., Kishida T., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A. 1981 Jan;78(1):129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Oku T., Imanishi J., Kishida T., Hayaishi O. Interferon: a mediator of indoleamine 2,3-dioxygenase induction by lipopolysaccharide, poly(I) X poly(C), and pokeweed mitogen in mouse lung. Arch Biochem Biophys. 1986 Sep;249(2):596–604. doi: 10.1016/0003-9861(86)90038-x. [DOI] [PubMed] [Google Scholar]



