Abstract
Alzheimer and prion diseases are neurodegenerative disorders characterised by the abnormal processing of amyloid-β (Aβ) peptide and prion protein (PrPC), respectively. Recent evidence indicates that PrPC may play a critical role in the pathogenesis of Alzheimer disease. PrPC interacts with and inhibits the β-secretase BACE1, the rate-limiting enzyme in the production of Aβ. More recently PrPC was identified as a receptor for Aβ oligomers and the expression of PrPC appears to be controlled by the amyloid intracellular domain (AICD). Here we review these observations and propose a feedback loop in the normal brain where PrPC exerts an inhibitory effect on BACE1 to decrease both Aβ and AICD production. In turn, the AICD upregulates PrPC expression, thus maintaining the inhibitory effect of PrPC on BACE1. In Alzheimer disease, this feedback loop is disrupted, and the increased level of Aβ oligomers bind to PrPC and prevent it from regulating BACE1 activity.
Key words: alzheimer disease, amyloid-β, Aβ oligomers, amyloid intracellular domain, BACE1, presenilin, prion protein
Introduction
Alzheimer disease (AD) is the most common form of dementia which currently affects more than 37 million people worldwide.1,2 The prevalence of AD will increase further with an aging population, bringing wide social and economic demands for the care and treatment of AD patients. AD is characterized pathologically by the formation of senile plaques composed of the amyloid-β (Aβ) peptide and neurofibrillary tangles composed of hyperphosphorylated Tau.3 However, it is the accumulation of Aβ in the brain that appears to be critical for the pathogenesis of AD.
The prion protein (PrP) is involved in neurodegeneration via its conversion from the normal cellular form, PrPC, to the infectious form, PrPSc, which is the causative agent of the transmissible spongiform encephalopathies (TSEs) including Creutzfeldt-Jakob disease (CJD).4 While there is an established role for PrPC in TSEs, the physiological role of PrPC has still not been fully established; roles in metal homeostasis, neuroprotective signalling, lymphocyte activation, neurite growth, synaptogenesis, cellular signalling, cell viability and in the cellular response to oxidative stress have all been proposed (reviewed in ref. 5).
There are a number of neuropathological similarities and genetic links between AD and prion diseases. The coexistence of AD pathology in CJD has been reported6 and PrPC has been shown to co-localise with Aβ in plaques.7 These compound PrPC-Aβ plaques were shown to be present in most CJD patients with associated AD-type pathology8 and it has been proposed that PrPC may promote Aβ plaque formation.9 A genetic correlation between PrPC and AD has also been reported. A systematic meta-analysis of AD genetic association studies revealed that the gene encoding PrPC (PRNP) is a potential AD susceptibility gene10 and the Met/Val 129 polymorphism in PRNP has been reported to be a risk factor for early-onset AD.8,11,12
Until recently, although such pathological and genetic links between AD and PrPC had been identified, there was no evidence of an interaction between the proteins involved in these diseases. However, in 2007 we reported an interaction between PrPC and the rate-limiting enzyme in the production of Aβ, the β-secretase BACE1,13 and more recently, two further studies have also found direct links; PrPC has been reported to be a receptor for Aβ oligomers14 and the expression of PrPC is controlled by the amyloid intracellular domain (AICD).15 In this review, we discuss these molecular and cellular links between AD and PrPC and propose a model to encompass these recent findings.
Proteolytic Production and Degradation of Amyloid-β
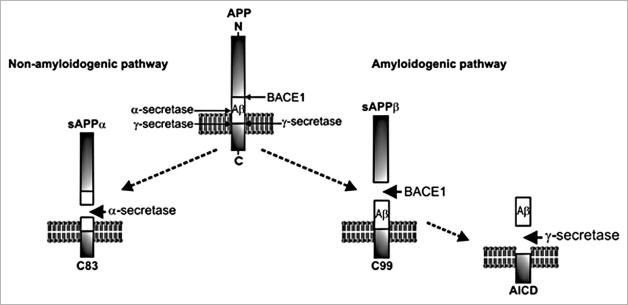
Aβ is formed by the proteolytic processing of the amyloid precursor protein (APP) (Fig. 1).16 The amyloidogenic pathway of APP processing involves the initial cleavage of APP by the β-secretase (BACE1; β-site APP cleaving enzyme-1), to release a soluble N-terminal fragment, sAPPβ. The residual short membrane-bound C-terminal fragment of APP (C99) is subsequently cleaved by the presenilin-containing γ-secretase complex to form Aβ and the amyloid intracellular domain (AICD). The amyloidogenic cleavage of APP results in a number of Aβ isoforms from 39–43 amino acids in length. Of these isoforms, Aβ40 and Aβ42 are the most commonly found. Aβ42 is the more amyloidogenic isoform as it aggregates more readily and it is this isoform that is predominantly found in senile plaques. Aβ peptides can self-assemble into small soluble oligomers or larger protofibrils and fibrils. While monomeric Aβ is generally non-toxic, there is growing evidence that Aβ oligomers are responsible for the synaptic dysfunction that occurs in AD.17–19 APP can alternatively be processed via the non-amyloidogenic pathway (Fig. 1) where the initial cleavage is by α-secretase, members of the ADAM (a disintegrin and metalloprotease) family.16 This α-cleavage occurs within the Aβ region, thus precluding the formation of the Aβ peptide.
Figure 1.
Proteolytic processing of APP. The amyloidogenic pathway involves the sequential cleavage of APP first by BACE1, producing sAPPβ and C99, and then by γ-secretase to release the amyloidogenic Aβ and the AICD. In the non-amyloidogenic pathway, α-secretase cleaves within the Aβ fragment, preventing the formation of Aβ peptides.
The amyloid cascade hypothesis, formulated in 1991,20 implies that amyloid deposition is the central event in the progression of AD and therefore indicates that an understanding of the mechanisms involved in Aβ generation, degradation and clearance are critical to understanding the development of the disease. In normal brains, Aβ is degraded by multiple peptidases, including neprilysin, endothelin-converting enzyme and insulin-degrading enzyme.21 While Aβ is commonly thought of as the main toxic agent in AD, there is emerging evidence of a normal physiological role for the peptide in the regulation of neuronal calcium and potassium channel currents.22,23 This, therefore, indicates that Aβ should only be considered as a toxic agent when levels increase as a result of an imbalance in its production and degradation/clearance, such as occurs in AD.
In late-onset, sporadic AD the amyloidogenic processing of APP is increased as a result of increased BACE1 activity and protein levels.24–26 As BACE1 is the rate-limiting step in Aβ production, an increase in the expression and activity of this protein will have a significant effect on Aβ levels. More recently, it has also been shown that γ-secretase activity may be enhanced in AD as presenilin-1 mRNA levels have been shown to be increased in AD brains.27 In addition to increased Aβ production, the degradation of Aβ is decreased due a reduction in the levels of the Aβ-degrading enzymes. For example, insulin-degrading enzyme is decreased in the brains of AD patients28 and more specifically in the hippocampus, an area primarily affected in AD29 and neprilysin levels decline in brain areas affected by AD.30
PrPC Regulates the β-Secretase Cleavage of APP
In 2007, we reported that PrPC decreased the amyloidogenic processing of APP thereby decreasing Aβ levels.13 Overexpression of PrPC in a human neuroblastoma cell line decreased both sAPPβ and Aβ levels, while PrPC depletion by siRNA in a murine neuroblastoma (N2a) cell line or by genetic knockout in mice resulted in increased Aβ levels. As the effect of PrPC on APP processing was seen at the level of β-secretase cleavage, indicated by the change in sAPPβ levels, it was concluded that PrPC mediates its effect on Aβ levels by decreasing the cleavage of APP by BACE1. We went on to investigate the mechanism involved in PrPC inhibition of BACE1, and were able to determine that the interaction between PrPC and BACE1 required localisation of PrPC to cholesterol-rich lipid rafts, where BACE1 cleavage of APP preferentially occurs,31,32 and found that the polybasic region at the extreme N-terminus of mature PrPC possibly through interactions with glycosaminoglycans is critical for the interaction with BACE1. To study the effect of the Met/Val129 polymorphism in human PRNP on Aβ production, we utilized mice whose endogenous Prnp gene was replaced with the human PRNP harboring either the MM or VV 129 genotypes.33 Interestingly, we found that there was a significant increase in the amount of Aβ40 in the brains of the MM mice compared to the VV mice.13 Further studies are required to determine whether a similar increase in Aβ occurs in humans homozygous for Met129 and if the polymorphism contributes to an increased risk of early onset AD.12 Together our results indicated that PrPC has a regulatory role in mediating Aβ production and suggested that PrPC is protective against AD.34
p53-Dependent Transcriptional Control of PrPC by Presenilins
Vincent et al.15 recently reported a link between PrPC expression and regulation by the presenilins, the catalytic subunits of the γ-secretase complex. The study revealed a direct link between γ-secretase activity and PrPC mRNA levels and protein expression. The authors determined that the AICD, resulting from the γ-secretase cleavage of APP (Fig. 1), plays a role in the regulation of PrPC expression. The mechanism proposed by the authors is that the AICD, in association with Tip60 and Fe65, translocates to the nucleus and acts as a transcription factor to regulate p53 expression. p53 was shown to regulate PrPC at the transcriptional level by interacting with its promoter, resulting in changes in PrPC mRNA and protein expression. Previous work by the same authors35 and others36 has reported that the AICD acts as a transcription factor and that one of its target genes is the Aβ-degrading enzyme neprilysin.
A Model for the Regulation of APP Processing by PrPC—A Feedback Loop
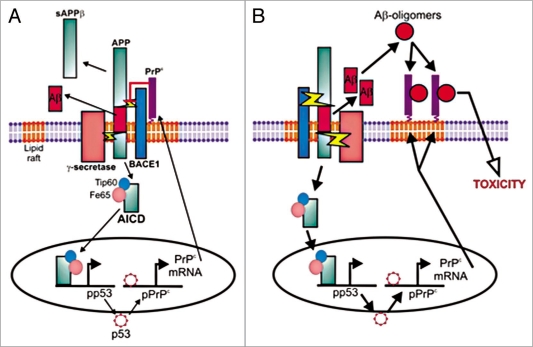
Our work showing that PrPC inhibits the β-secretase cleavage of APP and hence reduces Aβ production, along with the recent observation that the AICD from the γ-secretase cleavage of APP upregulates PrPC expression, suggests that there might be a potential feedback loop to control Aβ production (Fig. 2A). PrPC exerts an inhibitory effect on BACE1 to decrease the amyloidogenic processing of APP hence controlling both Aβ and AICD production. In turn, the amount of APP processing regulates the inhibitory effect of PrPC on BACE1 via the AICD regulating PrPC expression. Such a feedback loop would control Aβ production to maintain the balance between its production and degradation, so ensuring that there is adequate Aβ to maintain its normal physiological roles while preventing the toxicity that results from excessive Aβ. If the inhibitory effect of PrPC on BACE1 is reduced, resulting in higher Aβ and AICD levels, the increased AICD would lead to increased expression of PrPC, thus restoring the inhibition of BACE1 and lowering Aβ and AICD levels.
Figure 2.
A model for the regulation of APP processing by PrPC. (A) Aβ levels are kept in balance under physiological conditions via inhibition of BACE1 by PrPC and regulation of PrPC levels via AICD. (B) In AD, Aβ levels are increased as a result of increased production and/or decreased degradation and clearance resulting in the increased formation of Aβ-oligomers which are toxic via their interaction with PrPC. The binding of the Aβ-oligomers to PrPC may disrupt its regulation of BACE1, thereby further increasing APP processing and Aβ levels.
PrPC Mediates Impairment of Synaptic Plasticity by Aβ Oligomers
Lauren et al.14 recently reported the results from an expression cloning screen to identify potential binding sites for Aβ42-oligomers. Two-independent positive clones isolated from a total of 225,000 clones revealed that the Aβ42-oligomers bound to full-length PrPC and further investigation indicated that PrPC has high affinity and high selectivity for Aβ42-oligomers. Purified recombinant PrPC was shown to interact directly with Aβ42-oligomers in a pull-down assay and the binding of synthetic Aβ42-oligomers to neurons was shown to be decreased in PrPC-null mice, suggesting that PrPC acts as a receptor for Aβ42-oligomers. To determine the region of PrPC involved in the interaction, several mutated forms of PrPC were generated lacking specific domains; the results identified that a specific charge cluster region (amino acids 95–110) within the unstructured central region of PrPC was the principal site for Aβ42-oligomer binding. The critical role of this charge cluster region in binding Aβ42-oligomers was confirmed using antibodies against specific epitopes of PrPC. The authors also examined the effects of the interaction between PrPC and Aβ42-oligomers on long-term potentiation (LTP) using hippocampal slices from wild-type and PrPC-null mice. Soluble Aβ42-oligomers reduced LTP in the wild-type mice but not in the PrPC-null mice, indicating that PrPC is required to mediate one of the toxic effects of Aβ. This study concluded that there is a direct interaction between PrPC and Aβ42-oligomers and that this interaction affects synaptic plasticity. This suggests, therefore, that PrPC mediates a toxic effect of the Aβ42-oligomers and thereby plays an important role in the neurodegeneration associated with AD. The implication from this is that higher levels of PrPC would be detrimental in AD as they would allow for further Aβ42-oligomer interaction and thus greater neuronal toxicity, while a reduction in PrPC levels would be protective.
Disruption in AD of the Feedback Loop by which PrPC Regulates APP Processing
With the observation that PrPC exerts a regulatory effect on Aβ production, it is perhaps surprising that it also may be a mediator of neuronal toxicity by acting as a receptor for Aβ42-oligomers. So, does PrPC have a protective role in AD or does it mediate the neuronal toxicity of Aβ? The identification of PrPC as a regulator of BACE1 activity may reflect a physiological role for PrPC to control Aβ generation in the ‘normal’ brain. The binding of Aβ42-oligomers to PrPC may be more reflective of the later stages of AD when Aβ levels are elevated in the brain.
As discussed above, in AD Aβ levels increase above that seen in age-matched normal brains, and specifically, Aβ42 levels have been shown to increase in AD patients37 which may result in an increased pool of Aβ42 which can then aggregate to form toxic oligomers. These oligomers then mediate their toxic effects in part via PrPC. In addition, the increased proteolytic cleavage of APP would also increase AICD generation which would further increase PrPC levels providing more receptors for the Aβ42-oligomers resulting in further cell toxicity (Fig. 2B). But why can't the increased level of PrPC regulate the BACE1 cleavage of APP? One possibility is that binding of the Aβ42-oligomers to PrPC sterically prevents it from interacting with BACE1. Alternatively, binding of Aβ42-oligomers may, for example, result in the removal of PrPC from lipid rafts, through enhancing its endocytosis, thus preventing PrPC from interacting with BACE1. The other possibility is that in the AD brain there is a disruption in the feedback loop by which the AICD normally upregulates PrPC expression. In support of this latter possibility is the observation that the amount of PrPC in the brains of AD patients was significantly lower than in age-matched controls.38 (Whitehouse and Hooper NM, unpublished). A recent brief report39 that showed a decrease of PrPC in the hippocampus, frontal cortex and temporal cortex in AD would also appear to support this. However, the number of cases (two AD and three controls) was too small for statistical analysis and whether the AD cases were sporadic and appropriately age matched with the controls not reported. Like many biological changes that occur as a consequence of the pathologic cascade in AD, this reduction in PrPC could simply be explained by downstream events associated with the neurodegeneration and further studies are required to clarify this.
Conclusions
The recent data reviewed here hints at two potential roles for PrPC in AD: first, a role in the physiological regulation of APP processing via its interaction with BACE1; and second, a role in the pathological progression of AD by mediating Aβ toxicity by binding Aβ42-oligomers. The feedback loop between PrPC, BACE1, APP and AICD described here provides a model linking these recent observations. However, several questions remain to be answered, including what effect does Aβ42-oligomer binding have on the functions of PrPC, how do the levels of PrPC compare in the brains of AD patients and age-matched controls, and what is the effect of altering PrPC levels in mouse models of AD. Clearly understanding the molecular and cellular mechanisms involved in the interactions between PrPC and APP/Aβ is crucial to our understanding of AD pathogenesis and warrants urgent further investigation.
Acknowledgements
We gratefully acknowledge the financial support of the Alzheimer Research Trust and the Medical Research Council of Great Britain.
Abbreviations
- Aβ
amyloid-β peptide
- AD
Alzheimer disease
- AICD
amyloid intracellular domain
- APP
amyloid precursor protein
- BACE1
β-site APP cleaving enzyme-1
- CJD
Creutzfeldt-Jakob disease
- LTP
long-term potentiation
- PrPC
cellular form of the prion protein
- PrPSc
infectious form of the prion protein
- sAPPβ
soluble ectodomain fragment of APP after cleavage by β-secretase
- TSE
transmissible spongiform encephalopathy
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/9980
References
- 1.Mount C, Downton C. Alzheimer disease: progress or profit? Nat Med. 2006;12:780–784. doi: 10.1038/nm0706-780. [DOI] [PubMed] [Google Scholar]
- 2.Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338:467–471. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 3.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid [beta]-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 4.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 5.Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- 6.Hainfellner JA, Wanschitz J, Jellinger K, Liberski PP, Gullotta F, Budka H. Coexistence of Alzheimer-type neuropathology in Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 1998;96:116–122. doi: 10.1007/s004010050870. [DOI] [PubMed] [Google Scholar]
- 7.Voigtlander T, Kloppel S, Birner P, Jarius C, Flicker H, Verghese-Nikolakaki S, et al. Marked increase of neuronal prion protein immunoreactivity in Alzheimer's disease and human prion diseases. Acta Neuropathol (Berl) 2001;101:417–423. doi: 10.1007/s004010100405. [DOI] [PubMed] [Google Scholar]
- 8.Del Bo R, Scarlato M, Ghezzi S, Martinelli-Boneschi F, Fenoglio C, Galimberti G, et al. Is M129V of PRNP gene associated with Alzheimer's disease? A case-control study and a meta-analysis. Neurobiol Aging. 2006;27:770–775. doi: 10.1016/j.neurobiolaging.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Schwarze-Eicker K, Keyvani K, Gortz N, Westaway D, Sachser N, Paulus W. Prion protein (PrPc) promotes beta-amyloid plaque formation. Neurobiol Aging. 2005;26:1177–1182. doi: 10.1016/j.neurobiolaging.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 11.Dermaut B, Croes EA, Rademakers R, Van den Broeck M, Cruts M, Hofman A, et al. PRNP Val129 homozygosity increases risk for early-onset Alzheimer's disease. Ann Neurol. 2003;53:409–412. doi: 10.1002/ana.10507. [DOI] [PubMed] [Google Scholar]
- 12.Riemenschneider M, Klopp N, Xiang W, Wagenpfeil S, Vollmert C, Muller U, et al. Prion protein codon 129 polymorphism and risk of Alzheimer disease. Neurology. 2004;63:364–366. doi: 10.1212/01.wnl.0000130198.72589.69. [DOI] [PubMed] [Google Scholar]
- 13.Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, et al. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc Natl Acad Sci USA. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent B, Sunyach C, Orzechowski H-D, St George-Hyslop P, Checler F. p53-Dependent transcriptional control of cellular prion by presenilins. J Neurosci. 2009;29:6752–6760. doi: 10.1523/JNEUROSCI.0789-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vardy ERLC, Catto AJ, Hooper NM. Proteolytic mechanisms in amyloid-beta metabolism: therapeutic implications for Alzheimer's disease. Trends Mol Med. 2005;11:464–472. doi: 10.1016/j.molmed.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 18.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 20.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 21.Carson JA, Turner AJ. β-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases? J Neurochem. 2002;81:1–8. doi: 10.1046/j.1471-4159.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramsden M, Plant LD, Webster NJ, Vaughan PF, Henderson Z, Pearson HA. Differential effects of unaggregated and aggregated amyloid beta protein (1–40) on K+ channel currents in primary cultures of rat cerebellar granule and cortical neurones. J Neurochem. 2001;79:699–712. doi: 10.1046/j.1471-4159.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 23.Plant LD, Webster NJ, Boyle JP, Ramsden M, Freir DB, Peers C, et al. Amyloid beta peptide as a physiological modulator of neuronal ‘A’-type K+ curren. Neurobiol Aging. 2006;27:1673–1683. doi: 10.1016/j.neurobiolaging.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 24.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 25.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 26.Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, et al. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer's disease patients. Proc Natl Acad Sci USA. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui T, Ingelsson M, Fukumoto H, Ramasamy K, Kowa H, Frosch MP, et al. Expression of APP pathway mRNAs and proteins in Alzheimer's disease. Brain Res. 2007;1161:116–123. doi: 10.1016/j.brainres.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 28.Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, et al. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer's disease is associated with the apolipoprotein E-epsilon4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O'Connor T, et al. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer's disease pathogenesis. J Neurosci. 2007;27:3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama H, Kondo H, Ikeda K, Kato M, McGeer PL. Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid beta-protein (Abeta) deposition. Brain Res. 2001;902:277–281. doi: 10.1016/s0006-8993(01)02390-3. [DOI] [PubMed] [Google Scholar]
- 31.Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition upregulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, Thomson V, et al. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006;5:393–398. doi: 10.1016/S1474-4422(06)70413-6. [DOI] [PubMed] [Google Scholar]
- 34.Hooper NM, Turner AJ. A new take on prions: preventing Alzheimer's disease. Trends Biochem Sci. 2008;33:151–155. doi: 10.1016/j.tibs.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Pardossi-Piquard R, Petit A, Kawarai T, Sunyach C, Alves da Costa C, Vincent B, et al. Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron. 2005;46:541–554. doi: 10.1016/j.neuron.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Belyaev ND, Nalivaeva NN, Makova NZ, Turner AJ. Neprilysin gene expression requires binding of the amyloid precursor protein intracellular domain to its promoter: implications for Alzheimer disease. EMBO Rep. 2009;10:94–100. doi: 10.1038/embor.2008.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, et al. Quantitation of amyloid beta-protein (Ab) in the cortex during aging and in Alzheimer's disease. Am J Pathol. 1998;152:1633–1640. [PMC free article] [PubMed] [Google Scholar]
- 38.Rezaie P, Pontikis CC, Hudson L, Cairns NJ, Lantos PL. Expression of cellular prion protein in the frontal and occipital lobe in Alzheimer's disease, diffuse Lewy body disease, and in normal brain: an immunohistochemical study. J Histochem Cytochem. 2005;53:929–940. doi: 10.1369/jhc.4A6551.2005. [DOI] [PubMed] [Google Scholar]
- 39.Velayos JL, Irujo A, Cuadrado M, Paternain B, Moleres FJ, Ferrer V. The cellular prion protein and its role in Alzheimer's disease. Prion. 2009;3:110–117. doi: 10.4161/pri.3.2.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]